Abstract
Objective
To compare the diagnostic accuracies of Lipiodol computed tomography (CT) and helical biphasic CT as preoperative imaging modalities for hepatocellular carcinoma (HCC).
Summary Background Data
Lipiodol CT after digital subtraction angiography has long been used as a highly sensitive imaging modality for HCC. The recent advent of helical CT has allowed scanning the entire liver during both the arterial and portal venous phase of contrast enhancement.
Methods
The authors analyzed data from 164 patients who underwent hepatic resection for HCC to calculate the sensitivity and specificity of these modalities. Findings of intraoperative ultrasonography followed by histologic confirmation were set as the gold standard.
Results
Although sensitivity decreased with both modalities as tumors became small and well differentiated, helical CT showed a higher sensitivity than Lipiodol CT in detecting well-differentiated HCC nodules smaller than 2 cm. In contrast, Lipiodol CT was superior to helical CT for the detection of small but moderately to poorly differentiated nodules. The overall sensitivity of helical CT was higher than that of Lipiodol CT. These findings suggest that helical CT is superior in delineating early HCC, whereas Lipiodol CT is specific to the detection of intrahepatic metastases. In terms of specificity, helical CT was superior to Lipiodol CT.
Conclusions
Helical CT and Lipiodol CT are complementary modalities. At present, helical biphasic CT does not obviate the need for invasive techniques such as angiography and Lipiodol CT as preoperative examinations for HCC.
Advances in various imaging modalities including ultrasonography and computed tomography (CT) have facilitated the detection of hepatocellular carcinoma (HCC) in a preclinical stage. 1 As a result, the resectability of HCC has markedly increased, thereby significantly improving survival during the past two decades. 2–5 HCC nodules are often multifocal and frequently accompanied by intrahepatic metastatic nodules. 6–8 Underestimation of these lesions may lead to inappropriate surgical resection. Therefore, accurate preoperative imaging evaluation of HCC nodules is essential for selecting appropriate patients for surgical intervention and for determining the extent of hepatectomy. Because typical HCCs are hypervascular tumors that have only an hepatic arterial blood supply (i.e., there is no portal venous flow 9), hepatic angiography has been widely used as an imaging technique for HCC. 10 Moreover, Lipiodol CT, which involves CT after intrahepatic arterial injection of iodized oil (Lipiodol Ultrafluid; Andre Guerbet, Aulnay-Soubois, France), has been reported to be the most sensitive preoperative imaging modality for HCC, 11–20 especially in detecting intrahepatic metastatic nodules. 12,14,18,19
However, the recent advent of helical CT has allowed scanning of the entire liver during both the arterial and the portal venous phase of contrast enhancement. 21 Several studies have shown a significant increase in the detection of HCC nodules with the biphasic contrast-enhanced technique using helical CT compared with conventional CT. 22–24 However, consensus has not been reached as to whether helical biphasic CT obviates the need for other invasive imaging modalities such as digital subtraction angiography (DSA) and Lipiodol CT. Likewise, the abilities of various imaging modalities to detect early HCCs (i.e., <2 cm, well differentiated) has not been adequately investigated. 25,26 This is a major clinical concern because well-differentiated HCCs, as is frequently the case with small HCCs, are often hypovascular and thus theoretically less detectable by these imaging modalities than more advanced HCCs. 25
In the 6 years from 1993 through 1998, we routinely carried out DSA and Lipiodol CT in addition to ultrasonography and enhanced CT (with or without helical biphasic) as preoperative imaging studies to determine resectability and surgical procedures. In total, 164 patients underwent these examinations. In all cases, the final surgical decision was made based on the findings of intraoperative ultrasound. In this report, we retrospectively analyze the data collected from these 164 patients to compare the diagnostic accuracy of helical biphasic CT, DSA, and Lipiodol CT in detecting HCC nodules.
PATIENTS AND METHODS
Patients
Between January 1993 and December 1998, 164 patients with HCC underwent preoperative DSA and Lipiodol CT examination at the First Department of Surgery, Shinshu University School of Medicine. These patients form the basis of this report. The methods that first led to the diagnosis of HCC were ultrasound, CT (with or without biphasic contrast enhancement), and/or tumor markers such as alfa-fetoprotein and des-γ-carboxy prothrombin. There were 121 men and 43 women, ranging in age from 21 to 82 years (mean 63.8). Helical biphasic CT was carried out in the last 69 consecutive patients; in other words, this modality was not available when the first 95 patients were evaluated.
