Abstract
Objective
To determine the risks and benefits of gastric bypass-induced weight loss on severe venous stasis disease in morbid obesity.
Summary Background Data
Severe obesity is associated with a risk of lower extremity venous stasis disease, pretibial ulceration, cellulitis, and bronze edema.
Methods
The GBP database was queried for venous stasis disease including pretibial venous stasis ulcers, bronze edema, and cellulitis.
Results
Of 1,976 patients undergoing GBP, 64 (45% female) met the criteria. Mean age was 44 ± 10 years. Thirty-seven patients had pretibial venous stasis ulcers, 4 had bronze edema, 23 had both, and 17 had recurrent cellulitis. All had 2 to 4+ pitting pretibial edema. Mean preoperative body mass index (BMI) was 61 ± 12 kg/m2 and weight was 179 ± 39 kg (270 ± 51% ideal body weight), significantly greater than in patients who underwent GBP without venous stasis disease. Two patients had a pulmonary embolus and four had Greenfield filters in the remote past. Additional comorbidities included obesity hypoventilation syndrome, sleep apnea syndrome, hypertension, gastroesophageal reflux, degenerative joint disease symptoms, type 2 diabetes mellitus, pseudotumor cerebri, and urinary incontinence. Comorbidities were significantly more frequent in the patients with venous stasis disease than for those without. At 3.9 ± 4 years after surgery, patients lost 55 ± 21% of excess weight, 62 ± 33 kg, reaching 40 ± 9 kg/m2 BMI or 176 ± 41% ideal body weight. Venous stasis ulcers resolved in all but three patients. Complications included anastomotic leaks with peritonitis and death, fatal pulmonary embolism, fatal respiratory arrest, wound infections or seromas, staple line disruptions, marginal ulcerations treated with acid suppression, stomal stenoses treated with endoscopic dilatation, late small bowel obstructions, and incisional hernias. There were six other late deaths.
Conclusions
Severe venous stasis disease was associated with a significantly greater weight, BMI, male sex, age, comorbidity, and surgical risk (pulmonary embolus, leak, death, incisional hernia) than in other patients who underwent GBP. Surgically induced weight loss corrected the venous stasis disease in almost all patients as well as their other obesity-related problems.
Severe obesity is associated with many medical problems, one of which is severe venous stasis disease. 1 Many severely obese patients have pitting pretibial edema, but some have refractory pretibial venous stasis ulcers or pretibial bronze edema from extravasation of red blood cells. Treatment has consisted of compression boots 2 and split-thickness skin grafts. However, in our experience the ulcers usually do not remain healed in these patients. This retrospective study was designed to analyze the risks and benefits of bariatric surgery for severe venous stasis disease, including pretibial venous stasis ulcers, recurrent lower extremity cellulitis, and bronze edema at the Medical College of Virginia Hospitals.
METHODS
The bariatric surgery database was queried for venous stasis disease, including subjective severity of pitting edema, lower extremity venous stasis ulcers, bronze edema, and deep vein thrombosis, as well as gender, age, preoperative and postoperative weight, body mass index (BMI) in kg/m2, percentage of ideal body weight (%IBW), and percentage of excess weight loss (%EWL). Pre- and postoperative obesity-related comorbidities were also analyzed, including obesity hypoventilation syndrome (OHS; defined as PaO2 ≤ 55 mmHg and/or PaCO2 ≥ 65 mmHg), sleep apnea syndrome (SAS; defined as a respiratory disturbance index ≥ 10 hypopneic and/or apneic episodes per hour of sleep), type 2 diabetes mellitus (defined as a fasting blood sugar ≥ 150 mg/dL or primary care physician-prescribed oral hypoglycemics or insulin), degenerative joint disease (DJD; defined as complaints of pain in the weight-bearing joints [hips, knees, ankles, or lower back]), gastroesophageal reflux (symptoms of heartburn without confirmatory studies of esophageal 24-hour pH, manometry, or endoscopy), pseudotumor cerebri (documented by an elevated cerebrospinal fluid pressure of ≥ 200 cm H2O with a normal cerebral magnetic resonance imaging or computed tomography scan except for a dilated sella turcica), systemic hypertension (systolic blood pressure ≥ 150 mmHg and/or diastolic blood pressure ≥ 90 mmHg with a wide blood pressure cuff or physician-prescribed antihypertensive medications), and urinary incontinence (history from women of difficulty controlling their urine or need to wear a perineal pad).
