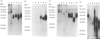Abstract
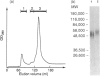
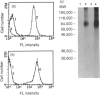
Porcine CD44 was identified by the cross-reactive anti-human CD44 monoclonal antibody (mAb) Hermes-1. Material which inhibited the Hermes-1 mAb binding to porcine lymphocytes was found in porcine intestinal efferent lymph and demonstrated to be a soluble form of porcine CD44. A protocol using non-affinity techniques was established to isolate this material from porcine lymph. The isolated soluble CD44 molecule consists of a single peptide with an apparent MW of about 50,000, containing the Hermes-1 epitope. The soluble and membrane-bound forms of porcine CD44 were also affinity purified and characterized. The soluble CD44 preparation comprised a major component of 48,000-70,000 MW peptide and a minor band of 120,000 MW. The membrane-bound CD44 preparation contained a 90,000 MW major and a 120,000 MW minor component. Both the major components of the soluble and membrane-bound forms of porcine CD44 contained N-linked glycosylation. The 48,000-70,000 MW component of the soluble CD44 molecules consisted of two subsets discriminated by their binding capacity for lentil lectin and a slight difference in molecular weights. Soluble CD44 molecules were also identified in the sera of sheep, goats, horses, dogs and humans. Like the porcine counterpart, these molecules could be enriched by anion-exchange chromatography, suggesting that they have similar biochemical properties.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Bazil V., Horejsí V. Shedding of the CD44 adhesion molecule from leukocytes induced by anti-CD44 monoclonal antibody simulating the effect of a natural receptor ligand. J Immunol. 1992 Aug 1;149(3):747–753. [PubMed] [Google Scholar]
- Belitsos P. C., Hildreth J. E., August J. T. Homotypic cell aggregation induced by anti-CD44(Pgp-1) monoclonal antibodies and related to CD44(Pgp-1) expression. J Immunol. 1990 Mar 1;144(5):1661–1670. [PubMed] [Google Scholar]
- Chin Y. H., Rasmussen R. A., Woodruff J. J., Easton T. G. A monoclonal anti-HEBFPP antibody with specificity for lymphocyte surface molecules mediating adhesion to Peyer's patch high endothelium of the rat. J Immunol. 1986 Apr 1;136(7):2556–2561. [PubMed] [Google Scholar]
- Culty M., Miyake K., Kincade P. W., Sikorski E., Butcher E. C., Underhill C., Silorski E. The hyaluronate receptor is a member of the CD44 (H-CAM) family of cell surface glycoproteins. J Cell Biol. 1990 Dec;111(6 Pt 1):2765–2774. doi: 10.1083/jcb.111.6.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan B. F., Dalchau R., Allen A. K., Daar A. S., Fabre J. W. Chemical composition and tissue distribution of the human CDw44 glycoprotein. Immunology. 1989 Jun;67(2):167–175. [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. A., Zhou D. F., Picker L. J., Minty C. N., Bargatze R. F., Ding J. F., Butcher E. C. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989 Mar 24;56(6):1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Hale L. P., Singer K. H., Haynes B. F. CD44 antibody against In(Lu)-related p80, lymphocyte-homing receptor molecule inhibits the binding of human erythrocytes to T cells. J Immunol. 1989 Dec 15;143(12):3944–3948. [PubMed] [Google Scholar]
- Haynes B. F., Telen M. J., Hale L. P., Denning S. M. CD44--a molecule involved in leukocyte adherence and T-cell activation. Immunol Today. 1989 Dec;10(12):423–428. doi: 10.1016/0167-5699(89)90040-6. [DOI] [PubMed] [Google Scholar]
- Horst E., Meijer C. J., Duijvestijn A. M., Hartwig N., Van der Harten H. J., Pals S. T. The ontogeny of human lymphocyte recirculation: high endothelial cell antigen (HECA-452) and CD44 homing receptor expression in the development of the immune system. Eur J Immunol. 1990 Jul;20(7):1483–1489. doi: 10.1002/eji.1830200712. [DOI] [PubMed] [Google Scholar]
