Abstract
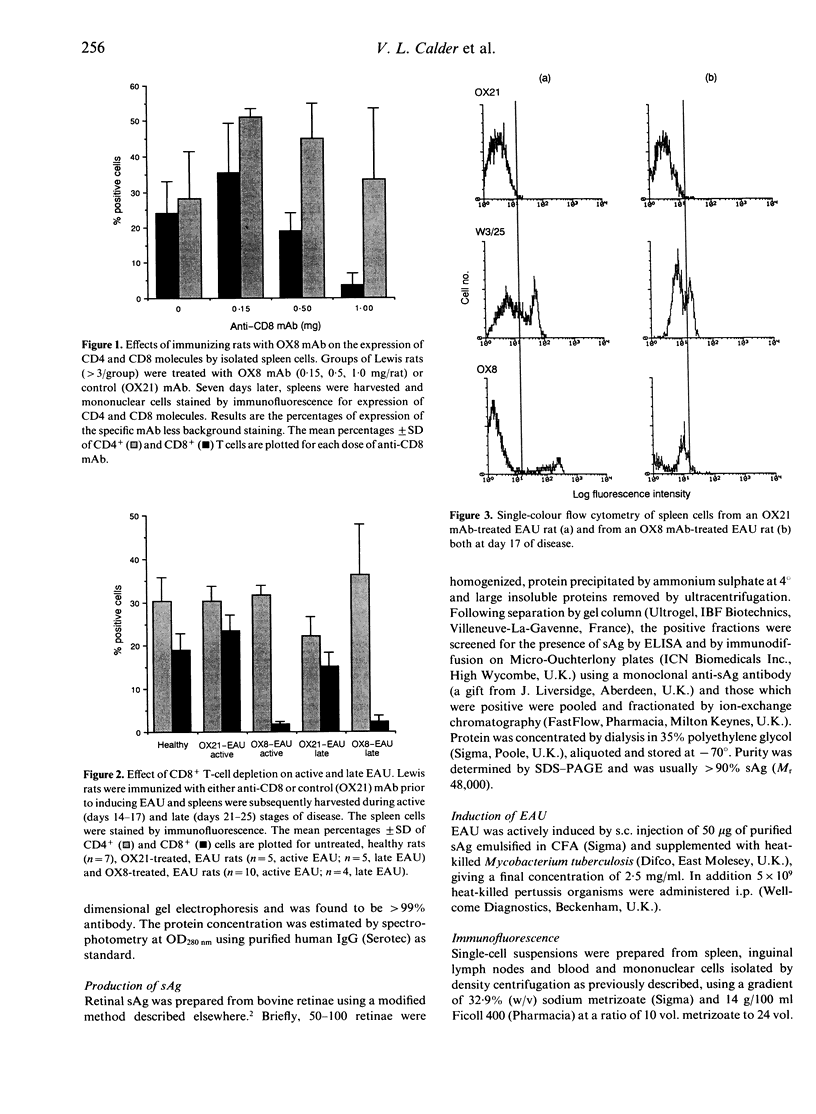
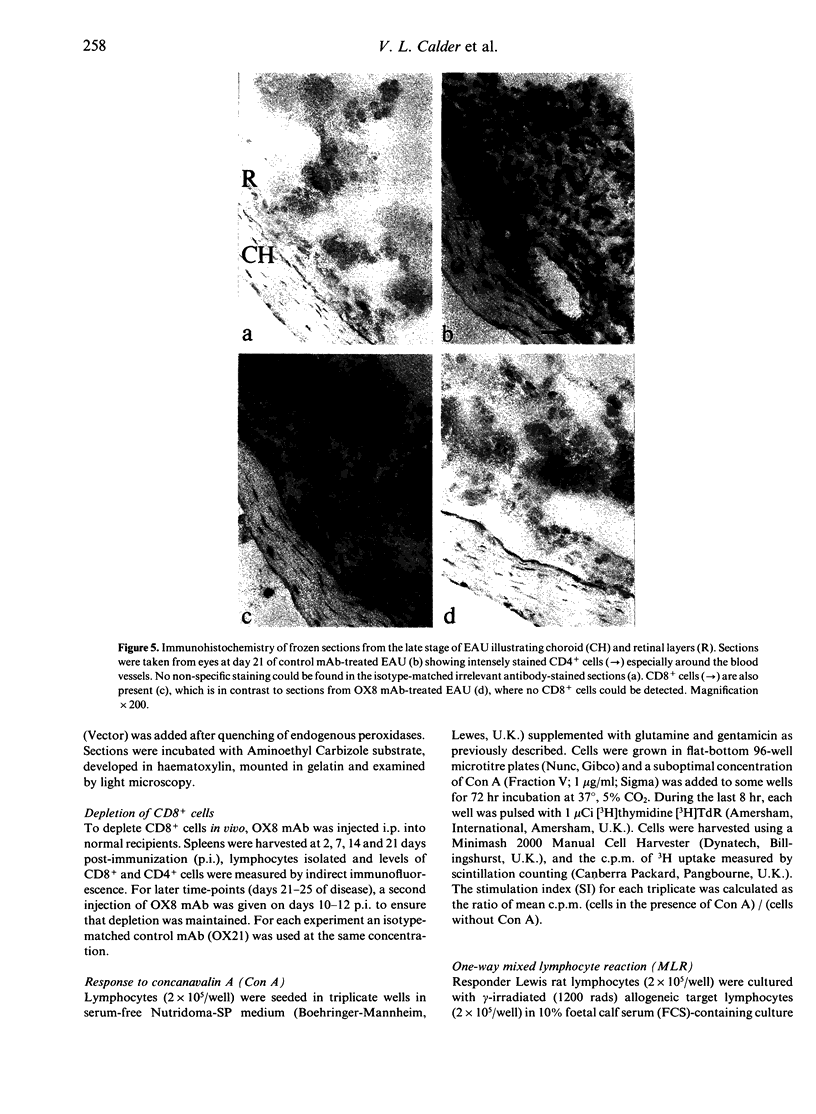
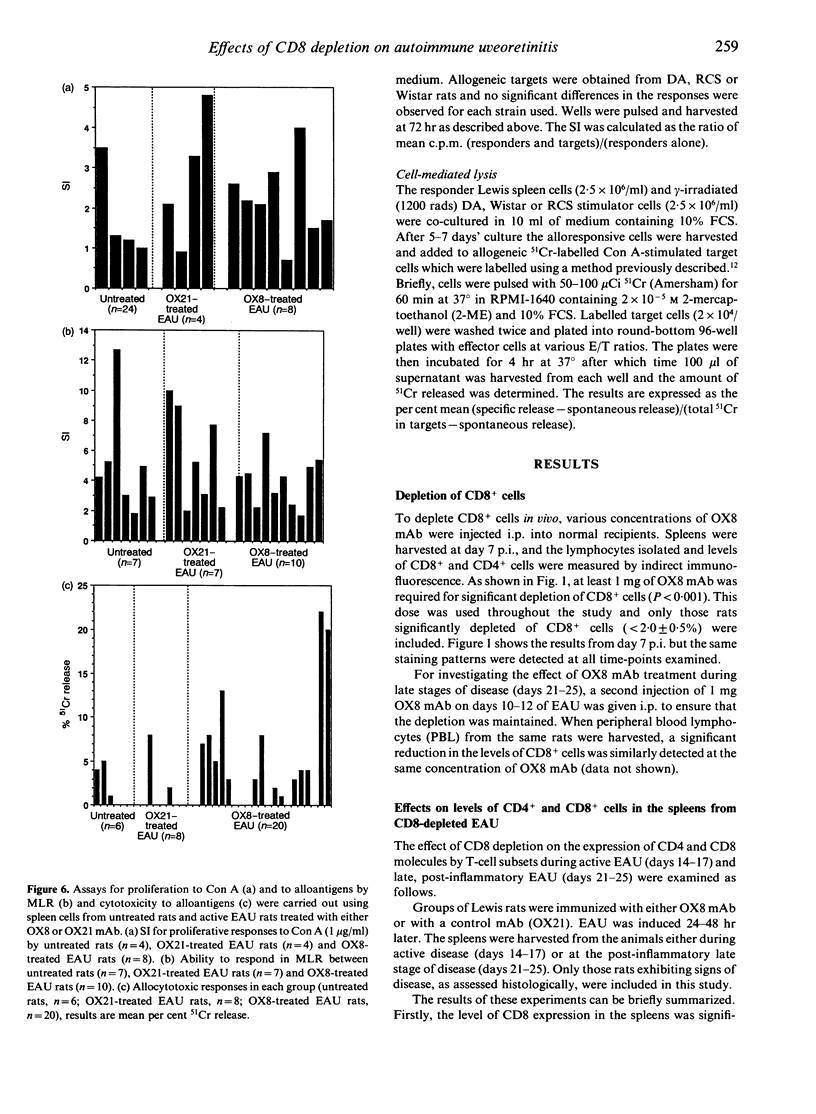
During the later stages of soluble-antigen (sAg)-induced experimental autoimmune uveoretinitis (EAU), an increase in the relative number of CD8+ lymphocytes has been observed at the site of inflammation in the retina. It has been suggested that these late-appearing CD8+ cells might down-regulate this acute disease process. To determine the role of the CD8+ cells in EAU, Lewis rats were depleted of CD8+ cells prior to and during disease and the enucleated eyes examined histologically. The spleen cells from CD8-depleted rats were also examined for their ability to respond to concanavalin A (Con A) and to allogeneic targets as determined by mixed lymphocyte reaction (MLR) and cytotoxicity assays. The results suggest that depleting CD8+ cells had no effect on the course of disease and that CD8+ cells do not play a crucial role in the immunoregulation of EAU.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alters S. E., Sakai K., Steinman L., Oi V. T. Mechanisms of anti-CD4-mediated depletion and immunotherapy. A study using a set of chimeric anti-CD4 antibodies. J Immunol. 1990 Jun 15;144(12):4587–4592. [PubMed] [Google Scholar]
- Atalla L., Linker-Israeli M., Steinman L., Rao N. A. Inhibition of autoimmune uveitis by anti-CD4 antibody. Invest Ophthalmol Vis Sci. 1990 Jul;31(7):1264–1270. [PubMed] [Google Scholar]