Enhanced CT or Helical Biphasic CT
We used conventional CT (CT 9800; General Electric Medical Systems, Milwaukee, WI), before the introduction of helical CT, as a preoperative examination consisting of 1-cm contiguous sections through the entire liver, before and after intravenous infusion of 100 mL iohexol (Omnipaque 300; Daiichi Pharmaceutical Co., Tokyo, Japan). After the introduction of helical CT, the patients underwent unenhanced and biphasic helical CT scanning (Hispeed Advantage; General Electric Medical Systems), which involves the arterial and portal venous phases after intravenous infusion of contrast media, as follows. First, patients were imaged with a helical CT scanner with a 7- to 10-mm collimation and a 1:1 pitch in a cranial-caudal direction beginning at the top of the liver. Then, 100 mL iohexol was administered at a rate of 3 mL/s. CT was initiated 30 seconds after infusion. The entire liver was imaged in 20 to 30 seconds (the arterial phase). Images from the second pass of contrast agent through the liver were obtained beginning at 60 to 70 seconds after infusion. Again, 20 to 30 seconds was required to image the entire liver (the portal venous phase).
DSA and Lipiodol CT
We first obtained arterial portograms using the superior mesenteric artery to confirm the presence or absence of tumor thrombus in the portal vein after previous assessment by ultrasound and/or CT. Next, hepatic arteriography, for which images were obtained through the conventional method and DSA, was performed. We injected an approximately 5-mL suspension of iodized oil into the proper hepatic artery or selectively into each left, middle, and right hepatic artery. CT examination of the liver was performed an average of 15.3 days after intraarterial injection of iodized oil to evaluate areas of Lipiodol retention.
Intraoperative Ultrasonography and Pathologic Examination
Surgery was performed on average 20.3 days after the Lipiodol CT examination. The gold standard for the presence or absence of HCC lesions was provided by findings at visual inspection of the liver surface, bimanual palpation, and intraoperative ultrasound (SSD 650 CL and/or SSD 2000; Aloka, Tokyo, Japan) with a 7.5-MHz linear probe. Operators were aware of the results of all preoperative imaging studies. Lesions detected for the first time by intraoperative ultrasound were resected whenever possible together with the tumors identified before surgery. If hepatic resection of these lesions was deemed inappropriate because of the tumor location, ultrasound-guided biopsy followed by immediate histologic examination was performed. The tumors were treated with intraoperative ethanol injection or left untreated, according to the results of histologic examination.
Data Analysis
The images for each radiologic study were interpreted by at least two attending radiologists before surgery. The examiners were aware of the results of previously performed imaging studies. Because retention of iodized oil is also known to occur in hemangiomas and areas of arterioportal shunt, 16,27 Lipiodol deposits in these lesions were not counted as positive nodules if they had been detected by other imaging techniques.
A comparison between preoperative imaging data and those from the intraoperative ultrasound and pathologic examinations was undertaken to determine the accuracy of various preoperative imaging modalities for diagnosing HCC nodules. True-positive, false-positive, and false-negative nodules detected with each preoperative imaging modality were assessed according to lesion-by-lesion analysis by setting nodules confirmed to be HCC with intraoperative ultrasound and pathologic examination as the gold standard. Thus, sensitivity and positive predictive value were calculated in this study population, lesion by lesion, as follows: sensitivity = true-positive nodules/(true-positive nodules + false-negative nodules), and positive predictive value = true-positive nodules/(true-positive nodules + false-positive nodules).
Because true-negative nodules could not be defined based on lesion-by-lesion analysis, true-negative cases were defined based on patient-by-patient analysis as any patient in whom the area without HCC was correctly predicted by preoperative imaging. Then, the specificity of each imaging modality was calculated, patient by patient, as true-negative cases/(true-negative cases + false-positive cases).
The sensitivity and specificity of the imaging modalities were compared by the Fisher exact test. We also conducted the generalized estimating equation approach to account for the correlation in results from the same lesion, considering that such a correlation may overestimate the difference determined by the Fisher exact test. P < .05 was accepted as a significant difference.
