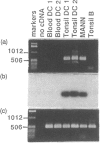
Abstract
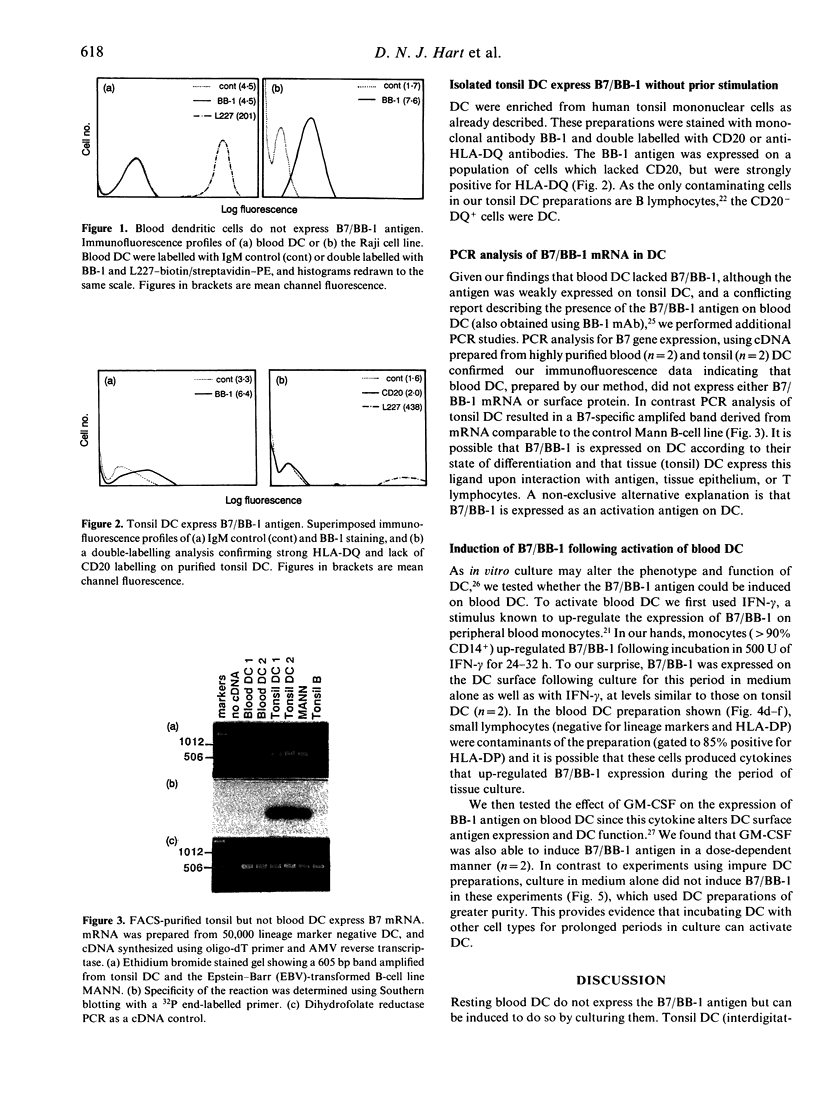
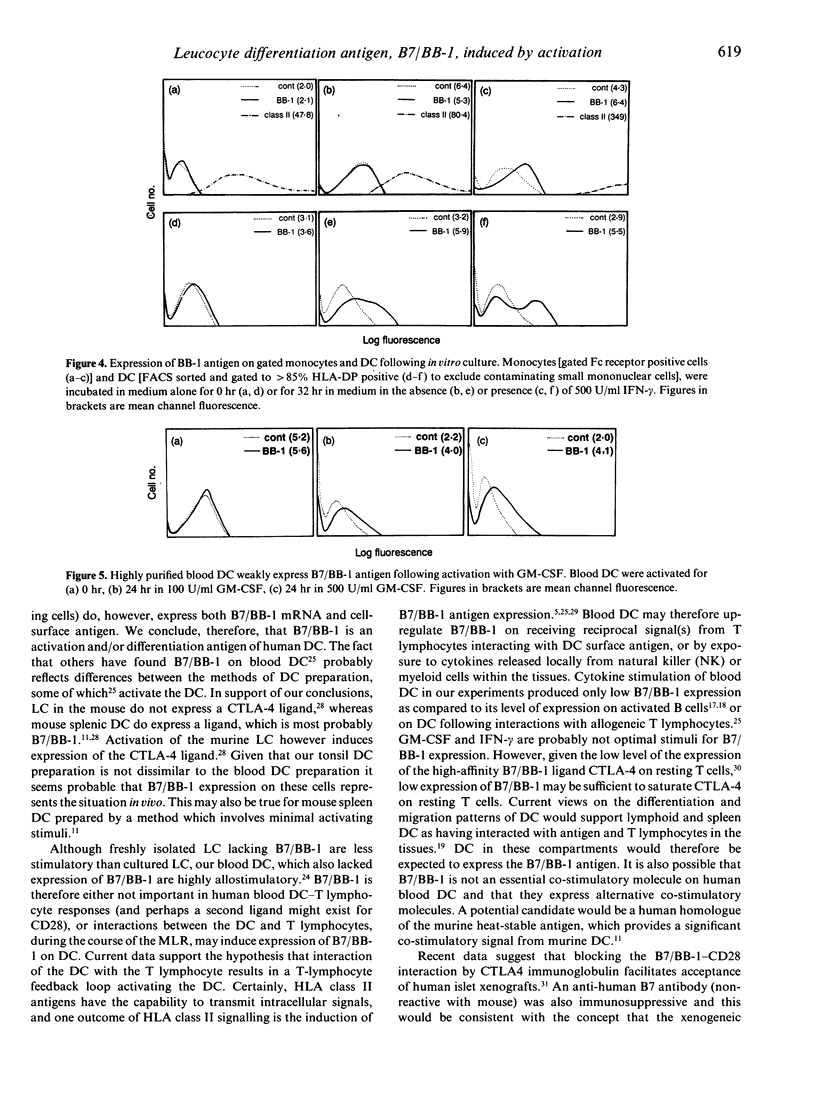
Activation of a primary T-lymphocyte response requires additional signals apart from interaction of the T-cell receptor (TcR)/CD3 complex with major histocompatibility complex (MHC) antigens on the antigen-presenting cell. The CD28 antigen on T lymphocytes provides an important co-stimulatory signal to T lymphocytes and we therefore searched for the presence of its ligand, the B7/BB-1 antigen, on blood and tonsil dendritic cells (DC). Blood DC, prepared from peripheral blood mononuclear cells with a minimal period of in vitro culture, did not stain with the monoclonal antibody BB-1 using flow cytometry analysis. In contrast, tonsil DC stained weakly for B7/BB-1 compared to positive control cell lines. Polymerase chain reaction (PCR) was used to amplify a 605 base pair (bp) fragment from human B7/BB-1 mRNA and demonstrated significant amounts of B7/BB-1 mRNA in tonsil DC but no specific product was obtained from blood DC, confirming the surface-staining results. Weak expression of B7/BB-1 antigen was detected by immunofluorescence analysis following culture of blood DC with either interferon-gamma (IFN-gamma) or granulocyte-macrophage colony-stimulating factor (GM-CSF). These data support the concept that blood DC give rise to tissue and/or lymphoid DC, which acquire co-stimulatory ligands as a result of activation and/or differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma M., Cayabyab M., Buck D., Phillips J. H., Lanier L. L. CD28 interaction with B7 costimulates primary allogeneic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. J Exp Med. 1992 Feb 1;175(2):353–360. doi: 10.1084/jem.175.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle N. K., Doyle L. V., Grosmaire L. S., Ledbetter J. A. Differential regulatory signals delivered by antibody binding to the CD28 (Tp44) molecule during the activation of human T lymphocytes. J Immunol. 1988 Mar 15;140(6):1753–1761. [PubMed] [Google Scholar]
- Fraser J. D., Irving B. A., Crabtree G. R., Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991 Jan 18;251(4991):313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Freeman G. J., Rhynhart K., Nadler L. M. Selective induction of B7/BB-1 on interferon-gamma stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol. 1991 Oct 15;137(2):429–437. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Freeman G., Horowitz J. C., Daley J., Nadler L. M. B7, a B-cell-restricted antigen that identifies preactivated B cells. J Immunol. 1987 Nov 15;139(10):3260–3267. [PubMed] [Google Scholar]
