Abstract
Objective
To examine the relationship between preoperative biliary drainage and the morbidity and mortality associated with pancreaticoduodenectomy.
Summary Background Data
Recent reports have suggested that preoperative biliary drainage increases the perioperative morbidity and mortality rates of pancreaticoduodenectomy.
Methods
Peri-operative morbidity and mortality were evaluated in 300 consecutive patients who underwent pancreaticoduodenectomy. Univariate and multivariate logistic regression analyses were done to evaluate the relationship between preoperative biliary decompression and the following end points: any complication, any major complication, infectious complications, intraabdominal abscess, pancreaticojejunal anastomotic leak, wound infection, and postoperative death.
Results
Preoperative prosthetic biliary drainage was performed in 172 patients (57%) (stent group), 35 patients (12%) underwent surgical biliary bypass performed during prereferral laparotomy, and the remaining 93 patients (31%) (no-stent group) did not undergo any form of preoperative biliary decompression. The overall surgical death rate was 1% (four patients); the number of deaths was too small for multivariate analysis. By multivariate logistic regression, no differences were found between the stent and no-stent groups in the incidence of all complications, major complications, infectious complications, intraabdominal abscess, or pancreaticojejunal anastomotic leak. Wound infections were more common in the stent group than the no-stent group.
Conclusions
Preoperative biliary decompression increases the risk for postoperative wound infections after pancreaticoduodenectomy. However, there was no increase in the risk of major postoperative complications or death associated with preoperative stent placement. Patients with extrahepatic biliary obstruction do not necessarily require immediate laparotomy to undergo pancreaticoduodenectomy with acceptable morbidity and mortality rates; such patients can be treated by endoscopic biliary drainage without concern for increased major complications and death associated with subsequent pancreaticoduodenectomy.
As institutions have acquired significant experience with various complex pancreaticobiliary surgical procedures, 1–3 there has been renewed interest in investigating the risk/benefit ratio for preoperative biliary drainage. 4–9 In contrast to the putative benefits of preoperative biliary drainage that formed the basis for the first generation of controlled trials, 10–15 recent retrospective studies have suggested that the placement of biliary drains and subsequent bacterial colonization of the biliary tree may increase the rates of morbidity 4,8,16 and mortality 4 of pancreaticoduodenectomy. A recent comprehensive report from Memorial Sloan-Kettering Cancer Center of 240 consecutive patients who underwent pancreaticoduodenectomy showed that preoperative biliary drainage was associated with increased perioperative rates of morbidity and mortality. 4 Preoperative biliary drainage was found to be the only statistically significant variable associated with overall complications, infectious complications, intraabdominal abscess, and postoperative death. A four-fold increase in operative mortality rate among patients with stents caused the authors to recommend that preoperative biliary drainage be avoided whenever possible. The authors suggested instead that prompt pancreaticoduodenectomy may improve patient outcome after pancreaticoduodenectomy. These observations may have a significant impact on staging and referral practices for physicians who evaluate patients with obstructive jaundice of presumed malignant extrahepatic etiology.
The objective of this study was to examine the potential relationship between preoperative biliary drainage and subsequent morbidity and mortality after pancreaticoduodenectomy performed in a tertiary care cancer center. During the past decade, we have maintained an active clinical research program of multimodality therapy for localized pancreatic cancer. To receive preoperative (neoadjuvant) treatment, patients with obstructive jaundice required biliary decompression. Thus, if a significant relationship between preoperative biliary drainage and surgical morbidity and mortality exists, we should be able to identify the association given the large number of patients who have undergone biliary decompression prior to pre-operative combined-modality therapy and pancreaticoduodenectomy at our institution.
METHODS
Patients
Using a pancreatic and periampullary cancer database, we identified 300 consecutive patients who underwent pancreaticoduodenectomy for malignancy or suspected malignancy of the pancreas or periampullary region between May 1, 1990, and July 1, 1999 at M. D. Anderson. Patients who underwent total pancreatectomy or distal pancreatectomy were not included in this analysis.
Preoperative Evaluation and Treatment
All patients were required to have potentially resectable disease based on physical examination and previously published objective radiographic criteria. 17 Pretreatment biliary decompression was performed in virtually all patients with biliary obstruction to facilitate protocol-based preoperative chemoradiation. Preoperative biliary drainage in the early era was performed by percutaneous transhepatic catheter (PTC) placement; endobiliary stent (EBS) placement became the preferred approach for biliary decompression after therapeutic endoscopists (I.R., S.L.) joined our multidisciplinary Pancreatic Tumor Study Group. Patients who had undergone laparotomy with common bile duct exploration and placement of a common bile duct catheter (T tube) underwent placement of an EBS with removal of the T tube (n = 8).
Biliary instrumentation was defined as any attempt to perform cholangiography (with or without biliary drainage) by either an anterograde or retrograde approach. Prosthetic biliary drainage (PBD) was defined as successful placement of a PTC or EBS into the biliary tree before pancreaticoduodenectomy. Patients treated with a surgical biliary bypass procedure (BBP) before definitive pancreaticoduodenectomy included patients who underwent prereferral choledochojejunostomy, choledochoduodenostomy, or cholecystojejunostomy. The PBD group was analyzed separately and in combination with the BBP group to minimize the possible confounding effects of reoperative biliary surgery on complication rates.