RESULTS
Intraoperative ultrasound followed by histologic confirmation identified 268 HCC nodules in 164 patients. Of these patients, 108 had one tumor, 29 had two tumors, 14 had three tumors, and 12 had four or more tumors. The mean diameter of these nodules was 25.8 mm (range 2–135, median 20). There were 66 well-differentiated nodules, 165 moderately differentiated nodules, and 37 poorly differentiated nodules. Figure 1 shows the relationship between tumor size and cellular differentiation of HCC nodules. The diameters of well-differentiated, moderately differentiated, and poorly differentiated HCC nodules (mean ± SD) were 1.62 ± 0.96, 2.75 ± 1.9, and 3.45 ± 3.0 cm, respectively. Likewise, median diameters of these three groups of HCC nodules were 1.4, 2.5, and 2.5 cm, respectively. Although tumors became less differentiated as their size increased (r = 0.31, P < .0001, by Spearman rank analysis), 36% (12/33) of poorly differentiated nodules were smaller than 2 cm in diameter.
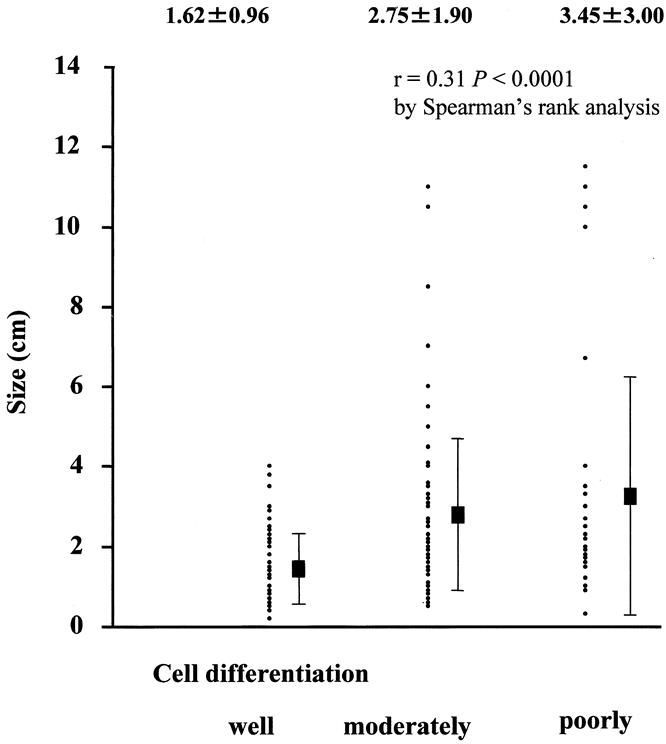
Figure 1. The relationship between tumor size and cellular differentiation of hepatocellular carcinoma nodules. Tumors become less differentiated as the their size increases. Values are expressed as mean ± standard deviation (cm).
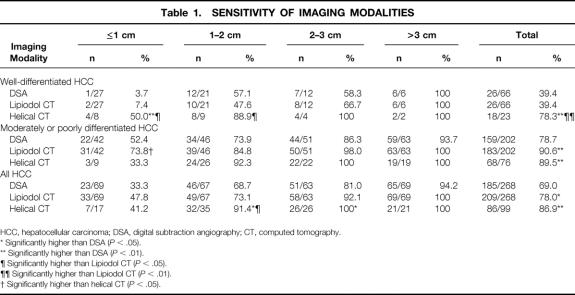
In the lesion-by-lesion and patient-by-patient analyses (Tables 1 and 2), all robust probability values by the generalized estimating equation approach were virtually the same as the naive ones calculated by the Fisher exact test. Thus, data were expressed as naive values calculated by Fisher exact tests. Table 1 shows the sensitivities of the various imaging modalities according to tumor size and the degree of tumor differentiation. In general, the sensitivity of either imaging method decreased as the tumors became smaller and better differentiated. Nonetheless, the sensitivity of helical CT in detecting fairly small (≤2 cm), well-differentiated HCC nodules was significantly higher (50% for nodules ≤1cm, 89% for those 1–2 cm) than that of DSA (4% for nodules ≤1cm, P < .01; 57% for those 1–2 cm, P = NS) or that of Lipiodol CT (7% for nodules ≤1cm, P < .05; 48% for those 1–2 cm, P < .05). On the contrary, Lipiodol CT showed higher sensitivity than DSA and helical CT for the detection of moderately to poorly differentiated HCC nodules with a diameter of 1 cm or less (74%, 52%, and 33%, respectively;P < .05 for Lipiodol CT vs. helical CT). The overall sensitivities of DSA, Lipiodol CT, and helical CT in detecting HCC were 69%, 78%, and 87%, respectively. The differences in sensitivity between DSA and Lipiodol CT (P < .05) and between helical CT and DSA (P < .01) were statistically significant. Although the difference in overall sensitivity between Lipiodol CT and helical CT was not statistically significant by the Fisher exact test, the McNemar test on data exclusively from the last 69 consecutive patients who underwent both of these modalities showed a significant difference in sensitivities (78% vs. 87%, respectively;P < .05).