- Bakhiet M., Olsson T., van der Meide P., Kristensson K. Depletion of CD8+ T cells suppresses growth of Trypanosoma brucei brucei and interferon-gamma) production in infected rats. Clin Exp Immunol. 1990 Aug;81(2):195–199. doi: 10.1111/j.1365-2249.1990.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Carteron N. L., Schimenti C. L., Wofsy D. Treatment of murine lupus with F(ab')2 fragments of monoclonal antibody to L3T4. Suppression of autoimmunity does not depend on T helper cell depletion. J Immunol. 1989 Mar 1;142(5):1470–1475. [PubMed] [Google Scholar]
- Caspi R. R., Roberge F. G., McAllister C. G., el-Saied M., Kuwabara T., Gery I., Hanna E., Nussenblatt R. B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [PubMed] [Google Scholar]
- Chan C. C., Mochizuki M., Nussenblatt R. B., Palestine A. G., McAllister C., Gery I., BenEzra D. T-lymphocyte subsets in experimental autoimmune uveitis. Clin Immunol Immunopathol. 1985 Apr;35(1):103–110. doi: 10.1016/0090-1229(85)90083-2. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Mochizuki M., Palestine A. G., BenEzra D., Gery I., Nussenblatt R. B. Kinetics of T-lymphocyte subsets in the eyes of Lewis rats with experimental autoimmune uveitis. Cell Immunol. 1985 Dec;96(2):430–434. doi: 10.1016/0008-8749(85)90373-9. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Martin G., Waldmann H. The induction of skin graft tolerance in major histocompatibility complex-mismatched or primed recipients: primed T cells can be tolerized in the periphery with anti-CD4 and anti-CD8 antibodies. Eur J Immunol. 1990 Dec;20(12):2747–2755. doi: 10.1002/eji.1830201232. [DOI] [PubMed] [Google Scholar]