Thigh-length intermittent venous compression boots placed before the induction of anesthesia have been used since the start of the bariatric surgical program in 1980. These boots were used until the patient was fully ambulatory. Early ambulation on the evening of surgery was mandated since the inception of the program except in patients with OHS or SAS who required postoperative mechanical ventilation. Low-molecular-weight heparin (enoxaparin, 40 mg subcutaneously 30 minutes before surgery) was instituted in 1992.
Healing of venous stasis ulcers was evaluated, including ulcer recurrence, by direct observation. Insertion of a Greenfield inferior vena caval filter was also analyzed, including the timing of and reason for insertion. Postoperative complications were recorded, including death and its cause, anastomotic leak, pulmonary embolism (PE), wound infection (severe defined as the need to extend the hospital stay or necessitate readmission for wound care, mild as outpatient treatment), marginal ulcer or stomal stenosis (diagnosed endoscopically) and the method of treatment, staple line disruption necessitating revisional surgery, small bowel obstruction necessitating laparotomy and lysis of adhesions, or incisional hernia.
The data were compared with those from patients without venous stasis disease and were analyzed for statistical significance using the nonpaired t test. Significance was set at P < .05.
RESULTS
Of 1,976 patients who underwent bariatric surgery between September 1981 and September 1999, 64 patients were identified with venous stasis disease: 37 had venous stasis ulcers, 4 had bronze edema, 23 had both, and 17 had recurrent episodes of lower extremity cellulitis. All had 2 to 4+ pitting pretibial edema. Deep vein thrombosis was documented by Doppler sonography in eight patients before surgery. Mean preoperative BMI was 61 ± 12 kg/m2, weight was 179 ± 39 kg (270 ± 51% IBW), and the mean age was 44 ± 10 years (Table 1). These were all greater (P < .001) than the corresponding figures in obesity surgery patients without venous stasis disease, who weighed 142 ± 30 kg, had a BMI of 51 ± 10 kg/m2 (226 ± 41% IBW), and were 39 ± 10 years old. Many of these patients were extremely obese, with BMIs of 50 or more in 20, 60 or more in 16, 70 or more in 13, and 80 or more in 3 patients. Only 45% of the patients with venous stasis disease were female, in contrast to 82% in patients without this problem (P < .001).
Table 1. PREOPERATIVE DEMOGRAPHICS

BMI, body mass index; IBW, ideal body weight.
*P < .001.
Patients with venous stasis disease had a much higher frequency (P < .001) of comorbidities than patients without this problem (Table 2). Greenfield inferior vena caval filters were inserted in 4 patients before and in 12 patients with OHS at the time of bariatric surgery. Two patients had PEs before obesity surgery.
Table 2. OBESITY COMORBIDITIES
*P < .001, patients with vs. without venous stasis disease.
**P < .001 after vs. before surgically induced weight loss.
Procedures performed on these patients included 18 primary standard gastric bypass operations (GBPs), 2 conversions from a horizontal gastroplasty and 4 conversions from a vertical banded gastroplasty to a standard GBP with a 45-cm Roux limb, 34 long-limb GBPs with a 75-cm standard GBPs biliary tract and a 150-cm Roux limb, 3 and 6 primary and 4 gastroplasty operations converted to a malabsorptive partial biliopancreatic bypass procedure without distal gastrectomy, 4,5 called a distal GBP. Two of these patients required revision to a less malabsorptive gastric bypass because of severe malnutrition.
