Abstract
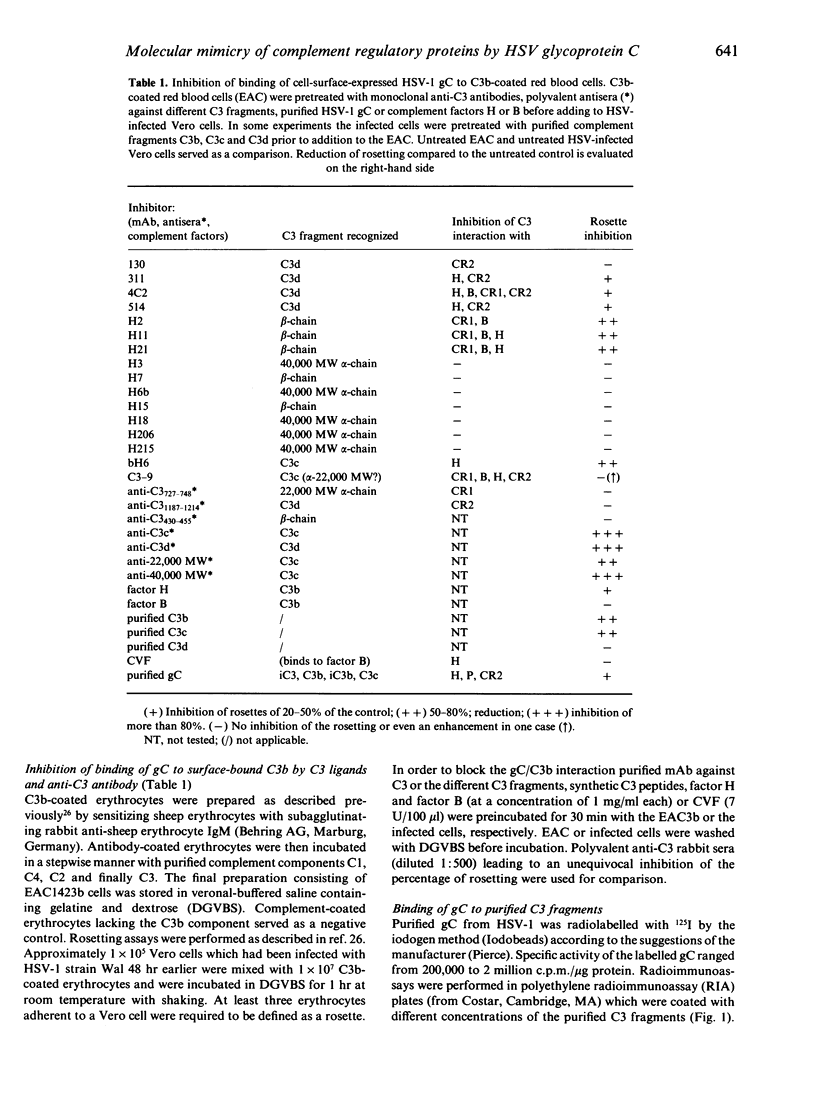
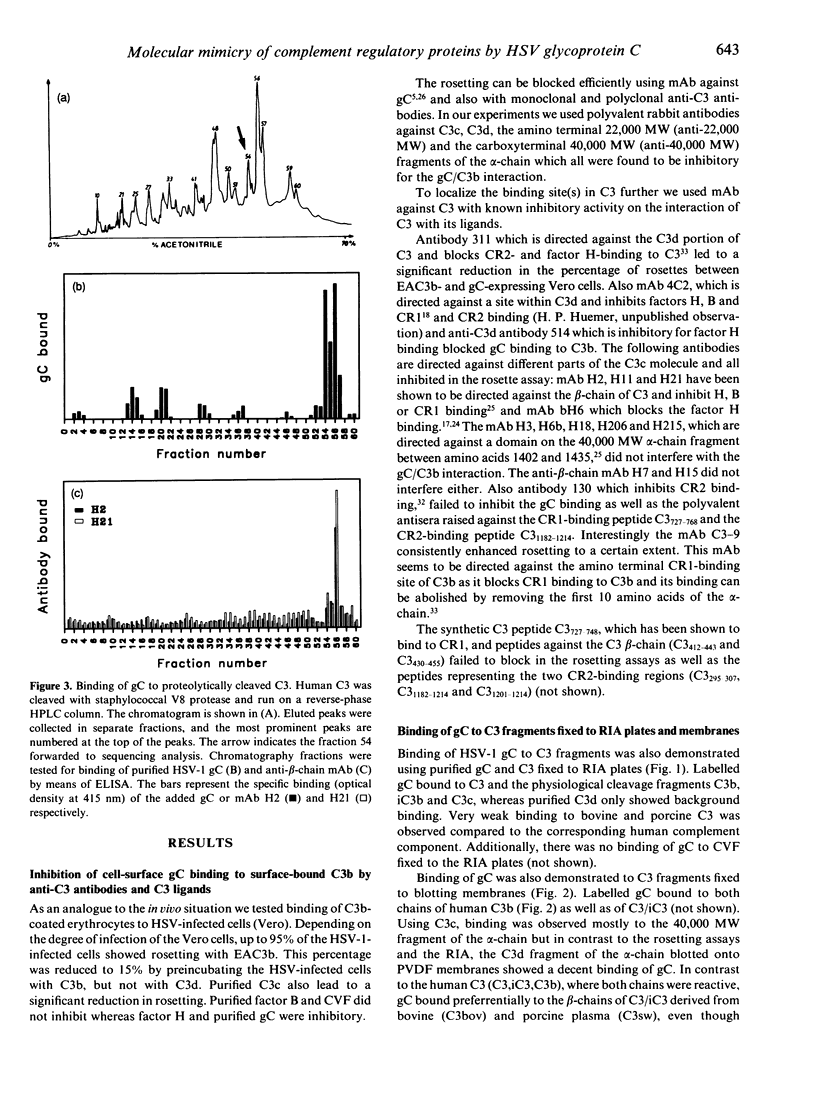
Herpes simplex virus (HSV) encodes a protein, glycoprotein C (gC), which binds to the third complement component, the central mediator of complement activation. In this study the structural and functional relationships of gC from HSV type 1 (HSV-1) and known human complement regulatory proteins factor H, properdin, factor B, complement receptor 1 (CR1) and 2 (CR2) were investigated. The interaction of gC with C3b was studied using purified complement components, synthetic peptides, antisera against different C3 fragments and anti-C3 monoclonal antibodies (mAb) with known inhibitory effects on C3-ligand interactions. All the mAb that inhibited gC/C3b interactions, in a differential manner, also prevented binding of C3 fragments to factors H, B, CR1 or CR2. No blocking was observed with synthetic peptides representing different C3 regions or with factor B and C3d, whereas C3b, C3c and factor H were inhibitory, as well as purified gC. There was no binding of gC to cobra venom factor (CVF), a C3c-like fragment derived from cobra gland. Purified gC bound to iC3, iC3b and C3c, but failed to bind to C3d. Glycoprotein C bound only weakly to iC3 derived from bovine and porcine plasma, thus indicating a preference of the viral protein for the appropriate host. Binding of gC was also observed to proteolytic C3 fragments, especially to the beta-chain, thus suggesting the importance of the C3 region as a binding site. Purified gC from HSV-1, but not HSV-2, inhibited the binding of factor H and properdin but not of CR1 to C3b. The binding of iC3b to CR2, a molecule involved in B-cell activation and binding of the Epstein-Barr virus, was also inhibited by the HSV-1 protein. As factor H and properdin, the binding of which was inhibited by gC, are important regulators of the alternative complement pathway, these data further support a role of gC in the evasion of HSV from a major first-line host defence mechanism, i.e. the complement system. In addition, the inhibition of the C3/CR2 interaction may suggest a possible immunoregulatory role of HSV glycoprotein C.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsenz J., Avila D., Huemer H. P., Esparza I., Becherer J. D., Kinoshita T., Wang Y., Oppermann S., Lambris J. D. Phylogeny of the third component of complement, C3: analysis of the conservation of human CR1, CR2, H, and B binding sites, concanavalin A binding sites, and thiolester bond in the C3 from different species. Dev Comp Immunol. 1992 Jan-Feb;16(1):63–76. doi: 10.1016/0145-305x(92)90052-e. [DOI] [PubMed] [Google Scholar]
- Becherer J. D., Alsenz J., Esparza I., Hack C. E., Lambris J. D. Segment spanning residues 727-768 of the complement C3 sequence contains a neoantigenic site and accommodates the binding of CR1, factor H, and factor B. Biochemistry. 1992 Feb 18;31(6):1787–1794. doi: 10.1021/bi00121a029. [DOI] [PubMed] [Google Scholar]