Many patients in this report underwent preoperative chemoradiation in either a protocol or off-protocol setting using either standard-fractionation (50.4 Gy in 28 fractions) or rapid-fractionation (30 Gy in10 fractions) radiation therapy. Preoperative treatment protocols included standard-fractionation radiation with 5-fluorouracil 18,19 and rapid-fractionation radiation with 5-fluorouracil, 20 paclitaxel, 21 or gemcitabine. 22
Technique of Pancreaticoduodenectomy
All patients underwent preoperative bowel preparation with a polyelectrolyte solution and oral antibiotics. Patients also received perioperative intravenous antibiotic prophylaxis using a second-generation cephalosporin with or without metronidazole; penicillin-allergic patients received ciprofloxacin and metronidazole.
All patients underwent standard pancreaticoduodenectomy without pylorus preservation. 23 When possible, a soft 12-mm occluding bulldog clamp (Soft Surgical Spring Clip, Applied Medical Resources, Laguna Hills, CA) was positioned across the transected common hepatic duct to minimize intraperitoneal accumulation of bile during the period between division of the common hepatic duct and subsequent biliary-enteric reconstruction. Reconstruction was by end-to-side pancreaticojejunostomy.
Postoperative care was as described. 23 Patients with unexplained postoperative fever, leukocytosis, or worrisome findings on physical examination underwent postoperative CT. When necessary, intraabdominal fluid collections were drained percutaneously, and aspirated fluid was sent for culture and amylase assay.
Preoperative, Perioperative, and Postoperative Clinical Data
Preoperative, perioperative, and postoperative clinical data were retrospectively collected by chart review. Preoperative comorbidities were recorded, including a history of jaundice, diabetes mellitus, hypertension, or coronary artery disease. Perioperative data recorded included surgical time, intraoperative blood loss (as recorded in the anesthesia record), intraoperative transfusion of red blood cells, and details of the surgical procedure itself, including the need for portal or superior mesenteric venous resection/reconstruction and/or contiguous organ resection (colectomy, hepatectomy, appendectomy, or nephrectomy).
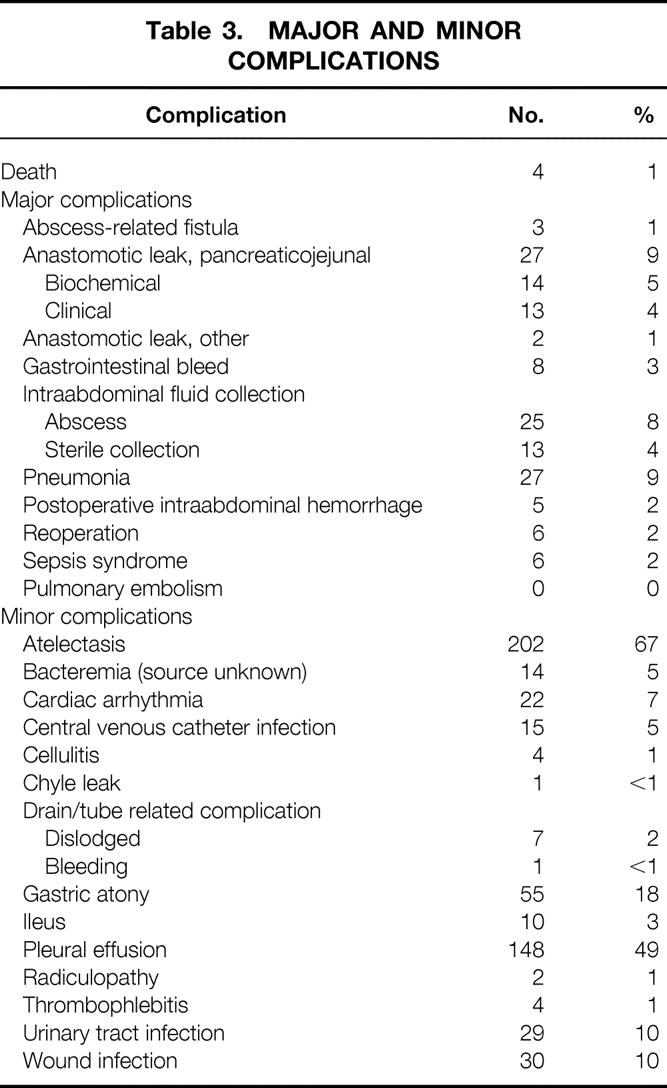
Major complications recorded in the postoperative period included postoperative death (death during the hospital stay for surgery or within 30 days of surgery); reoperation (during the hospital stay for surgery); postoperative intraabdominal hemorrhage (distinct from gastrointestinal bleeding); intraabdominal abscess (postoperative fluid collection with positive fluid culture results); pancreaticojejunal anastomotic leak, (biochemical [drain amylase level more than 2.5 times the upper limit of normal for serum amylase on postoperative day 3 or beyond without clinical sequelae] or clinical [biochemical leak associated with clinical sequelae such as fever, leukocytosis, fistula, or abscess]); other anastomotic leaks (from the biliary-enteric or gastrojejunal anastomosis); sepsis syndrome (positive blood cultures in the setting of fever without apparent source); pneumonia (clinical or radiographically significant pneumonia associated with pulmonary infiltrate with or without positive sputum cultures); intraluminal gastrointestinal bleeding (distinct from postoperative intraabdominal hemorrhage); catheter-related sepsis (positive blood cultures with positive cultures from the central venous catheter tip); and pulmonary embolism.