- Freeman G. J., Freedman A. S., Segil J. M., Lee G., Whitman J. F., Nadler L. M. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989 Oct 15;143(8):2714–2722. [PubMed] [Google Scholar]
- Freeman G. J., Gray G. S., Gimmi C. D., Lombard D. B., Zhou L. J., White M., Fingeroth J. D., Gribben J. G., Nadler L. M. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991 Sep 1;174(3):625–631. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girolomoni G., Simon J. C., Bergstresser P. R., Cruz P. D., Jr Freshly isolated spleen dendritic cells and epidermal Langerhans cells undergo similar phenotypic and functional changes during short-term culture. J Immunol. 1990 Nov 1;145(9):2820–2826. [PubMed] [Google Scholar]
- Harding F. A., McArthur J. G., Gross J. A., Raulet D. H., Allison J. P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992 Apr 16;356(6370):607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- Hart D. N., McKenzie J. L. Interstitial dendritic cells. Int Rev Immunol. 1990;6(2-3):127–138. doi: 10.3109/08830189009056624. [DOI] [PubMed] [Google Scholar]
- Hart D. N., McKenzie J. L. Isolation and characterization of human tonsil dendritic cells. J Exp Med. 1988 Jul 1;168(1):157–170. doi: 10.1084/jem.168.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. N., Prickett T. C. Intercellular adhesion molecule-2 (ICAM-2) expression on human dendritic cells. Cell Immunol. 1993 May;148(2):447–454. doi: 10.1006/cimm.1993.1126. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Taylor P. S., Norton S. D., Urdahl K. B. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991 Oct 15;147(8):2461–2466. [PubMed] [Google Scholar]
- Jenkins M. K. The role of cell division in the induction of clonal anergy. Immunol Today. 1992 Feb;13(2):69–73. doi: 10.1016/0167-5699(92)90137-V. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Gillespie M. M., Lindsten T., Thompson C. B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987 Dec;7(12):4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulova L., Clark E. A., Shu G., Dupont B. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991 Mar 1;173(3):759–762. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. P., Ritchie S. C., Pearson T. C., Linsley P. S., Lowry R. P. Functional expression of the costimulatory molecule, B7/BB1, on murine dendritic cell populations. J Exp Med. 1992 Oct 1;176(4):1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow D. J., Zeng Y., Thistlethwaite J. R., Montag A., Brady W., Gibson M. G., Linsley P. S., Bluestone J. A. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992 Aug 7;257(5071):789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Clark E. A., Ledbetter J. A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Greene J. L., Tan P., Bradshaw J., Ledbetter J. A., Anasetti C., Damle N. K. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992 Dec 1;176(6):1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Janeway C. A., Jr Cells that present both specific ligand and costimulatory activity are the most efficient inducers of clonal expansion of normal CD4 T cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3845–3849. doi: 10.1073/pnas.89.9.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jones B., Brady W., Janeway C. A., Jr, Linsley P. S., Linley P. S. Co-stimulation of murine CD4 T cell growth: cooperation between B7 and heat-stable antigen. Eur J Immunol. 1992 Nov;22(11):2855–2859. doi: 10.1002/eji.1830221115. [DOI] [PubMed] [Google Scholar]
- MacPherson G. G., Fossum S., Harrison B. Properties of lymph-borne (veiled) dendritic cells in culture. II. Expression of the IL-2 receptor: role of GM-CSF. Immunology. 1989 Sep;68(1):108–113. [PMC free article] [PubMed] [Google Scholar]
- Nabavi N., Freeman G. J., Gault A., Godfrey D., Nadler L. M., Glimcher L. H. Signalling through the MHC class II cytoplasmic domain is required for antigen presentation and induces B7 expression. Nature. 1992 Nov 19;360(6401):266–268. doi: 10.1038/360266a0. [DOI] [PubMed] [Google Scholar]
- Romani N., Lenz A., Glassel H., Stössel H., Stanzl U., Majdic O., Fritsch P., Schuler G. Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol. 1989 Nov;93(5):600–609. doi: 10.1111/1523-1747.ep12319727. [DOI] [PubMed] [Google Scholar]
- Yokochi T., Holly R. D., Clark E. A. B lymphoblast antigen (BB-1) expressed on Epstein-Barr virus-activated B cell blasts, B lymphoblastoid cell lines, and Burkitt's lymphomas. J Immunol. 1982 Feb;128(2):823–827. [PubMed] [Google Scholar]
- Young J. W., Koulova L., Soergel S. A., Clark E. A., Steinman R. M., Dupont B. The B7/BB1 antigen provides one of several costimulatory signals for the activation of CD4+ T lymphocytes by human blood dendritic cells in vitro. J Clin Invest. 1992 Jul;90(1):229–237. doi: 10.1172/JCI115840. [DOI] [PMC free article] [PubMed] [Google Scholar]