Table 1. SENSITIVITY OF IMAGING MODALITIES
HCC, hepatocellular carcinoma; DSA, digital subtraction angiography; CT, computed tomography.
* Significantly higher than DSA (P < .05).
** Significantly higher than DSA (P < .01).
¶ Significantly higher than Lipiodol CT (P < .05).
¶¶ Significantly higher than Lipiodol CT (P < .01).
† Significantly higher than helical CT (P < .05).
Table 2. SPECIFICITY OF IMAGING MODALITIES
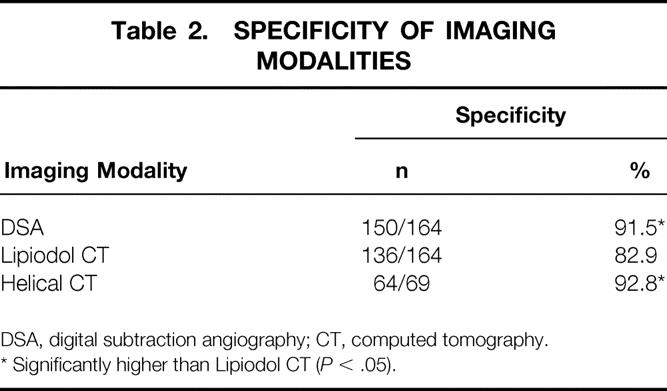
DSA, digital subtraction angiography; CT, computed tomography.
* Significantly higher than Lipiodol CT (P < .05).
DSA examination resulted in 15 false-positive nodules in 164 patients: one nodule was diagnosed as hemangioma by intraoperative ultrasound and histologic examination, and the remaining 14 were not identified by ultrasound. The 36 false-positive nodules by Lipiodol CT included seven hemangiomas, two regenerative nodules, one arterioportal shunt, and 26 deposits not identified by ultrasound. Helical CT examination resulted in five false-positive nodules in 69 patients. All five were defined as false positive because intraoperative ultrasound could not detect them. Table 2 shows the specificities of various imaging modalities according to patient-by-patient analysis. The specificity of Lipiodol CT (83%) was significantly lower than that of DSA (92%) and helical CT (93%) (P < .05, respectively).
The positive predictive values according to lesion-by-lesion analysis were as follows: DSA, 93%; Lipiodol CT, 85%; and helical CT, 95%. The differences between Lipiodol CT and DSA and that between Lipiodol CT and helical CT were statistically significant (P < .05).
Figure 2 shows a representative patients in whom the HCC nodule was correctly identified by Lipiodol CT but not by helical CT. Figure 3 shows another patient in whom helical CT identified the HCC nodule not detected by Lipiodol CT.
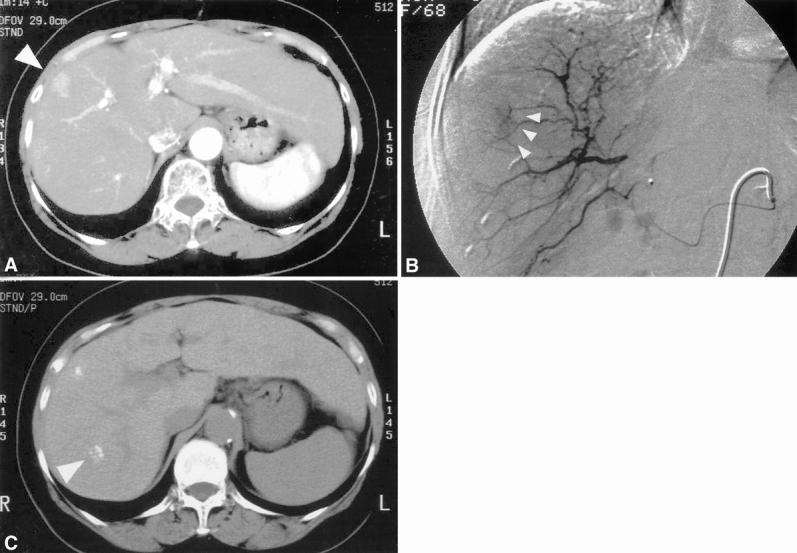
Figure 2. A 68-year-old woman in whom an hepatocellular carcinoma (HCC) nodule was correctly identified by Lipiodol computed tomography (CT) but not by helical CT. (A) Helical CT scan during the arterial phase shows a 1.5-cm hypervascular tumor in S8 (arrowhead). (B) Digital subtraction angiography confirmed the helical CT findings (arrowheads). (C) Lipiodol deposits were identified in S7 (arrowhead) in addition to S8. Both lesions were confirmed to be HCC by intraoperative ultrasound and postoperative histologic evaluation.