- Fallis R. J., McFarlin D. E. Chronic relapsing experimental allergic encephalomyelitis. Cytotoxicity effected by a class II restricted T cell line specific for an encephalitogenic epitope. J Immunol. 1989 Oct 1;143(7):2160–2165. [PubMed] [Google Scholar]
- Holmdahl R., Olsson T., Moran T., Klareskog L. In vivo treatment of rats with monoclonal anti-T-cell antibodies. Immunohistochemical and functional analysis in normal rats and in experimental allergic neuritis. Scand J Immunol. 1985 Aug;22(2):157–169. doi: 10.1111/j.1365-3083.1985.tb01868.x. [DOI] [PubMed] [Google Scholar]
- Hsiung L., Barclay A. N., Brandon M. R., Sim E., Porter R. R. Purification of human C3b inactivator by monoclonal-antibody affinity chromatography. Biochem J. 1982 Apr 1;203(1):293–298. doi: 10.1042/bj2030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone A. M., Powis S. J., Diamond A. G., Butcher G. W., Howard J. C. A trans-acting major histocompatibility complex-linked gene whose alleles determine gain and loss changes in the antigenic structure of a classical class I molecule. J Exp Med. 1989 Sep 1;170(3):777–795. doi: 10.1084/jem.170.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Mathieson P. W., Stapleton K. J., Oliveira D. B., Lockwood C. M. Immunoregulation of mercuric chloride-induced autoimmunity in Brown Norway rats: a role for CD8+ T cells revealed by in vivo depletion studies. Eur J Immunol. 1991 Sep;21(9):2105–2109. doi: 10.1002/eji.1830210919. [DOI] [PubMed] [Google Scholar]