At 3.9 ± 4 years after surgery, patients lost 55 ± 21% EWL, 62 ± 33 kg (P < .001), reaching 40 ± 9 kg/m2 BMI (P < .001) or 176 ± 41% IBW (P < .001). Venous stasis ulcers resolved in all but one patient within 1 year after surgery (P < .001) but recurred in two patients with large amounts of regained weight (Figs. 1–4). Bronze discolora tion persisted in all, but the pitting pretibial edema resolved in all but four patients. There was a significant (P < .001) decrease in obesity comorbidities, with persistent problems in 22 of 46 patients with hypertension, 22 of 44 with DJD, 4 of 17 with diabetes, 2 of 15 with urinary incontinence, and complete resolution of OHS, SAS, gastroesophageal reflux, and pseudotumor cerebri (see Table 2). As of this writing, 18 patients were current with their follow-up, 15 were 1 to 2 years overdue, 7 were 3 to 5 years overdue, and 12 had not been seen in more than 5 years.

Figure 1. Pretibial venous stasis ulcers before gastric bypass-induced weight loss in a severely obese 40-year-old woman weighing 175 kg, body mass index 68 kg/m2, 310% ideal body weight (Reprinted with permission from Sugerman HJ. Obesity. In: Wilmore DW, ed. Care of the surgical patient. Vol. 2, Elective care. Section VII, Special problems. 1st ed. New York: Scientific American; 1994:1–13).

Figure 2. Healed venous stasis ulcers 1 year after the loss of 66 kg with gastric bypass-induced weight loss. Ulcers remained healed for 13 years when she died of metastatic colon adenocarcinoma (Reprinted with permission from Kellum JM, DeMaria EJ, Sugerman HJ. The surgical treatment of morbid obesity. Curr Probl Surg 1998; 35:791–858).
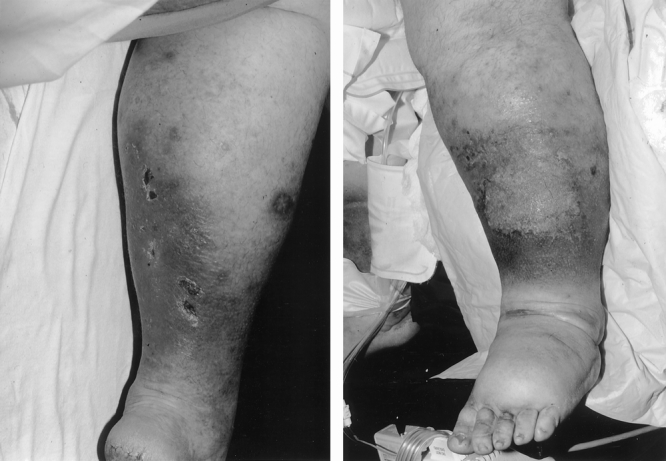
Figure 3. Anterior and posterior views of venous stasis ulcers and bronze edema before long-limb gastric bypass in a 30-year-old man weighing 203 kg, body mass index 75 kg/m2, 310% ideal body weight.

Figure 4. Healed venous stasis ulcers and improved bronze edema at 3 years after the loss of 110 kg with long-limb gastric bypass surgery.