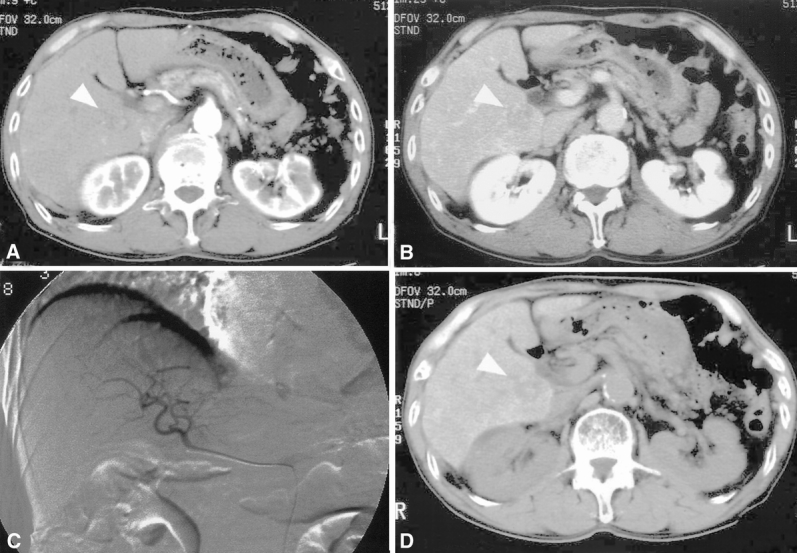
Figure 3. A 78-old-year man in whom helical computed tomography (CT) identified an hepatocellular carcinoma nodule not detected by Lipiodol CT. (A, B) A nodule measuring 2.3 cm in diameter (arrowhead) was depicted in the caudate lobe as a low-density lesion during both the arterial (A) and the portal venous (B) phase by helical CT. (C) This lesion was not detected by digital subtraction angiography. (D) No Lipiodol deposit was seen in the lesion (arrowhead). This lesion was confirmed to be well-differentiated hepatocellular carcinoma by postoperative histologic examination.
DISCUSSION
Several studies have evaluated the accuracy of Lipiodol CT imaging in detecting HCC nodules. 13,15–19,28–33 They used histologic assessment of liver biopsy specimens, 17 liver parenchyma taken by partial liver resection, 13,15,18,19 or explanted liver at the time of liver transplantation as references 28–33 and have reported a wide variety of sensitivities (40% to >90%). 13,15–19,28–33 Still, the question of whether Lipiodol CT provides information beyond that obtained with other noninvasive modalities, as preoperative evaluation in the era of helical biphasic CT, remains to be addressed. Although a few studies have assessed the diagnostic accuracy of helical biphasic CT in comparison with that of Lipiodol CT, they set the findings of Lipiodol CT as the gold standard. 34–36 With these in mind, we reviewed our institutional experiences with DSA, Lipiodol CT, and helical biphasic CT performed before liver resection for HCC. In this study, intraoperative ultrasound findings, followed by histologic confirmation, served as the gold standard. This procedure is thought to be appropriate from the practical viewpoint of preoperative evaluation because it is accepted that liver resection is the mainstay of HCC treatment and that intraoperative ultrasound is the most sensitive imaging technique currently available. 3,15
There are several notable findings in this investigation pertaining to both advantages and disadvantages of Lipiodol CT (see Table 1). First, there is a general trend toward reduced detection ability with both imaging techniques as the tumors become smaller and more differentiated. This observation is in line with that reported by Spreafico et al 31 for Lipiodol CT and by Ohashi et al 37 for dynamic incremental CT. Nonetheless, the sensitivity of helical CT for well-differentiated HCC is superior to that of Lipiodol CT or DSA, especially when tumors are small, whereas Lipiodol CT shows sensitivity superior to that of helical CT in detecting small but moderately to poorly differentiated HCCs. This finding agrees with those of Ohashi et al 37 and Raby et al, 38 who reported that Lipiodol CT could detect HCC as small as 3 mm, provided that the nodule is hypervascular. It is not possible to discriminate completely between daughter (i.e., intrahepatic metastatic) and multifocal nodules based solely on imaging or pathologic findings. However, it is generally accepted that a tumor becomes less differentiated (i.e., well to moderately or poorly) as its size increases 39 (also shown in Fig. 1), whereas daughter nodules are small but their grade of differentiation is moderate to poor. 20,40 In light of these considerations, the present results strongly suggest that helical CT is superior to Lipiodol CT in delineating early HCC nodules, but the latter method is superior for detecting intrahepatic metastatic lesions.