- Huet S., Groux H., Caillou B., Valentin H., Prieur A. M., Bernard A. CD44 contributes to T cell activation. J Immunol. 1989 Aug 1;143(3):798–801. [PubMed] [Google Scholar]
- Idzerda R. L., Carter W. G., Nottenburg C., Wayner E. A., Gallatin W. M., St John T. Isolation and DNA sequence of a cDNA clone encoding a lymphocyte adhesion receptor for high endothelium. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4659–4663. doi: 10.1073/pnas.86.12.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M., Bargatze R., Tammi M., Butcher E. C. Biochemical properties of glycoproteins involved in lymphocyte recognition of high endothelial venules in man. J Immunol. 1988 Sep 1;141(5):1615–1623. [PubMed] [Google Scholar]
- Koopman G., van Kooyk Y., de Graaff M., Meyer C. J., Figdor C. G., Pals S. T. Triggering of the CD44 antigen on T lymphocytes promotes T cell adhesion through the LFA-1 pathway. J Immunol. 1990 Dec 1;145(11):3589–3593. [PubMed] [Google Scholar]
- Kornfeld S., Rogers J., Gregory W. The nature of the cell surface receptor site for Lens culinaris phytohemagglutinin. J Biol Chem. 1971 Nov;246(21):6581–6586. [PubMed] [Google Scholar]
- Lesley J., Schulte R., Hyman R. Binding of hyaluronic acid to lymphoid cell lines is inhibited by monoclonal antibodies against Pgp-1. Exp Cell Res. 1990 Apr;187(2):224–233. doi: 10.1016/0014-4827(90)90085-o. [DOI] [PubMed] [Google Scholar]
- Lucas M. G., Green A. M., Telen M. J. Characterization of the serum In(Lu)-related antigen: identification of a serum protein related to erythrocyte p80. Blood. 1989 Feb;73(2):596–600. [PubMed] [Google Scholar]
- Miyake K., Medina K. L., Hayashi S., Ono S., Hamaoka T., Kincade P. W. Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med. 1990 Feb 1;171(2):477–488. doi: 10.1084/jem.171.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Underhill C. B., Lesley J., Kincade P. W. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990 Jul 1;172(1):69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottenburg C., Rees G., St John T. Isolation of mouse CD44 cDNA: structural features are distinct from the primate cDNA. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8521–8525. doi: 10.1073/pnas.86.21.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecanda A., Walcheck B., Bishop D. K., Jutila M. A. Rapid activation-independent shedding of leukocyte L-selectin induced by cross-linking of the surface antigen. Eur J Immunol. 1992 May;22(5):1279–1286. doi: 10.1002/eji.1830220524. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Siraganian R., Wahl L., Shaw S. Dual role of the CD44 molecule in T cell adhesion and activation. J Immunol. 1989 Oct 15;143(8):2457–2463. [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Aruffo A., Amiot M., Seed B. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO J. 1991 Feb;10(2):343–348. doi: 10.1002/j.1460-2075.1991.tb07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Guo Y. J., Stamenkovic I. Distinct effects of two CD44 isoforms on tumor growth in vivo. J Exp Med. 1991 Oct 1;174(4):859–866. doi: 10.1084/jem.174.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Optimizing hydrolysis of N-linked high-mannose oligosaccharides by endo-beta-N-acetylglucosaminidase H. Anal Biochem. 1984 Sep;141(2):515–522. doi: 10.1016/0003-2697(84)90080-0. [DOI] [PubMed] [Google Scholar]
- Zhou D. F., Ding J. F., Picker L. J., Bargatze R. F., Butcher E. C., Goeddel D. V. Molecular cloning and expression of Pgp-1. The mouse homolog of the human H-CAM (Hermes) lymphocyte homing receptor. J Immunol. 1989 Nov 15;143(10):3390–3395. [PubMed] [Google Scholar]