Minor complications recorded included allergic reaction, atelectasis (radiographic or clinical), cardiac arrhythmia, wound infection, wound cellulitis, cholangitis, gastrostomy or jejunostomy tube-related complications (including dislodging or clogging), delayed gastric emptying (gastrostomy tube output >1,000 mL on postoperative day 7 or inability to tolerate a postgastrectomy diet by postoperative day 10), ileus (absence of flatus and/or bowel sounds beyond postoperative day 7), infectious colitis (as documented by Clostridium difficile toxin assay), urinary tract infection (documented by positive urine culture), deep vein thrombosis, chylous ascites, and pleural effusion (radiographic or clinical).
Statistical Analysis
Results are expressed as the median and range or the number and percentage of patients. The Kruskal-Wallis test (nonparametric, one-way analysis of variance) was used to compare distributions of numeric variables between groups. The change in length of hospital stay over time was analyzed using robust linear regression (because of the skewed distribution). Univariate and multivariate associations between the biliary decompression groups and outcomes were analyzed via logistic regression analysis. Results are reported as odds ratios (ORs) with 95% confidence intervals (CIs) and probability values. P ≤ .05 was considered statistically significant. Because of the large numbers of comparisons and the fact that some of the comparisons lacked adequate statistical power, the probability values should be interpreted with caution. Significant probability values may represent false-positive conclusions, the likelihood of which is increased as the number of comparisons increases. Nonsignificant probability values may represent underpowered comparisons, which are indicated by CIs that may include clinically relevant values (e.g., OR < 0.3 or OR > 3.0) and thus cannot exclude the possibility of strong associations. Cutoffs for numeric variables were determined by recursive partitioning analysis using as an end point the occurrence of either intraabdominal abscess or pancreaticojejunal anastomotic leak.
RESULTS
Clinicopathologic Characteristics and Preoperative Biliary Instrumentation and Drainage
During the 9-year period studied, 300 consecutive patients underwent pancreaticoduodenectomy at M. D. Anderson Cancer Center. The demographic factors, comorbid conditions, histologic subtypes, preoperative treatments, and surgical variables for this cohort are outlined in Table 1. Pancreaticoduodenectomy was performed after prereferral tumor-related laparotomy in 58 patients (19%). Vascular resection and reconstruction were performed as part of the surgical procedure in 89 patients (30%).
Table 1. DEMOGRAPHIC FACTORS, COMORBID CONDITIONS, HISTOLOGIC SUBTYPES, AND TREATMENT-RELATED FACTORS
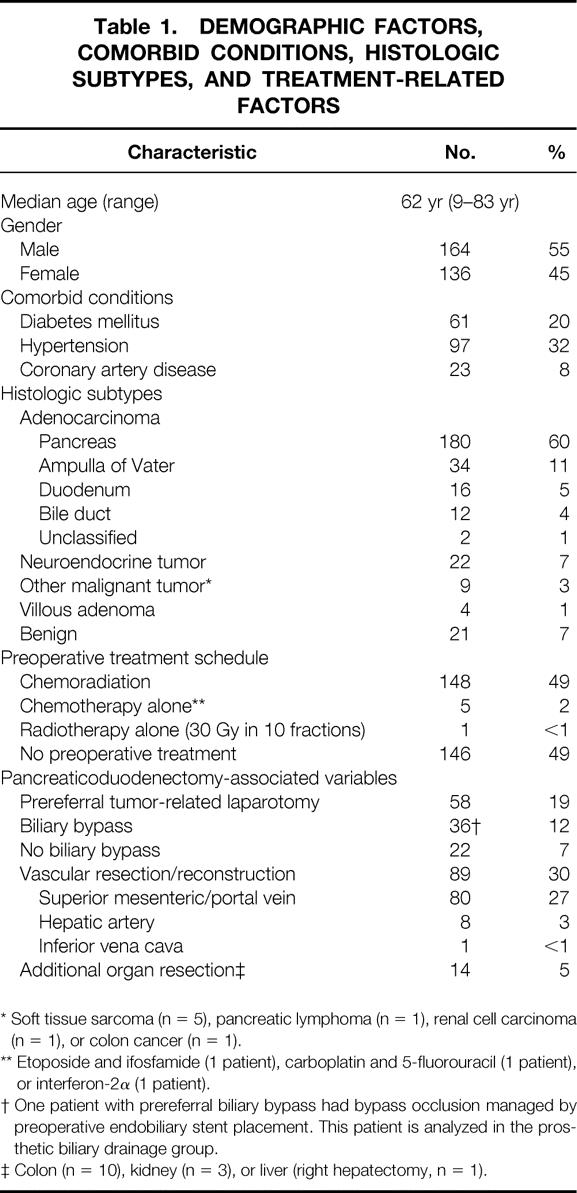
* Soft tissue sarcoma (n = 5), pancreatic lymphoma (n = 1), renal cell carcinoma (n = 1), or colon cancer (n = 1).