Despite several studies that reported the sensitivity of various imaging modalities including Lipiodol CT, 13,15–19,28–33 few if any have referred to the specificity of detecting lesions with these techniques. 16 The present results indicate the inferior specificity of Lipiodol CT compared with DSA or helical CT, even after what were presumed to be nonspecific Lipiodol deposits had been ruled out. This inferior specificity should be kept in mind when interpreting Lipiodol CT images.
Although the overall sensitivity and specificity of Lipiodol CT were inferior to those of helical biphasic CT in the present study, these modalities have diagnostic values specific to different clinical entities and are thought to be complementary. Detection of intrahepatic metastases has major clinical importance because this has been the factor most consistently reported to be indicative of a poor prognosis after hepatic resection. 4,8,41 Further, Lipiodol CT is always accompanied by angiography, and sensitivity when combined with DSA increases to as much as 82%. In our series of 69 patients who underwent both helical biphasic and Lipiodol CT, the latter identified new HCC nodules that had not been detected by helical biphasic CT in three patients. Further, during the study period, we had five patients who were initially candidates for hepatectomy but were judged to have inoperable disease because of lesions identified solely by Lipiodol CT. These patients underwent transcatheter arterial embolization and were ultimately confirmed by their clinical courses and later imaging studies as being “true positives.”
In conclusion, although the overall ability of helical biphasic CT scan to detect HCC nodules is superior to that of Lipiodol CT, these modalities appear to be complementary. At present, helical biphasic CT does not obviate the need for DSA and subsequent Lipiodol CT as a preoperative examination for HCC.
Footnotes
Correspondence: Hiroshi Imamura, MD, Division of Hepato-Biliary-Pancreatic Surgery, Department of Surgery, Graduate School of Medicine, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
E-mail: himamura-tky@umin.ac.jp
Dr. Imamura is currently at the Division of Hepato-Biliary-Pancreatic Surgery, Department of Surgery, Graduate School of Medicine, University of Tokyo, Tokyo, Japan.
Dr. Matsuyama is currently at the Department of Biostatics, Kyoto University School of Public Health, Kyoto, Japan.
Accepted for publication November 13, 2000.