- Becherer J. D., Alsenz J., Lambris J. D. Molecular aspects of C3 interactions and structural/functional analysis of C3 from different species. Curr Top Microbiol Immunol. 1990;153:45–72. doi: 10.1007/978-3-642-74977-3_3. [DOI] [PubMed] [Google Scholar]
- Becherer J. D., Lambris J. D. Identification of the C3b receptor-binding domain in third component of complement. J Biol Chem. 1988 Oct 5;263(28):14586–14591. [PubMed] [Google Scholar]
- Daoudaki M. E., Becherer J. D., Lambris J. D. A 34-amino acid peptide of the third component of complement mediates properdin binding. J Immunol. 1988 Mar 1;140(5):1577–1580. [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Friedman H. M., Fries L. F., Frank M. M., Hastings J. C., Cohen G. H. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb Pathog. 1987 Dec;3(6):423–435. doi: 10.1016/0882-4010(87)90012-x. [DOI] [PubMed] [Google Scholar]
- Esparza I., Becherer J. D., Alsenz J., De la Hera A., Lao Z., Tsoukas C. D., Lambris J. D. Evidence for multiple sites of interaction in C3 for complement receptor type 2 (C3d/EBV receptor, CD21). Eur J Immunol. 1991 Nov;21(11):2829–2838. doi: 10.1002/eji.1830211126. [DOI] [PubMed] [Google Scholar]
- Farries T. C., Finch J. T., Lachmann P. J., Harrison R. A. Resolution and analysis of 'native' and 'activated' properdin. Biochem J. 1987 Apr 15;243(2):507–517. doi: 10.1042/bj2430507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farries T. C., Seya T., Harrison R. A., Atkinson J. P. Competition for binding sites on C3b by CR1, CR2, MCP, factor B and factor H. Complement Inflamm. 1990;7(1):30–41. doi: 10.1159/000463124. [DOI] [PubMed] [Google Scholar]
- Fearon D. T. Anti-inflammatory and immunosuppressive effects of recombinant soluble complement receptors. Clin Exp Immunol. 1991 Oct;86 (Suppl 1):43–46. doi: 10.1111/j.1365-2249.1991.tb06206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Friedman H. M., Glorioso J. C., Cohen G. H., Hastings J. C., Harris S. L., Eisenberg R. J. Binding of complement component C3b to glycoprotein gC of herpes simplex virus type 1: mapping of gC-binding sites and demonstration of conserved C3b binding in low-passage clinical isolates. J Virol. 1986 Nov;60(2):470–475. doi: 10.1128/jvi.60.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries L. F., Friedman H. M., Cohen G. H., Eisenberg R. J., Hammer C. H., Frank M. M. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J Immunol. 1986 Sep 1;137(5):1636–1641. [PubMed] [Google Scholar]
- Garred P., Mollnes T. E., Kazatchkine M. D. Activation-dependent antigenic changes of human C3. Complement Inflamm. 1989;6(3):205–218. doi: 10.1159/000463094. [DOI] [PubMed] [Google Scholar]
- Garred P., Mollnes T. E., Lea T., Fischer E. Characterization of a monoclonal antibody MoAb bH6 reacting with a neoepitope of human C3 expressed on C3b, iC3b, and C3c. Scand J Immunol. 1988 Mar;27(3):319–327. doi: 10.1111/j.1365-3083.1988.tb02353.x. [DOI] [PubMed] [Google Scholar]