** Etoposide and ifosfamide (1 patient), carboplatin and 5-fluorouracil (1 patient), or interferon-2α (1 patient).
† One patient with prereferral biliary bypass had bypass occlusion managed by preoperative endobiliary stent placement. This patient is analyzed in the prosthetic biliary drainage group.
‡ Colon (n = 10), kidney (n = 3), or liver (right hepatectomy, n = 1).
Preoperative biliary instrumentation was performed in 183 patients (61%): 173 patients underwent ERCP, and 32 patients had PTC placement (22 patients underwent both). PBD via EBS or PTC was established in 172 patients (57% of the entire cohort): an EBS was used in 140 patients, a PTC was used in 28 patients, and other forms of biliary drainage (T-tube, n = 2; Wallstent, n = 1; cholecystostomy tube, n = 1) were used in 4 patients. Thirty-six patients (12%) had undergone a BBP prior to referral (choledochojejunostomy, n = 18; cholecystojejunostomy, n = 15; choledochoduodenostomy, n = 2). One patient with occlusion of a prereferral biliary bypass was managed by preoperative EBS placement; this patient is included in the PBD group for analysis.
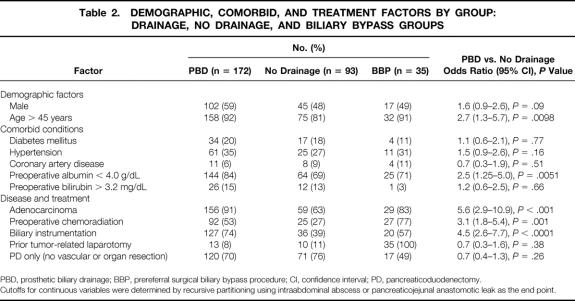
The demographics, co-morbid conditions, and treatment factors among the PBD, BBP, and no drainage groups and the relative frequency of these factors in the PBD versus no drainage groups are summarized in Table 2. The PBD and no drainage groups are not comparable in that PBD was utilized more frequently in patients who had preoperative biliary instrumentation (OR, 4.5; 95% CI, 2.6–7.7;P < 0.0001), who were older than 45 years (OR, 2.7; 95% CI, 1.3–5.7;P = 0.0098), who had adenocarcinoma (OR, 5.6; 95% CI, 2.9–10.9;P < 0.001), who were treated with preoperative chemoradiation (OR, 3.1; 95% CI, 1.8–5.4;P = 0.001), or who had a preoperative serum albumin level below 4.0gm/dL (OR, 2.5; 95% CI, 1.25–5.0;P = 0.0051) (cut points for continuous variables defined by recursive partitioning). There were no significant differences between these groups in operating room time (OR, 1.1; 95% CI, 0.7–1.9), P = 0.67), estimated blood loss (OR, 1.4; 95% CI, 0.8–2.3;P = 0.24), or transfusion requirement (OR, 1.3, 95% CI, 0.7–2.5, P = 0.35).
Table 2. DEMOGRAPHIC, COMORBID, AND TREATMENT FACTORS BY GROUP: DRAINAGE, NO DRAINAGE, AND BILIARY BYPASS GROUPS
PBD, prosthetic biliary drainage; BBP, prereferral surgical biliary bypass procedure; CI, confidence interval; PD, pancreaticoduodenectomy.
Cutoffs for continuous variables were determined by recursive partitioning using intraabdominal abscess or pancreaticojejunal anastomotic leak as the end point.
Postoperative Complications, Deaths, and Length of Hospital Stay
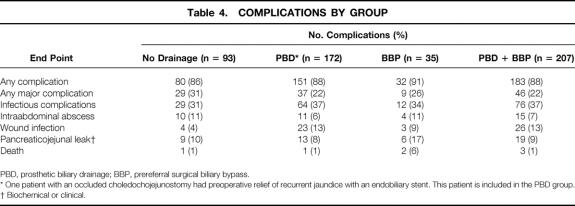
The major and minor complications observed after pancreaticoduodenectomy are outlined in Table 3. One or more complications occurred in 265 patients (88%). Postoperative death occurred in four patients (1.3%) at 2, 11, 39, and 95 days after surgery. The causes of postoperative death were myocardial infarction (n = 2) and multisystem organ failure (n = 2). The median hospital stay was 14 days (range 5–80) among the 296 patients who survived surgery. The length of stay declined over time at a rate of 1.2 days/year, and the median hospital stay in 1999 was 11.5 days.
Table 3. MAJOR AND MINOR COMPLICATIONS
Analysis of Factors Associated With Morbididty and Mortality
The frequency of major complications in the PBD, BBP, and no drainage groups is outlined in Table 4 We also tabulated the frequency of major complications in the PBD + BBP groups to facilitate comparison with prior reports. 4,8 Univariate regression analysis (data not shown) was used to compare the frequency of complications in the PBD versus no drainage groups, the BBP versus no drainage group, and the PBD plus BBP versus no drainage groups. The frequencies of any complication, any major complication, Univariate regression analysis (data not shown) was used to compare the frequency of complications in the PBD versus no drainage groups, the BBP versus no drainage group, and the PBD plus BBP versus no drainage groups. The frequencies of any complication, any major complication, infectious complications, and pancreaticojejunal anastomotic leak were similar in the PBD, BBP, PBD + BBP, and no drainage groups. However, wound infections were noted in 23 (13%) of 172 patients in the PBD group and 4 (4%) of 93 patients in the no drainage group (OR, 3.4; 95% CI, 1.2–10.2, P = .028). There was also a statistically significantly higher wound infection rate in the PBD plus BBP group than in the no drainage group (OR, 3.2; 95% CI, 1.1–9.4;P = 0.036).