References
- 1.Kobayashi K, Sugimoto T, Makino H, et al. Screening methods for early detection of hepatocellular carcinoma. Hepatology 1985; 5: 1100–1105. [DOI] [PubMed] [Google Scholar]
- 2.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985; 56: 918–928. [DOI] [PubMed] [Google Scholar]
- 3.Makuuchi M, Hasegawa H, Yamazaki S, et al. The use of operative ultrasound as an aid to liver resection in patients with hepatocellular carcinoma. World J Surg 1987; 11: 615–621. [DOI] [PubMed] [Google Scholar]
- 4.Arii S, Okamoto E, Imamura M. Registries in Japan: current status of hepatocellular carcinoma in Japan. Liver Cancer Study Group of Japan. Semin Surg Oncol 1996; 12: 204–211. [DOI] [PubMed] [Google Scholar]
- 5.Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 1985; 161: 346–350. [PubMed] [Google Scholar]
- 6.Lin TY, Lee CS, Chen KM, Chen CC. Role of surgery in the treatment of primary carcinoma of the liver: a 31-year experience. Br J Surg 1987; 74: 839–842. [DOI] [PubMed] [Google Scholar]
- 7.Kanematsu T, Matsumata T, Takenaka K, et al. Clinical management of recurrent hepatocellular carcinoma after primary resection. Br J Surg 1988; 75: 203–206. [DOI] [PubMed] [Google Scholar]
- 8.Imamura H, Matsuyama Y, Miyagawa Y, et al. Prognostic significance of anatomical resection and des-γ-carboxy prothrombin in patients with hepatocellular carcinoma. Br J Surg 1999; 86: 1032–1038 [DOI] [PubMed] [Google Scholar]
- 9.Matsui O, Kadoya M, Kameyama T, et al. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology 1991; 178: 493–497. [DOI] [PubMed] [Google Scholar]
- 10.Sumida M, Ohto M, Ebara M, et al. Accuracy of angiography in the diagnosis of small hepatocellular carcinoma. AJR Am J Roentgenol 1986; 147: 531–536. [DOI] [PubMed] [Google Scholar]
- 11.Konno T, Maeda H, Iwai K, et al. Effect of arterial administration of high-molecular weight anticancer agent SMANCS with lipid limphographic agent on hepatoma: a preliminary report. Eur J Cancer Clin Oncol 1983; 19: 1053–1065. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Tashiro S, Hiraoka T, et al. Hepatocellular carcinoma and metastatic cancer detected by Iodized oil. Radiology 1985; 154: 15–17. [DOI] [PubMed] [Google Scholar]
- 13.Yumoto Y, Jinno K, Tokuyama K, et al. Hepatocellular carcinoma detected by iodized oil. Radiology 1985; 154: 19–24. [DOI] [PubMed] [Google Scholar]
- 14.Ohishi H, Uchida H, Yoshimura H, et al. Hepatocellular carcinoma detected by iodized oil. Use of anticancer agents. Radiology 1985; 154: 25–29. [DOI] [PubMed] [Google Scholar]
- 15.Takayasu K, Moriyama N, Muramatsu Y, et al. The diagnosis of small hepatocellular carcinomas: efficacy of various imaging procedures in 100 patients. AJR Am J Roentgenol 1990; 155: 49–54. [DOI] [PubMed] [Google Scholar]
- 16.Ngan H. Lipiodol computerized tomography: how sensitive and specific is the techmique in the diagnosis of hepatocellular carcinoma? Br J Radiol 1990; 63: 771–775. [DOI] [PubMed] [Google Scholar]
- 17.De Santis M, Romangnoli R, Cristani A, et al. MRI of small hepatocellular carcinoma: comparison with US, CT, DSA, and Lipiodol-CT. J Comput Assist Tomogr 1992; 16: 189–197. [DOI] [PubMed] [Google Scholar]
- 18.Bartolozzi C, Lencioni R, Caramera D, et al. Small hepatocellular carcinoma detection with US, CT, MR imaging, DSA, and Lipiodol-CT. Acta Radiol 1996; 37: 69–74. [DOI] [PubMed] [Google Scholar]
- 19.Lencioni R, Pinto F, Armillotta N, et al. Intrahepatic metastatic nodules of hepatocellular carcinoma detected at Lipiodol-CT: imaging-pathologic correlation. Abdom Imaging 1997; 22: 253–258. [DOI] [PubMed] [Google Scholar]
- 20.Itai Y. Lipiodol-CT for hepatocellular carcinoma. Abdom Imaging 1997; 22: 259–260. [DOI] [PubMed] [Google Scholar]
- 21.Baron RL. Understanding and optimizing use of contrast material for CT of the liver. AJR Am J Roentgenol 1994; 163: 323–331. [DOI] [PubMed] [Google Scholar]
- 22.Hollett MD, Jeffrey RB Jr, Nino-Murcia M, et al. Dual-phase helical CT of the liver: value of arterial phase scans in the detection of small (≤1.5cm) malignant hepatic neoplasmas. AJR Am J Roentgenol 1995; 164: 879–884. [DOI] [PubMed] [Google Scholar]