Acute complications (Table 3)included two anastomotic leaks with peritonitis and death (one a new long-limb GBP, BMI 72 kg/m2, and one conversion gastroplasty to long-limb GBP, BMI 62 kg/m2), two fatal PEs (one before and the other 1.5 months after discharge from the hospital, BMIs 56 and 83 kg/m2, respectively), one fatal respiratory arrest secondary to a mucus plug obstruction of a tracheostomy tube that had been inserted for severe OHS and SAS (respiratory disturbance index 110, BMI 59 kg/m2), and five major and six minor wound infections. There were no recognized nonfatal PEs in the venous stasis group but 15 in the patients without venous stasis. This death rate of almost 8% was significantly (P < .001) higher than that in our series of GBP patients without venous stasis disease, which averaged 0.5%. Late complications included eight marginal ulcers treated with acid reduction medications; seven stomal stenoses treated with endoscopic dilatation; two staple line disruptions that required surgical revision; three small bowel obstructions, one of which required surgical correction; and 23 incisional hernias, or 40% of the 59 patients who survived the surgery. This frequency of incisional hernia is significantly higher (P < .001) than the 20% seen in our overall GBP series. 6 There were six other late deaths: one probable arrhythmia at 2 months, 2 myocardial infarctions at 5 and 11 years, 1 motor vehicle accident at 3 years, 1 non-Hodgkin’s lymphoma at 3 years, and 1 colon cancer at 13 years after GBP without recurrence of her venous stasis ulcers.
Table 3. COMPLICATIONS

* Number of patients eligible for complication: vena caval filters inserted before or during surgery in 16/64 patients with venous stasis disease, leaving 48 at risk, and 45/1,915 without venous stasis disease, leaving 1,870 at risk for fatal pulmonary embolism; 4/64 operative deaths (+ 1 death after revision gastric bypass), leaving 60 patients with and 16/1,915 patients without venous stasis disease, leaving 1,899 at risk for late complications.
**P < .05 comparing patients with vs. without venous stasis disease.
***P < .01 comparing patients with vs. without venous stasis disease.
† Only 1 patient required laparotomy for lysis of adhesions.
DISCUSSION
Severe venous stasis disease is a serious but uncommon complication of severe obesity. The frequency in this series was 0.3%. This study confirms the efficacy of gastric surgery-induced weight loss on the healing of severe venous stasis disease. Several of these patients had undergone treatment with rigid medicated (Una) boots and multiple split-thickness skin grafts without resolution of their disease. Patients in this study with severe venous stasis disease were much heavier and somewhat older than our average patient without this problem who underwent obesity surgery. This suggests that the longer one is severely obese, the more likely one is to have venous stasis disease. They also had a much greater problem with severe obesity comorbidities. Presumably, these problems were related to the complications associated with central obesity, including the metabolic syndrome with type 2 diabetes mellitus and hypercholesterolemia 7,8 and the increased intraabdominal pressure with OHS, pseudotumor cerebri, systemic hypertension, urinary incontinence in women, and gastroesophageal reflux. 6,9–16 In addition to correcting their venous stasis disease, surgically induced weight loss was associated with complete resolution or marked improvement in their obesity comorbidities.
Superobesity has been defined by some 5 as a BMI of 50 kg/m2 or more. The patients with venous stasis disease in this study had a BMI of 61 ± 12 kg/m2, which was 270 ± 51% IBW. One might call these patients super-super-obese. This extreme degree of obesity was also probably responsible for the significant increase in preoperative comorbidity and the frequency of serious complications after surgical treatment, including fatal PE, deaths from anastomotic leaks, and a marked increased risk of incisional hernias. We previously published data noting a significant increase in the death rate of 2.2% in patients with respiratory insufficiency of obesity (OHS and/or SAS) in contrast to 0.2% in patients without. 16 Many of the patients with venous stasis disease in this study also had respiratory insufficiency of obesity. However, these patients had an even greater death rate of 8%. Patients with this extreme degree of obesity should be informed of the increased risk of surgical treatment.