Table 4. COMPLICATIONS BY GROUP
PBD, prosthetic biliary drainage; BBP, prereferral surgical biliary bypass.
* One patient with an occluded choledochojejunostomy had preoperative relief of recurrent jaundice with an endobiliary stent. This patient is included in the PBD group.
† Biochemical or clinical.
Perioperative death occurred in 1 (0.6%) of 172 patients in the PDB group, 1 (1%) of 93 patients in the no drainage group, and 2 (6%) of 35 patients in the BBP group. These differences were not statistically significant by univariate regression (OR, 1.3; 95% CI, 0.1–13.0;P = 0.79).
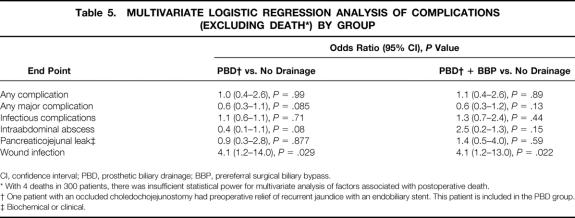
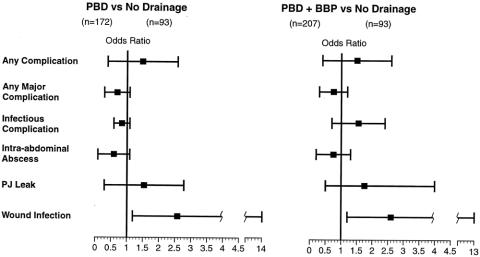
Multivariate logistic regression analyses were performed for the end points of any complication, any major complication, infectious complications, intraabdominal abscess, pancreaticojejunal anastomotic leak, and wound infection (Table 5, Fig. 1). By multivariate analysis, there were no significant differences between the PBD and no drainage groups or the PBD + BBP and no drainage groups for any endpoint except wound infection. The wound infection risk differed significantly between the PBD versus no drainage groups (OR, 4.1; 95% CI; 1.2–14.0;P = .029) and the PBD + BBP versus no drainage groups (OR, 4.1; 95% CI; 1.2–13.0;P = .022). We did not perform multivariate regression analyses for death because with only four deaths, such analyses would be statistically underpowered.
Table 5. MULTIVARIATE LOGISTIC REGRESSION ANALYSIS OF COMPLICATIONS (EXCLUDING DEATH*) BY GROUP
CI, confidence interval; PBD, prosthetic biliary drainage; BBP, prereferral surgical biliary bypass.
* With 4 deaths in 300 patients, there was insufficient statistical power for multivariate analysis of factors associated with postoperative death.
† One patient with an occluded choledochojejunostomy had preoperative relief of recurrent jaundice with an endobiliary stent. This patient is included in the PBD group.
‡ Biochemical or clinical.
Figure 1. Results of multivariate analyses for the postoperative morbidity end points. There were only 4 deaths among the 300 patients, so there was insufficient statistical power for a multivariate analysis of factors associated with postoperative death. Results for each of the other end points are presented as the odds ratio (box) and 95% confidence interval (bar). Wound infections were significantly more common in patients who underwent preoperative biliary drainage. No other complications were significantly different between groups. PBD, prosthetic biliary drainage; BBP, biliary bypass procedure; PJ, pancreaticojejunal.
DISCUSSION
We report death and complication rates in 300 consecutive patients who underwent pancreaticoduodenectomy at a tertiary care cancer center. The observed overall rate of major complications is comparable to that in other large series recently reported. 2 The observed mortality rate of 1.3% is comparable to the rate of less than 3% reported by other institutions. 2,23–26 Unique to this series was that 19% of patients had undergone laparotomy with unsuccessful attempted tumor resection before referral, 30% underwent simultaneous major vascular resection and reconstruction during pancreaticoduodenectomy, and 5% underwent adjacent organ resection.
In an evidence-based approach to interpretation of the medical literature, randomized trials are given increased weight owing to the presumed balanced nature of known prognostic factors between treatment and control groups. However, the existing phase III trials 10–15 evaluating preoperative biliary drainage included only small numbers of patients who underwent pancreaticoduodenectomy; the largest trial had only 29 such patients. 12 Consequently, specific subset analyses of pancreaticoduodenectomy-associated morbidity and mortality were impossible to perform in these trials. In the absence of larger randomized trials limited to patients undergoing pancreaticoduodenectomy, the current findings and the existing body of retrospective evidence assume increased importance. 4–6,8,28,29
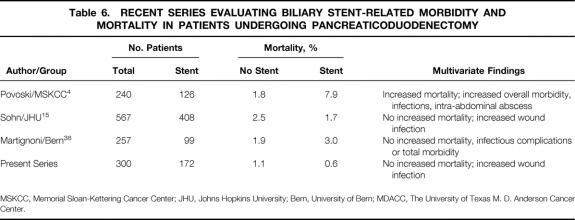
In the study from MSKCC by Povoski and colleagues the potential associations between an extensive group of demographic, co-morbid, operative, and biliary drain-related parameters were evaluated for four specific endpoints: overall complications, infectious complications, intra-abdominal abscess, and postoperative death. 4 Multiple individual univariate comparisons and multivariate analyses demonstrated that preoperative biliary drainage was independently associated with all four endpoints. Ten postoperative deaths (7.9%) were observed among the 126 patients who underwent preoperative biliary drainage compared with two deaths (1.8%) among the 114 patients who did not undergo preoperative biliary decompression (multivariate P < .037).