- Hack C. E., Paardekooper J., Smeenk R. J., Abbink J., Eerenberg A. J., Nuijens J. H. Disruption of the internal thioester bond in the third component of complement (C3) results in the exposure of neodeterminants also present on activation products of C3. An analysis with monoclonal antibodies. J Immunol. 1988 Sep 1;141(5):1602–1609. [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Harris S. L., Frank I., Yee A., Cohen G. H., Eisenberg R. J., Friedman H. M. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis. 1990 Aug;162(2):331–337. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- Hebell T., Ahearn J. M., Fearon D. T. Suppression of the immune response by a soluble complement receptor of B lymphocytes. Science. 1991 Oct 4;254(5028):102–105. doi: 10.1126/science.1718035. [DOI] [PubMed] [Google Scholar]
- Hidaka Y., Sakai Y., Toh Y., Mori R. Glycoprotein C of herpes simplex virus type 1 is essential for the virus to evade antibody-independent complement-mediated virus inactivation and lysis of virus-infected cells. J Gen Virol. 1991 Apr;72(Pt 4):915–921. doi: 10.1099/0022-1317-72-4-915. [DOI] [PubMed] [Google Scholar]
- Hidaka Y., Sakuma S., Kumano Y., Minagawa H., Mori R. Characterization of glycoprotein C-negative mutants of herpes simplex virus type 1 isolated from a patient with keratitis. Arch Virol. 1990;113(3-4):195–207. doi: 10.1007/BF01316673. [DOI] [PubMed] [Google Scholar]
- Holland T. C., Homa F. L., Marlin S. D., Levine M., Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984 Nov;52(2):566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huemer H. P., Bröker M., Larcher C., Lambris J. D., Dierich M. P. The central segment of herpes simplex virus type 1 glycoprotein C (gC) is not involved in C3b binding: demonstration by using monoclonal antibodies and recombinant gC expressed in Escherichia coli. J Gen Virol. 1989 Jun;70(Pt 6):1571–1578. doi: 10.1099/0022-1317-70-6-1571. [DOI] [PubMed] [Google Scholar]
- Huemer H. P., Larcher C., Coe N. E. Pseudorabies virus glycoprotein III derived from virions and infected cells binds to the third component of complement. Virus Res. 1992 May;23(3):271–280. doi: 10.1016/0168-1702(92)90113-n. [DOI] [PubMed] [Google Scholar]
- Huemer H. P., Larcher C., Dierich M. P., Falke D. Factors influencing the interaction of herpes simplex virus glycoprotein C with the third component of complement. Arch Virol. 1992;127(1-4):291–303. doi: 10.1007/BF01309591. [DOI] [PubMed] [Google Scholar]
- Hung S. L., Srinivasan S., Friedman H. M., Eisenberg R. J., Cohen G. H. Structural basis of C3b binding by glycoprotein C of herpes simplex virus. J Virol. 1992 Jul;66(7):4013–4027. doi: 10.1128/jvi.66.7.4013-4027.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., McDermott M. R., Chrisp C., Glorioso J. C. Pathogenicity in mice of herpes simplex virus type 2 mutants unable to express glycoprotein C. J Virol. 1986 Apr;58(1):36–42. doi: 10.1128/jvi.58.1.36-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinen V., Wessberg S., Leikola J. Common binding region of complement factors B, H and CR1 on C3b revealed by monoclonal anti-C3d. Complement Inflamm. 1989;6(4):270–280. doi: 10.1159/000463102. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Gaither T. A., Cason J., O'Shea J. J., Lawley T. J. Characterization of the C3 receptor induced by herpes simplex virus type 1 infection of human epidermal, endothelial, and A431 cells. J Immunol. 1987 Feb 15;138(4):1137–1142. [PubMed] [Google Scholar]