We have inserted prophylactic Greenfield inferior vena caval filters in patients with OHS and a mean pulmonary artery pressure of 40 mmHg or more because of the significant potential danger of a fatal PE in patients with primary pulmonary hypertension and pickwickian syndrome. 17,18 This is not based on any randomized, prospective data. Because 16 patients had Greenfield inferior vena caval filters inserted before or at the time of obesity surgery in the current series, this left 48 patients at risk for a postoperative PE. A fatal postoperative PE occurred in two patients for a frequency of 4%. Both of these patients had been treated with thigh-length intermittent venous compression boots and preoperative low-molecular-weight heparin. It is possible that the dose of enoxaparin was inadequate for this severely obese population, although no randomized, prospective trial has validated the risks or benefits of high-dose enoxaparin in this population. The risk of a fatal PE in these patients was significantly (P < .001) greater than the 0.2% risk of a fatal PE in patients who underwent gastric surgery for obesity in our series without venous stasis disease. These data suggest that a prophylactic inferior vena caval filter should be considered at the time of gastric surgery for obesity in patients with severe venous stasis disease. Venous thrombosis was documented in only eight patients by sonography before surgery. The frequency of venous thrombosis was probably much greater, however, because these patients did not undergo routine preoperative sonographic study for lower leg deep venous blood flow.
We hypothesize that venous stasis disease is secondary to the increased intraabdominal pressure associated with central obesity, producing an increase in inferior vena caval and femoral venous pressures. 6,9–15 We have documented increased urinary bladder pressures as a surrogate for intraabdominal pressure in a group of severely obese patients before GBP surgery, and these pressures were directly and significantly correlated with the BMI, sagittal abdominal diameter, and waist circumference but not with waist/hip ratios. 9 Based on that study, we can presume that the patients in this study with very high BMIs also had significantly increased urinary bladder pressures. These pressures returned to normal when measured 1 year after surgically induced weight loss. 10 Porcine studies have shown that increased intraabdominal pressure produces increased inferior vena caval and femoral venous pressure by both a direct effect on the vena cava and an indirect effect through increased intrathoracic pressure. 11,12 The increased venous pressure presumably leads to venous valve failure with extravasation of red blood cells, producing the bronze edema, and impaired epidermal nutrient flow, leading to skin breakdown and ulceration and an increased risk of venous thrombosis. Presumably, this is why the venous stasis disease resolves after surgically induced weight loss. Unfortunately, we measured only urinary bladder pressure, sagittal abdominal diameters, and waist circumferences in one patient in this study (all markedly elevated); therefore, this study is not helpful in validating this hypothesis. It is also possible that the massive lower extremities seen in some of these patients impaired venous return, leading to the venous incompetence and stasis ulcers, although the massive lower extremities could be due to venous and lymphatic edema as well as to fatty tissue. However, only 4 of these 64 patients had massive lower extremity enlargement. Most were like the extremity shown in Figure 1. Others have shown that increased intraabdominal pressure is probably the cause of OHS. 13 We have also hypothesized that the increased intraabdominal pressure is the cause for the increased risk of incisional hernia after obesity surgery 6 and of pseudotumor cerebri in obese women; both clinical and porcine studies support the latter hypothesis. 14,15
In conclusion, gastric surgery for obesity was effective in treating severe venous stasis disease that had been refractory to other forms of treatment in the vast majority of patients with this debilitating problem. These patients were much heavier, were somewhat older, and were more likely to be males than our average patient who underwent surgical treatment of obesity. Unfortunately, surgery in these patients was associated with a significantly increased risk of serious complications, including death. Because of the increased risk of a fatal PE, insertion of an inferior vena caval filter should be considered at the time of obesity surgery.
Footnotes
Correspondence: Harvey J. Sugerman, MD, Box 980519, Richmond, Virginia 23298-0519.
E-mail: hsugerma@hsc.vcu.edu
Accepted for publication November 30, 2000.