Direct comparison of the Memorial Sloan-Kettering results to our findings is limited by the fact that Povoski et al did not analyze other complications such as wound infection or anastomotic leak and included 10 patients in the preoperative biliary drainage group who underwent prereferral BBP. One assumes that the authors included patients with prior BBP because a preoperative biliary-enteric anastomosis results in bacterial colonization of the biliary tree and therefore a risk for biliary drainage-related complications similar to that with prosthetic biliary stents. However, inclusion of the subset of patients with prior BBP may confound the interpretation of biliary drain-related complications because it may be difficult, if not impossible, to distinguish between the potential added morbidity and mortality from reoperative biliary surgery and the potential morbidity/mortality from biliary decompression itself. To address this possibility, we have analyzed our data using two distinct approaches: excluding patients who underwent prereferral BBP (i.e., PBD vs. no biliary drainage), and comparing any form of biliary drainage (PBD + BBP) with no drainage (i.e., an identical approach to that used by Povoski et al 4). Using either approach and analyzing for several end points, we found that biliary decompression significantly increased only the risk of wound infection (OR, 4.1; 95% CI, 1.2–13.0;P = .022). Thus, in our experience, preoperative biliary decompression did not result in a significant increase in the rates of major complications after pancreaticoduodenectomy. The wide confidence intervals observed in the univariate logistic regression analysis for the risk of postoperative death with biliary decompression (OR, 1.3; 95% CI, 0.1–13.0;P = .79) are due to the small number of deaths. With 4 deaths in 300 patients, we did not have sufficient statistical power for a multivariate analysis of factors predicting postoperative death.
Sohn et al 16 recently reported results of a comprehensive analysis of 567 patients who underwent pancreaticoduodenectomy at Johns Hopkins. Patients who underwent prior BBP (30 patients) were excluded from the analysis to eliminate the potential confounding effects of reoperative biliary surgery on the assessment of biliary stent-related morbidity and mortality. The surgical mortality rate in the 408 patients who underwent preoperative biliary decompression was 1.7%, comparable to our rate of 0.6% in these patients. Comprehensive univariate and multivariate analyses were used for rates of overall complications, pancreatic fistula, wound infection, delayed gastric emptying, intraabdominal abscess, cholangitis, bile leak, pneumonia, pancreatitis, and reoperation. The only complication associated with preoperative biliary decompression (by PTC or EBS) by multivariate analysis was wound infection (P = .03). Sohn et al did not report ORs or CIs, making direct comparison with our results difficult. Nonetheless, analysis of our results with inclusion or exclusion of patients who had prereferral BBP yielded observations similar to those reported by the Johns Hopkins investigators.
The issue of whether there is increased risk for postoperative death associated with biliary stent placement is of major importance (Table 6). The Memorial Sloan-Kettering investigators found a fourfold increase in the surgical mortality rate associated with preoperative biliary drainage (1.8% in the no stent group vs. 7.9% in the biliary drainage group). 4 The authors concluded that preoperative biliary drainage should be avoided whenever possible in patients with potentially resectable pancreatic and peripancreatic lesions. Their data suggest that instead of endobiliary stent placement, jaundiced patients should undergo prompt laparotomy for attempted tumor resection and definitive operative management of their jaundice; this allows pancreaticoduodenectomy to be performed with the lowest possible mortality rate. In contrast, our experience suggests that patients with extrahepatic jaundice can be safely managed with prereferral nonsurgical biliary drainage. This facilitates timely palliation of symptomatic jaundice and allows time for careful treatment planning. As advocated by others, 30 such treatment planning should include referral to a regional center that specializes in pancreatic cancer treatment for more detailed pretreatment staging and stage-specific multimodality therapy. Referral to a high-volume center is important given recent reports from Birkmeyer et al 31,32 and others 33-37 documenting high morbidity and mortality rates associated with pancreaticoduodenectomy performed outside high-volume centers. Indeed, a policy of stent placement and referral to a regional center may be the safest and ultimately the most cost-effective strategy for these patients. Physicians caring for patients with presumed malignant extrahepatic biliary obstruction should not be concerned that they are increasing the risks of subsequent pancreaticoduodenectomy by proceeding with cholangiography and therapeutic biliary drainage before prompt referral. Similarly, patients treated with preoperative chemoradiation who require pretreatment nonoperative biliary decompression should not be concerned that the procedure will increase their risk for major complications or death during preoperative treatment 38 or following pancreaticoduodenectomy.
