Abstract
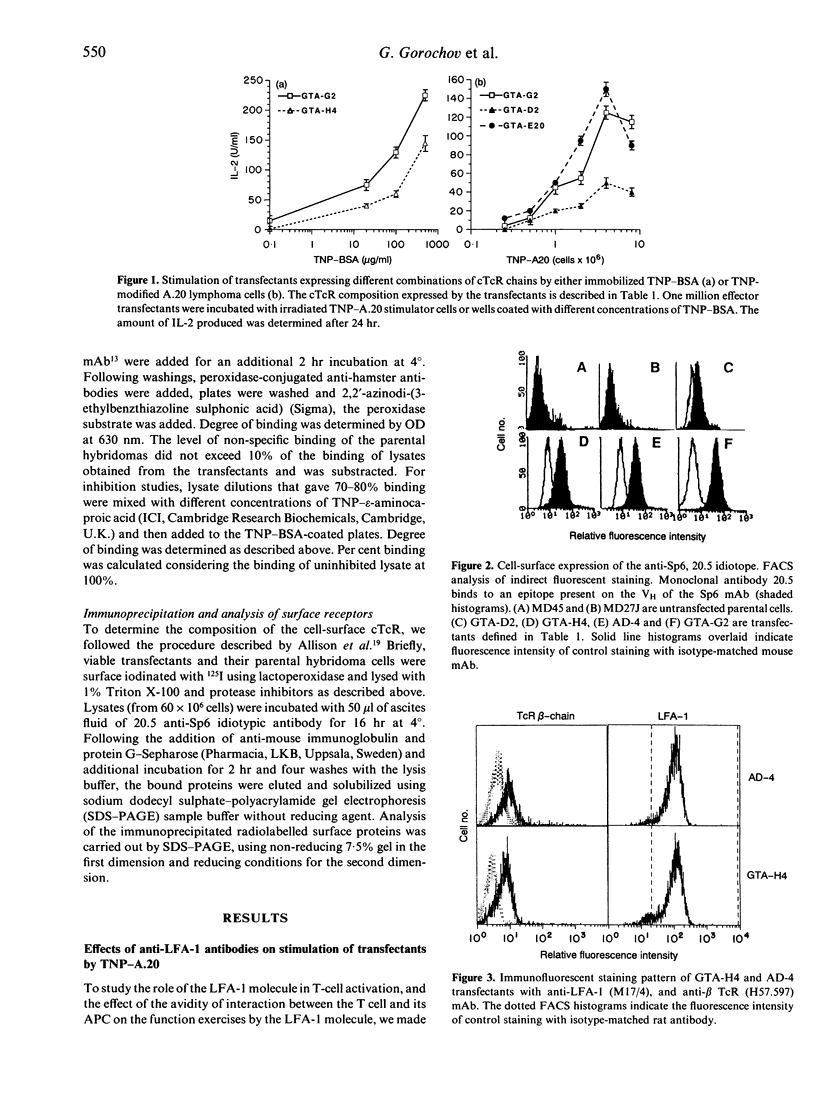
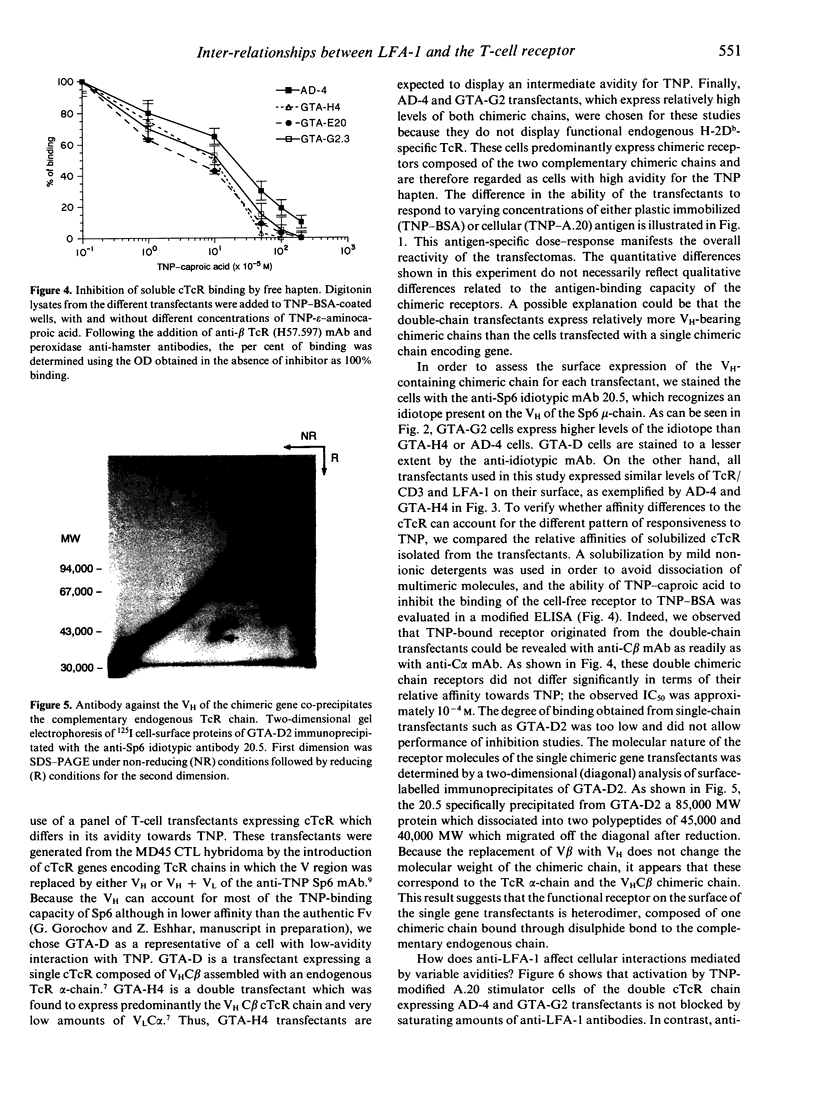
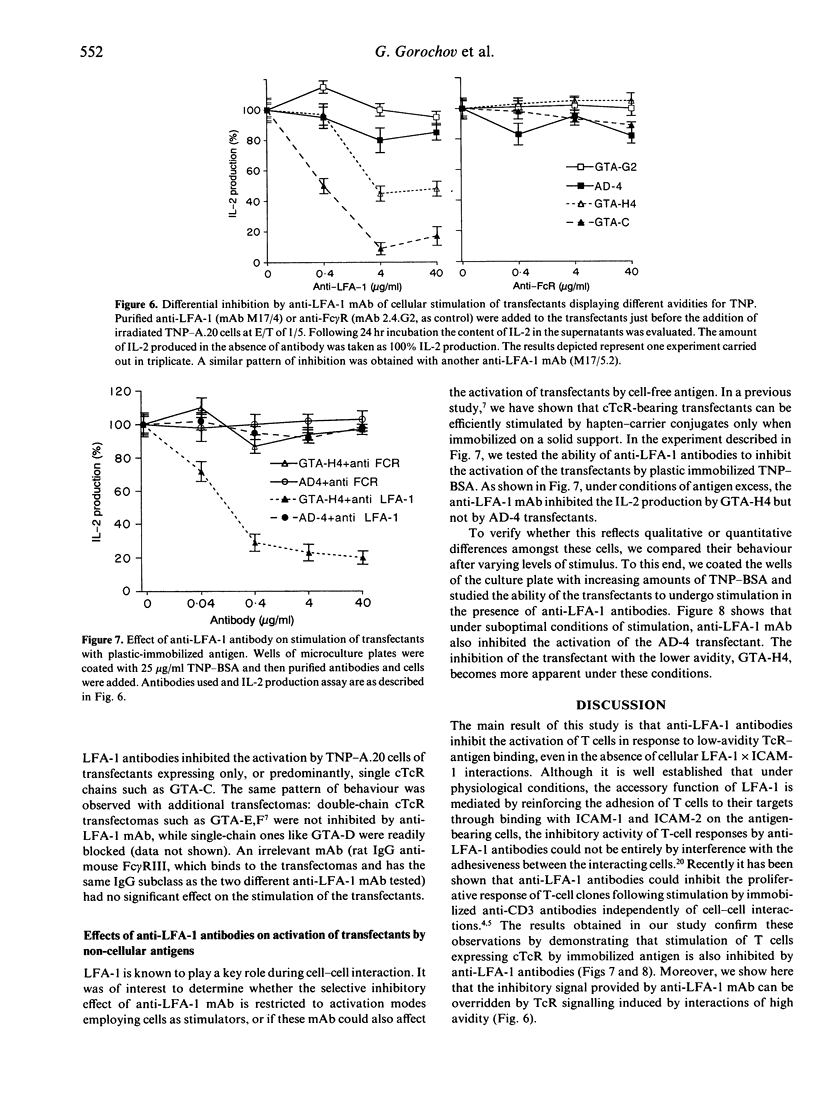
Anti-leucocyte function-associated antigen-1 (LFA-1) antibodies can provide either stimulatory or inhibitory signals to T cells, depending on the epitope they recognize, type and stage of activation of the T cells, and nature of the activation stimulus. Because of the low affinity of interaction between the T-cell receptor (TcR) and the antigen/major histocompatibility complex (MHC), it was proposed that the LFA-1 molecule strengthens the adhesion between the interacting cells, thus contributing in an additive manner to TcR-specific interactions. To check if high-avidity, TcR-specific interactions still require the accessory function of the adhesion molecule, we studied the effect of anti-LFA-1 antibodies on T-cell triggering mediated through chimeric receptors composed of an Fv of an antibody and a constant region of the TcR. Such chimeric TcR (cTcR) confer on T cells antibody-type specificity and affinity. We made use of transfected T-cell hybridomas expressing various amounts of either one cTcR chain (composed of VH linked to C beta) or double-chain cTcR (VHC beta + VLC alpha). When such transfectants were stimulated with hapten-modified cells, anti-LFA-1 antibodies inhibited activation predominantly mediated through cTcR composed of a single chimeric chain and did not inhibit stimulation of the double-chain transfectants. Moreover, these anti-LFA-1 antibodies blocked antigen-specific T-cell activation regardless of whether the stimulus was adhesion dependent or not, such as in the case of stimulation by immobilized hapten-protein conjugates. These studies show that the 'off-signal' provided by anti-LFA-1 antibodies is adhesion independent and affects mainly low-avidity TcR-antigen interactions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. P., McIntyre B. W., Bloch D. Tumor-specific antigen of murine T-lymphoma defined with monoclonal antibody. J Immunol. 1982 Nov;129(5):2293–2300. [PubMed] [Google Scholar]