- Saoudi A., Bellon B., de Kozak Y., Kuhn J., Vial M. C., Thillaye B., Druet P. Prevention of experimental autoimmune uveoretinitis and experimental autoimmune pinealitis in (Lewis x Brown-Norway) F1 rats by HgCl2 injections. Immunology. 1991 Oct;74(2):348–354. [PMC free article] [PubMed] [Google Scholar]
- Sedgwick J. D. Long-term depletion of CD8+ T cells in vivo in the rat: no observed role for CD8+ (cytotoxic/suppressor) cells in the immunoregulation of experimental allergic encephalomyelitis. Eur J Immunol. 1988 Apr;18(4):495–502. doi: 10.1002/eji.1830180402. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., MacPhee I. A., Puklavec M. Isolation of encephalitogenic CD4+ T cell clones in the rat. Cloning methodology and interferon-gamma secretion. J Immunol Methods. 1989 Jul 26;121(2):185–196. doi: 10.1016/0022-1759(89)90159-2. [DOI] [PubMed] [Google Scholar]
- Shinohara N., Huang Y. Y., Muroyama A. Specific suppression of antibody responses by soluble protein-specific, class II-restricted cytolytic T lymphocyte clones. Eur J Immunol. 1991 Jan;21(1):23–27. doi: 10.1002/eji.1830210105. [DOI] [PubMed] [Google Scholar]
- Wacker W. B., Donoso L. A., Kalsow C. M., Yankeelov J. A., Jr, Organisciak D. T. Experimental allergic uveitis. Isolation, characterization, and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol. 1977 Dec;119(6):1949–1958. [PubMed] [Google Scholar]