Table 6. RECENT SERIES EVALUATING BILIARY STENT-RELATED MORBIDITY AND MORTALITY IN PATIENTS UNDERGOING PANCREATICODUODENECTOMY
MSKCC, Memorial Sloan-Kettering Cancer Center; JHU, Johns Hopkins University; Bern, University of Bern; MDACC, The University of Texas M. D. Anderson Cancer Center.
In the absence of meaningful randomized data addressing the issue of pancreaticoduodenectomy-specific stent-related morbidity and mortality, it is important to acknowledge the limitations of the retrospective nonrandomized nature of this report and the other retrospective studies addressing this issue. 4,6,8,16,28,29 Significant clinical judgment (that results in selection bias) occurs during the clinical evaluation of jaundiced patients. Patients who are older, appear to have malnutrition, or have significant comorbid conditions, suboptimal performance status, or other organ function compromise secondary to biliary obstruction are more likely to undergo preoperative biliary decompression. The bias introduced by performing biliary decompression on patients with an inferior performance status is probably significant but is impossible to quantitate accurately in a retrospective fashion. Nevertheless, because patients with an inferior performance status tend to undergo biliary decompression first, whereas low-risk, good-performance-status patients are generally taken directly to surgery, the comparison of perioperative complication and death rates in biliary decompression versus no drainage groups in all retrospective studies is biased in favor of the no drainage group. In the current study, patients who underwent PBD were significantly older (P = .0098) and had a lower preoperative serum albumin level (P = .0051). Such selection bias-related differences between the biliary decompression and no drainage groups are still discernible in the current report despite an institutional commitment to protocol-based neoadjuvant therapy that extended the usual criteria used to select patients for preoperative biliary decompression (i.e., patients who would otherwise proceed to surgery without preoperative biliary decompression underwent biliary decompression solely to permit preoperative chemoradiation).
In summary, our analysis of 300 consecutive patients who underwent pancreaticoduodenectomy shows that preoperative biliary drainage is associated with an increased risk for subsequent wound infection but not other complications, including intraabdominal abscess, pancreaticojejunal anastomotic leak, or death. Our data thus suggest that preoperative biliary stenting can be used safely without an increased risk for major postoperative complications or perioperative death. This is significant in the context of evaluating current staging practices for jaundiced patients with suspected pancreatic or periampullary malignancy; these patients do not require immediate laparotomy and attempted tumor resection to undergo pancreaticoduodenectomy with acceptably low morbidity and mortality. Given the current findings, those of Sohn et al, 16 and the well-established relationship between institutional surgical volume and pancreaticoduodenectomy-associated rates of morbidity and mortality, 31–37 patients with extrahepatic jaundice of presumed neoplastic origin should receive accurate pretreatment CT imaging, timely biliary decompression, and should be offered referral to a high-volume center experienced in the multidisciplinary management of pancreatic cancer. 39
Acknowledgments
The authors thank Adrienne Denny, Vivian Z. Garcia, and Melissa Burkett for help in the management of data and preparation of this manuscript.
Footnotes
Presented in part at the Papers Session of the 85th Annual American College of Surgeons Clinical Congress, San Francisco, California, October 1999.
Correspondence: Peter W. T. Pisters, MD, Department of Surgical Oncology, Box 444, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030-4095.
E-mail: ppisters@mdanderson.org
Supported by The University of Texas M. D. Anderson Cancer Center Various Donors Fund for Pancreatic Cancer Research.
Accepted for publication December 1, 2000.
References
- 1.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990; 211: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997; 226: 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichlmayr R, Weimann A, Klempnauer J, et al. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg 1996; 224: 628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Povoski SP, Karpeh MS, Conlon KC, et al. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg 1999; 230: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Povoski SP, Karpeh MS, Conlon KC, et al. Preoperative biliary drainage: impact on intraoperative bile cultures and infectious morbidity and mortality after pancreaticoduodenectomy. J Gastrointest Surg 1999; 3: 496–505. [DOI] [PubMed] [Google Scholar]
- 6.Karsten TM, Allema JH, Reinders M, et al. Preoperative biliary drainage, colonisation of bile and postoperative complications in patients with tumours of the pancreatic head: a retrospective analysis of 241 consecutive patients. Eur J Surg 1996; 162: 881–888. [PubMed] [Google Scholar]
- 7.Hochwald SN, Burke EC, Jarnagin WR, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg 1999; 134: 261–266. [DOI] [PubMed] [Google Scholar]
- 8.Heslin MJ, Brooks AD, Hochwald SN, et al. A preoperative biliary stent is associated with increased complications after pancreatoduodenectomy. Arch Surg 1998; 133: 149–154. [DOI] [PubMed] [Google Scholar]