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- Collins T., Krensky A. M., Clayberger C., Fiers W., Gimbrone M. A., Jr, Burakoff S. J., Pober J. S. Human cytolytic T lymphocyte interactions with vascular endothelium and fibroblasts: role of effector and target cell molecules. J Immunol. 1984 Oct;133(4):1878–1884. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Fischer A., Friedrich W., Fasth A., Blanche S., Le Deist F., Girault D., Veber F., Vossen J., Lopez M., Griscelli C. Reduction of graft failure by a monoclonal antibody (anti-LFA-1 CD11a) after HLA nonidentical bone marrow transplantation in children with immunodeficiencies, osteopetrosis, and Fanconi's anemia: a European Group for Immunodeficiency/European Group for Bone Marrow Transplantation report. Blood. 1991 Jan 15;77(2):249–256. [PubMed] [Google Scholar]
- Gross G., Gorochov G., Waks T., Eshhar Z. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant Proc. 1989 Feb;21(1 Pt 1):127–130. [PubMed] [Google Scholar]
- Gross G., Waks T., Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Berke G., Eshhar Z. Cytotoxic T lymphocyte hybridomas that mediate specific tumor-cell lysis in vitro. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2502–2506. doi: 10.1073/pnas.78.4.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Golstein P., Pierres M., Springer T. A., Eshhar Z. LFA-1 but not Lyt-2 is associated with killing activity of cytotoxic T lymphocyte hybridomas. Nature. 1982 Nov 25;300(5890):357–360. doi: 10.1038/300357a0. [DOI] [PubMed] [Google Scholar]
- Kuijpers K. C., Kuijpers T. W., Zeijlemaker W. P., Lucas C. J., van Lier R. A., Miedema F. Analysis of the role of leukocyte function-associated antigen-1 in activation of human influenza virus-specific T cell clones. J Immunol. 1990 May 1;144(9):3281–3287. [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu Rev Immunol. 1989;7:309–337. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J Exp Med. 1989 Nov 1;170(5):1697–1713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson R. S., Springer T. A. Structure and function of leukocyte integrins. Immunol Rev. 1990 Apr;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Matsui K., Boniface J. J., Reay P. A., Schild H., Fazekas de St Groth B., Davis M. M. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science. 1991 Dec 20;254(5039):1788–1791. doi: 10.1126/science.1763329. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Ochi A., Hawley R. G., Hawley T., Shulman M. J., Traunecker A., Köhler G., Hozumi N. Functional immunoglobulin M production after transfection of cloned immunoglobulin heavy and light chain genes into lymphoid cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6351–6355. doi: 10.1073/pnas.80.20.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi S., Köhler G. Transmission and expression of a specific pair of rearranged immunoglobulin mu and kappa genes in a transgenic mouse line. 1985 Mar 28-Apr 3Nature. 314(6009):330–334. doi: 10.1038/314330a0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Davignon D., Martz E., Springer T. A. Antigens involved in mouse cytolytic T-lymphocyte (CTL)-mediated killing: functional screening and topographic relationship. Cell Immunol. 1982 Oct;73(1):1–11. doi: 10.1016/0008-8749(82)90431-2. [DOI] [PubMed] [Google Scholar]
- Schmitt-Verhulst A. M., Pettinelli C. B., Henkart P. A., Lunney J. K., Shearer G. M. H-2-restricted cytotoxic effectors generated in vitro by the addition of trinitrophenyl-conjugated soluble proteins. J Exp Med. 1978 Feb 1;147(2):352–368. doi: 10.1084/jem.147.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Roles of adhesion molecules in T-cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990 Apr;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990 Jun 15;144(12):4579–4586. [PubMed] [Google Scholar]
- Ward E. S., Güssow D., Griffiths A. D., Jones P. T., Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989 Oct 12;341(6242):544–546. doi: 10.1038/341544a0. [DOI] [PubMed] [Google Scholar]
- van Noesel C., Miedema F., Brouwer M., de Rie M. A., Aarden L. A., van Lier R. A. Regulatory properties of LFA-1 alpha and beta chains in human T-lymphocyte activation. Nature. 1988 Jun 30;333(6176):850–852. doi: 10.1038/333850a0. [DOI] [PubMed] [Google Scholar]