References
- 1.Wipke-Tevis DD, Stotts NA. Nutrition, tissue oxygenation, and healing of venous leg ulcers. J Vasc Nurs 1998; 16: 48–56. [DOI] [PubMed] [Google Scholar]
- 2.Marston WA, Carlin RE, Passman MA, et al. Healing rates and cost efficacy of outpatient compression treatment for leg ulcers associated with venous insufficiency. J Vasc Surg 1999; 30: 491–498. [DOI] [PubMed] [Google Scholar]
- 3.Brolin RE, Kenler HA, Gorman JH, Cody RP. Long-limb gastric bypass in the superobese. A prospective randomized study. Ann Surg 1992; 215: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scopinaro N, Gianetta D, Adami G, et al. Biliopancreatic diversion for obesity at eighteen years. Surgery 1996; 119: 261–268. [DOI] [PubMed] [Google Scholar]
- 5.Sugerman HJ, Kellum JM, DeMaria EJ. Conversion of proximal to distal gastric bypass for failed gastric bypass for superobesity. J Gastrointest Surg 1997; 1: 517–25. [DOI] [PubMed] [Google Scholar]
- 6.Sugerman HJ, Kellum JM, Reines HD, et al. Greater risk of incisional hernia with morbidly obese than steroid dependent patients and low recurrence with prefascial polypropylene mesh. Am J Surg 1996; 17: 80–84. [DOI] [PubMed] [Google Scholar]
- 7.Kissebah AH, Vydelingum N, Murray R, et al. Relationship of body fat distribution to metabolic complications of obesity. J Clin Endocrin Metabol 1982; 54: 254–260. [DOI] [PubMed] [Google Scholar]
- 8.Björntrop P. Abdominal obesity and the metabolic syndrome. Ann Med 1992; 24: 465–468. [DOI] [PubMed] [Google Scholar]
- 9.Sugerman HJ, Windsor ACJ, Bessos MK, Wolfe L. Abdominal pressure, sagittal abdominal diameter and obesity co-morbidity. J Intern Med 1997; 241: 71–79. [DOI] [PubMed] [Google Scholar]
- 10.Sugerman H, Windsor A, DeMaria E, Kellum J. Effects of surgically induced weight loss on urinary bladder pressure, sagittal abdominal diameter and obesity co-morbidity. Int J Obesity Metab Disord 1998; 22: 230–235. [DOI] [PubMed] [Google Scholar]
- 11.Ridings PC, Bloomfield GL, Blocher CR, Sugerman HJ. Cardiopulmonary effects of raised intra-abdominal pressure before and after intravascular volume expansion. J Trauma 1995; 39: 1071–1075. [DOI] [PubMed] [Google Scholar]
- 12.Bloomfield G, Saggi B, Blocher C, Sugerman H. Physiologic effects of externally applied continuous negative abdominal pressure for intra-abdominal hypertension. J. Trauma 1999; 46: 1009–1014. [DOI] [PubMed] [Google Scholar]
- 13.Hackney JD, Crane MG, Collier CC, et al. Syndrome of extreme obesity and hypoventilation: studies of etiology. Ann Intern Med 1959; 51: 541–552. [DOI] [PubMed] [Google Scholar]
- 14.Bloomfield GL, Ridings PC, Sugerman HJ, Blocher CR. Increased pleural pressure mediates the effects of elevated intra-abdominal pressure upon the central nervous and cardiovascular systems. Crit Care Med 1997; 25: 496–503. [DOI] [PubMed] [Google Scholar]
- 15.Sugerman HJ, DeMaria EJ, Felton WL III, et al. Increased intra-abdominal pressure and cardiac filling pressures in obesity-associated pseudotumor cerebri. Neurology 1997; 49: 507–511. [DOI] [PubMed] [Google Scholar]
- 16.Sugerman HJ, Fairman RP, Sood RK. Long-term effects of gastric surgery for treating respiratory insufficiency of obesity. Am J Clin Nutr 1992; 55 (suppl): 597–601. [DOI] [PubMed] [Google Scholar]
- 17.Greenfield LJ, Scher LA, Elkins RC. KMA-Greenfield filter placement for chronic pulmonary hypertension. Ann Surg 1979; 189: 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacGregor MI, Block AJ, Ball WC. Topics in clinical medicine: serious complications and sudden death in pickwickian syndrome. Johns Hopkins Med J 1970; 126: 279–295. [PubMed] [Google Scholar]



