Abstract
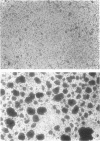
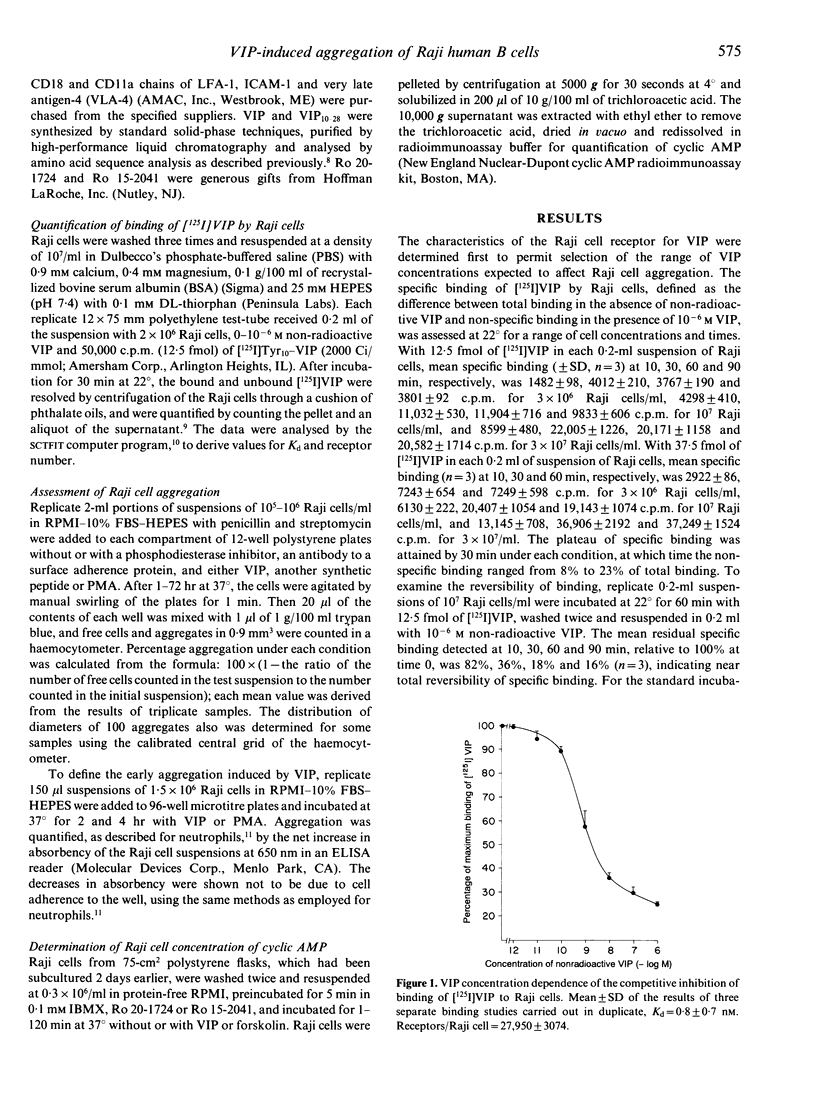
Subsets of neurons in the thymic cortex, Peyer's patches and lymphoid tissues of the respiratory system deliver vasoactive intestinal peptide (VIP) at nanomolar concentrations. The possible effects of VIP on B-cell adhesiveness in these tissues were examined in studies of the homotypic aggregation (HA) of human B-lymphoblastoid cells of the Raji line, which express a mean of 27,950 VIP receptors/cell with a mean Kd of 0.8 nM. Mean HA, assessed microscopically, attained a maximum of 54% after 8 hr with 0.1 microgram/ml of phorbol 12-myristate 13-acetate (PMA) (P < 0.01) and 31% after 24 hr with 10(-8) M VIP (P < 0.05), as contrasted with 13% and 20% at the respective times in medium alone, and both stimuli also increased the mean size of aggregates. The presence of the phosphodiesterase inhibitor Ro 20-1724 permitted 10(-9) M VIP, which had no effect alone, to raise the mean cyclic AMP content of Raji cells by more than 10-fold and concurrently to elevate mean HA from 55% in medium alone at 48 hr to 70% and from 55% at 72 hr to 68% (P < 0.05 for both). Monoclonal antibodies to lymphocyte function-associated (LFA-1) adhesive protein and to intercellular adherence molecule-1 (ICAM-1) suppressed significantly the HA of Raji cells induced by VIP and PMA. The effects of VIP on compartmental immunity in the lungs and intestines thus may be mediated in part by increases in lymphocyte adhesiveness, which could contribute to the regional accumulation of specifically immunocompetent cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett T. B., Shu G., Clark E. A. CD40 signaling activates CD11a/CD18 (LFA-1)-mediated adhesion in B cells. J Immunol. 1991 Mar 15;146(6):1722–1729. [PubMed] [Google Scholar]
- Cheng P. P., Sreedharan S. P., Kishiyama J. L., Goetzl E. J. The SKW 6.4 line of human B lymphocytes specifically binds and responds to vasoactive intestinal peptide. Immunology. 1993 May;79(1):64–68. [PMC free article] [PubMed] [Google Scholar]
- Dang L. H., Rock K. L. Stimulation of B lymphocytes through surface Ig receptors induces LFA-1 and ICAM-1-dependent adhesion. J Immunol. 1991 May 15;146(10):3273–3279. [PubMed] [Google Scholar]
- De Lean A., Hancock A. A., Lefkowitz R. J. Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol Pharmacol. 1982 Jan;21(1):5–16. [PubMed] [Google Scholar]
- Finch R. J., Sreedharan S. P., Goetzl E. J. High-affinity receptors for vasoactive intestinal peptide on human myeloma cells. J Immunol. 1989 Mar 15;142(6):1977–1981. [PubMed] [Google Scholar]
- Kavanaugh A. F., Lightfoot E., Lipsky P. E., Oppenheimer-Marks N. Role of CD11/CD18 in adhesion and transendothelial migration of T cells. Analysis utilizing CD18-deficient T cell clones. J Immunol. 1991 Jun 15;146(12):4149–4156. [PubMed] [Google Scholar]
- Ottaway C. A. In vitro alteration of receptors for vasoactive intestinal peptide changes the in vivo localization of mouse T cells. J Exp Med. 1984 Oct 1;160(4):1054–1069. doi: 10.1084/jem.160.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaway C. A., Lewis D. L., Asa S. L. Vasoactive intestinal peptide-containing nerves in Peyer's patches. Brain Behav Immun. 1987 Jun;1(2):148–158. doi: 10.1016/0889-1591(87)90017-1. [DOI] [PubMed] [Google Scholar]
- Ottaway C. A. Selective effects of vasoactive intestinal peptide on the mitogenic response of murine T cells. Immunology. 1987 Oct;62(2):291–297. [PMC free article] [PubMed] [Google Scholar]
- PULVERTAFT J. V. CYTOLOGY OF BURKITT'S TUMOUR (AFRICAN LYMPHOMA). Lancet. 1964 Feb 1;1(7327):238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- Stanisz A. M., Befus D., Bienenstock J. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer's patches, mesenteric lymph nodes, and spleen. J Immunol. 1986 Jan;136(1):152–156. [PubMed] [Google Scholar]
- van Kooyk Y., Weder P., Hogervorst F., Verhoeven A. J., van Seventer G., te Velde A. A., Borst J., Keizer G. D., Figdor C. G. Activation of LFA-1 through a Ca2(+)-dependent epitope stimulates lymphocyte adhesion. J Cell Biol. 1991 Jan;112(2):345–354. doi: 10.1083/jcb.112.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]