- Lambris J. D., Avila D., Becherer J. D., Müller-Eberhard H. J. A discontinuous factor H binding site in the third component of complement as delineated by synthetic peptides. J Biol Chem. 1988 Aug 25;263(24):12147–12150. [PubMed] [Google Scholar]
- Lambris J. D., Ganu V. S., Hirani S., Müller-Eberhard H. J. Mapping of the C3d receptor (CR2)-binding site and a neoantigenic site in the C3d domain of the third component of complement. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4235–4239. doi: 10.1073/pnas.82.12.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin D. M., Atkinson J. P. Decay-accelerating factor and membrane cofactor protein. Curr Top Microbiol Immunol. 1990;153:123–145. doi: 10.1007/978-3-642-74977-3_7. [DOI] [PubMed] [Google Scholar]
- McNearney T. A., Odell C., Holers V. M., Spear P. G., Atkinson J. P. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J Exp Med. 1987 Nov 1;166(5):1525–1535. doi: 10.1084/jem.166.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G. R., Mold C., Schwend V. K., Tollefson V., Cooper N. R. Identification of gp350 as the viral glycoprotein mediating attachment of Epstein-Barr virus (EBV) to the EBV/C3d receptor of B cells: sequence homology of gp350 and C3 complement fragment C3d. J Virol. 1987 May;61(5):1416–1420. doi: 10.1128/jvi.61.5.1416-1420.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Dondero D. V., Gallo D., Devlin V., Woodie J. D. Serological analysis of herpes simplex virus types 1 and 2 with monoclonal antibodies. Infect Immun. 1982 Jan;35(1):363–367. doi: 10.1128/iai.35.1.363-367.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröeder C. H., Engler H., Kirchner H. Protection of mice by an apathogenic strain HSV-1 against lethal infection by a pathogenic strain of HSV-1. J Gen Virol. 1981 Jan;52(Pt 1):159–161. doi: 10.1099/0022-1317-52-1-159. [DOI] [PubMed] [Google Scholar]
- Servis C., Lambris J. D. C3 synthetic peptides support growth of human CR2-positive lymphoblastoid B cells. J Immunol. 1989 Apr 1;142(7):2207–2212. [PubMed] [Google Scholar]
- Tanner J., Weis J., Fearon D., Whang Y., Kieff E. Epstein-Barr virus gp350/220 binding to the B lymphocyte C3d receptor mediates adsorption, capping, and endocytosis. Cell. 1987 Jul 17;50(2):203–213. doi: 10.1016/0092-8674(87)90216-9. [DOI] [PubMed] [Google Scholar]
- Wong W. W., Farrell S. A. Proposed structure of the F' allotype of human CR1. Loss of a C3b binding site may be associated with altered function. J Immunol. 1991 Jan 15;146(2):656–662. [PubMed] [Google Scholar]
- van Strijp J. A., van Kessel K. P., Miltenburg L. A., Fluit A. C., Verhoef J. Attachment of human polymorphonuclear leukocytes to herpes simplex virus-infected fibroblasts mediated by antibody-independent complement activation. J Virol. 1988 Mar;62(3):847–850. doi: 10.1128/jvi.62.3.847-850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]