- 9.Martignoni ME, Wagner M, Krahenbuhl L, et al. Effect of preoperative biliary drainage on surgical outcome after pancreaticoduodenectomy. Am J Surg 2001; 181: 52–59. [DOI] [PubMed] [Google Scholar]
- 10.Hatfield AR, Tobias R, Terblanche J, et al. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet 1982; 2: 896–899. [DOI] [PubMed] [Google Scholar]
- 11.McPherson GA, Benjamin IS, Hodgson HJ, et al. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg 1984; 71: 371–375. [DOI] [PubMed] [Google Scholar]
- 12.Lygidakis NJ, Lubbers MJ. Evaluation of preoperative biliary drainage in the surgical management of pancreatic head carcinoma. Am J Surg Scand 1987; 153: 665–668. [PubMed] [Google Scholar]
- 13.Lai EC, Mok FP, Fan ST, et al. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg 1994; 81: 1195–1198. [DOI] [PubMed] [Google Scholar]
- 14.Pitt HA, Gomes AS, Lois JF, et al. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg 1985; 201: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith RC, Pooley M, George CR, Faithful GR. Preoperative percutaneous transhepatic internal drainage in obstructive jaundice: a randomized, controlled trial examining renal function. Surgery 1985; 97: 641–648. [PubMed] [Google Scholar]
- 16.Sohn TA, Yeo CJ, Cameron JL, et al. Do preoperative biliary stents increase post-pancreaticoduodenectomy complications? J Gastrointest Surg 2000; 4: 258–268. [DOI] [PubMed] [Google Scholar]
- 17.Fuhrman GM, Charnsangavej C, Abbruzzese JL, et al. Thin-section contrast-enhanced computed tomography accurately predicts the resectability of malignant pancreatic neoplasms. Am J Surg 1994; 167: 104–111. [DOI] [PubMed] [Google Scholar]
- 18.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 1992; 127: 1335–1339. [DOI] [PubMed] [Google Scholar]
- 19.Staley CA, Lee JE, Cleary KR, et al. Preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for adenocarcinoma of the pancreatic head. Am J Surg 1996; 171: 118–124. [DOI] [PubMed] [Google Scholar]
- 20.Pisters PWT, Abbruzzese JL, Janjan NA, et al. Rapid-fractionation preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for resectable pancreatic adenocarcinoma. J Clin Oncol 1998; 16: 3843–3850. [DOI] [PubMed] [Google Scholar]
- 21.Pisters PWT, Abbruzzese JL, Janjan NA, et al. Comparative toxicities of preoperative paclitaxel vs 5-fluorouracil based rapid fractionation chemoradiation for resectable pancreatic adenocarcinoma. Proc Am Soc Clin Oncol 1999; 18: 939. [Google Scholar]
- 22.Wolff RA, Evans DB, Gravel DM, et al. Phase 2 trial of gemcitibin combined with radiation for the treatment of locally advanced pancreatic cancer. Proc Am Soc Clin Oncol 1998; 17: 283a. [Google Scholar]
- 23.Evans DB, Lee JE, Pisters PWT. Pancreaticoduodenectomy (Whipple operation), total pancreatectomy. In: Nyhus LM, Baker RJ, Fischer JE, eds. Mastery of surgery. New York: Little, Brown & Co; 1997: 1233–1249.
- 24.Schwarz RE, Keny H, Ellenhorn JDI. A mortality-free decade of pancreaticoduodenectomy: is quality independent of quantity? Am Surg 1999; 65: 949–954. [PubMed] [Google Scholar]
- 25.Rios G, Conrad A, Cole D, et al. Trends in indications and outcomes in the Whipple procedure over a 40-year period. Am Surg 1999; 65: 889–893. [PubMed] [Google Scholar]
- 26.Millikan KW, Deziel DJ, Silverstein JC, et al. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg 1999; 65: 618–623. [PubMed] [Google Scholar]
- 27.Fernandez del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg 1995; 130: 295–299. [DOI] [PubMed] [Google Scholar]
- 28.Andersen HB, Baden H, Brahe NE, Burcharth F. Pancreaticoduodenectomy for periampullary adenocarcinoma. J Am Coll Surg 1994; 179: 545–552. [PubMed] [Google Scholar]
- 29.Trede M, Schwall G. The complications of pancreatectomy. Ann Surg 1988; 207: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jimenez RE, Warshaw AL, Rattner DW, et al. Impact of laparoscopic staging in the treatment of pancreatic cancer. Arch Surg 2000; 135: 409–415. [DOI] [PubMed] [Google Scholar]
- 31.Birkmeyer JD, Warshaw AL, Finlayson SR, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery 1999; 126: 178–183. [PubMed] [Google Scholar]
- 32.Birkmeyer JD, Finlayson SR, Tosteson AN, et al. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery 1999; 125: 250–256. [PubMed] [Google Scholar]
- 33.Lieberman MD, Kilburn H, Lindsey M, Brennan MF. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg 1995; 222: 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon TA, Burleyson GP, Tielsch JM, Cameron JL. The effects of regionalization on cost and outcome for one general high-risk surgical procedure. Ann Surg 1995; 221: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon TA, Bowman HM, Tielsch JM, et al. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg 1998; 228: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg 1998; 228: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simunovic M, To T, Theriault M, Langer B. Relation between hospital surgical volume and outcome for pancreatic resection for neoplasm in a publicly funded health care system. Can Med Assoc J 1999; 160: 643–648. [PMC free article] [PubMed] [Google Scholar]
- 38.Pisters PWT, Hudec WA, Lee JE, et al. Preoperative chemoradiation for patients with pancreatic cancer: toxicity of endobiliary stents. J Clin Oncol 2000; 18: 860–867. [DOI] [PubMed] [Google Scholar]