- 23.Baron RL, Oliver JH III, Dodd GD III, et al. Hepatocellular carcinoma: evaluation with biphasic, contrast-enhanced, helical CT. Radiology 1996; 199: 505–511. [DOI] [PubMed] [Google Scholar]
- 24.Oliver JH III, Baron RL, Federle MP, Rockette HE Jr. Detecting hepatocellular carcinoma: value of unenhanced or arterial phase CT imaging or both used in conjunction with conventional portal venous phase contrast-enhanced CT imaging. AJR Am J Roentgenol 1996; 167: 71–77. [DOI] [PubMed] [Google Scholar]
- 25.Takayasu K, Furukawa H, Wakao F, et al. CT diagnosis of early hepatocellular carcinoma: sensitivity, findings and CT-pathologic correlation. AJR Am J Roentgenol 1995; 164: 885–890. [DOI] [PubMed] [Google Scholar]
- 26.Takayama T, Makuuchi M, Hirohashi S, et al. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology 1998; 28: 1241–1246. [DOI] [PubMed] [Google Scholar]
- 27.Kanazawa S, Niiya H, Mitogawa Y, et al. Cavernous hemangioma with arterioportal and arteriohapatic vein shunts coexisting with hepatocellular carcinoma. Radiat Med 1994; 12: 83–85. [PubMed] [Google Scholar]
- 28.Taourel PG, Pageaux GP, Coste V, et al. Small hepatocellular carcinoma in patients undergoing liver transplantation: detection with CT after injection of iodized oil. Radiology 1995; 197: 377–380. [DOI] [PubMed] [Google Scholar]
- 29.Valls C, Figueras J, Jaurrieta E, et al. Hepatocellular carcinoma: iodized oil CT TNM classification. AJR Am J Roentgenol 1996; 167: 477–481. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharya S, Dhillon AP, Rees J, et al. Small hepatocellular carcinomas in cirrhotic explant livers: identification by macroscopic examination and lipiodol localization. Hepatology 1997; 25: 613–618. [DOI] [PubMed] [Google Scholar]
- 31.Spreafico C, Marciano A, Mazzaferro V, et al. Hepatocellular carcinoma in patients who undergo liver transplantation: sensitivity of CT with iodized oil. Radiology 1997; 203: 457–460. [DOI] [PubMed] [Google Scholar]
- 32.Saada J, Bhattacharya S, Dhillon AP, et al. Detection of small hepatocellular carcinomas in cirrhotic livers using iodized oil computed tomography. Gut 1997; 41: 404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bizollon T, Rode A, Bancel B, et al. Diagnostic value and tolerance of lipiodol-computed tomography for the detection of small hepatocellular carcinoma: correlation with pathologic examination of explanted livers. J Hepatol 1998; 28: 491–496. [DOI] [PubMed] [Google Scholar]
- 34.Oi H, Murakami T, Kim T, et al. Dynamic MR imaging and early-phase helical CT for detecting small intrahepatic metastases of hepatocellular carcinoma. AJR Am J Roentgenol 1996; 166: 369–374. [DOI] [PubMed] [Google Scholar]
- 35.Choi BI, Lee HJ, Han JK, et al. Detection of hypervascular nodular hepatocellular carcinomas: value of triphasic helical CT compared with iodized-oil CT. AJR Am J Roentgenol 1997; 166: 219–224. [DOI] [PubMed] [Google Scholar]
- 36.Hori M, Murakami T, Oi H, et al. Sensitivity in detection of hypervascular hepatocellular carcinoma by helical CT with intra-arterial injection of contrast medium, and by helical CT and MR imaging with intravenous injection of contrast medium. Acta Radiol 1998; 39: 144–151. [DOI] [PubMed] [Google Scholar]
- 37.Ohashi I, Hanafusa K, Yoshida T. Small hepatocellular carcinomas: two-phase dynamic incremental CT in detection and evaluation. Radiology 1993; 189: 851–855. [DOI] [PubMed] [Google Scholar]
- 38.Raby N, Karani J, Michell M, et al. Lipiodol-enhanced CT scanning in assessment of hepatocellular carcinoma. Clin Radiol 1989; 40: 480–485. [DOI] [PubMed] [Google Scholar]
- 39.Kenmochi K, Sugihara S, Kojiro M. Relationship of histologic grade of hepatocellular carcinoma (HCC) to tumor size, and demonstration of tumor cells of multiple different grades in single small HCC. Liver 1987; 7: 18–26. [DOI] [PubMed] [Google Scholar]
- 40.Liver Cancer Study Group of Japan. Intrahepatic metastasis. In: The general rules for the clinical and pathological study of primary liver cancer, 3rd ed. Tokyo: Kanehara; 1992:26.
- 41.Okada S, Shimada K, Yamamoto J, et al. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology 1994; 106: 1618–1624. [DOI] [PubMed] [Google Scholar]