Abstract
Objective
To investigate whether the survival results after resection of hepatocellular carcinoma (HCC) have improved within the past decade by an analysis of a prospective cohort of patients over a 10-year period.
Summary Background Data
The surgical death rate after resection of HCC has greatly improved in recent years, but the long-term prognosis remains unsatisfactory. It remains unknown whether the survival results after resection of HCC have improved within the past decade.
Methods
The clinicopathologic and follow-up data of 377 patients who underwent curative resection of HCC between January 1989 and January 1999 were prospectively collected. These patients were categorized according to two time periods: before 1994 (group 1, n = 136) and after 1994 (group 2, n = 241). The two groups were compared for clinicopathologic data and survival results. The prognostic factors for disease-free survival were further analyzed to identify the factors that might have led to improved survival outcomes.
Results
The overall and disease-free survival results were significantly better in group 2 compared with group 1. Patients in group 2 had significantly higher proportions of subclinical presentation, small tumors, and tumors of early pTNM stage. There were also significantly lower frequencies of histologic margin involvement, less intraoperative blood loss, and a lower transfusion rate in group 2. By multivariate analysis, early pTNM stage, subclinical HCC, and no perioperative transfusion were independent favorable prognostic factors for disease-free survival.
Conclusions
Significant improvement of overall and disease-free survival results after resection of HCC has been achieved within the past decade as a result of advances in the diagnosis and surgical management of HCC. Earlier diagnosis of HCC by better imaging modalities, increased detection of subclinical HCC by screening of high-risk patients, and a reduced perioperative transfusion rate were identified as the major contributory factors for the improved outcomes.
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, and it is notorious for poor prognosis because of its invasiveness and frequent association with cirrhosis. Although liver transplantation has been established as an alternative curative treatment for HCCs of less than 5 cm, 1 its application is limited by the lack of donors, not only in Asia but also in Western countries. 2,3 Hepatic resection remains the treatment of choice for HCC. The role of surgical resection is becoming more important as advances in diagnostic imaging and wider use of screening programs have allowed early detection of small resectable HCCs. 4,5
During the past decade, hepatic surgeons have focused much effort on improving the surgical techniques and perioperative management for resection of HCC, resulting in greatly improved perioperative outcomes. By the end of the 1990s, studies from our institution and others have demonstrated that a zero hospital or surgical death rate could be achieved in large series of patients. 6,7 However, less progress has been made in the long-term prognosis after resection of HCC, which remains unsatisfactory. In particular, disease-free survival has been poor because of a high incidence of recurrence. A 5-year cumulative recurrence rate of 80% to 100% has been reported in recent series. 8–11 Several reports in the 1990s from Eastern and Western centers have documented a 5-year overall survival rate of 26% to 44% after resection of HCC, 3,12–19 which was apparently better than the 20% to 38% reported in the 1980s. 20–22 However, few studies have investigated whether there was significant improvement in the survival results in individual centers. 15,16 Whether the prognosis after hepatectomy for HCC has improved within the past decade remains to be clarified.
With the goal of continuously improving the outcome after hepatic resection of HCC, we have been prospectively collecting the survival data of all patients after resection of HCC in our institution since 1989. This study evaluated whether there was significant improvement in the survival results after resection of HCC by an analysis of a prospective cohort of 377 patients over a 10-year period.
METHODS
Between January 1989 and January 1999, 399 patients underwent hepatectomy for HCC in the Department of Surgery at Queen Mary Hospital, Hong Kong, China. Twenty-two patients who had gross residual tumors after resection were excluded from this analysis. The other 377 patients who had resection with a curative intent, defined as macroscopically complete removal of the tumors, were the subjects of this study. They were categorized into two 5-year periods: before January 1994 (group 1, n = 136) and after January 1994 (group 2, n = 241).
Preoperative Diagnosis and Assessment
The preoperative diagnosis of HCC in our patients was based mainly on typical imaging findings on computed tomography (CT) and hepatic arteriography, together with a serum alpha-fetoprotein (AFP) level of more than 400 ng/mL in some patients. Dual-phase helical CT was available only after 1994, and there has been increasing use of magnetic resonance imaging in recent years as well. Needle biopsy was not performed in patients with resectable disease to avoid needle tract seeding of tumor cells. All HCCs were subsequently confirmed by histologic examination after resection.
The criteria for resectability were the same over the 10-year period: absence of distant metastasis, anatomically resectable disease as evaluated by imaging studies, absence of main portal vein or inferior vena cava tumor thrombus, and adequate liver function reserve. During the period 1989 to 1993, hepatic function assessment was based mainly on the Child classification. After 1994, hepatic function assessment was based largely on the indocyanine green clearance test. 23
Surgical Techniques
The surgical techniques have been described in a previous report. 6 Briefly, after initial assessment of resectability, intraoperative ultrasound was routinely performed to detect any major vascular invasion or lesions in the contralateral lobe. After division of the inflow blood supply to the lobe or segment to be resected, parenchymal transection was performed using the finger-fracture technique between 1989 and 1992, and thereafter using an ultrasonic dissector. Intermittent Pringle maneuver was used to reduce bleeding if necessary. Hemostasis during parenchymal transection was achieved by diathermy coagulation, argon beam coagulation, and fine suturing. Blood transfusion was initiated when the hemoglobin level decreased to less than 8 g/dL. Meticulous attention was also paid to the preservation of function in the remnant liver by avoiding prolonged rotation, hypoxic injury, or venous congestion resulting from overloading of circulation. Hepatic resection was defined as major if three or more segments by the Couinaud classification were resected, 24 and minor if fewer than three segments were resected.
Adjuvant Therapy
Preoperative transarterial chemoembolization (TACE) was not used except in 18 patients who received TACE before referral to our center for hepatectomy. Thirty patients were recruited in a randomized trial of postoperative adjuvant chemotherapy from January 1991 to June 1995 and received a combination of intravenous epirubicin and transarterial lipiodolized cisplatin. 25 Another 14 patients received postoperative transarterial chemotherapy for histologic margin involvement. Otherwise, no postoperative adjuvant chemotherapy was given.
Follow-Up and Management of Recurrence
All patients were followed up monthly in the first year and thereafter quarterly, with regular monitoring of recurrence by serum AFP level and ultrasonography or CT of the liver. The diagnosis of recurrence was based on typical imaging findings on CT or arteriography, and if necessary percutaneous fine-needle aspiration cytology. Postoperative recurrence was managed aggressively using a multimodality approach detailed in a previous report. 26 Patients with anatomically resectable intrahepatic recurrence and adequate liver function reserve were managed by reresection, and those with unresectable recurrence were managed by either TACE or percutaneous ethanol injection therapy. Patients with isolated extrahepatic recurrence were also considered for surgical excision. 27 Systemic chemotherapy using intravenous epirubicin was offered to selected patients with widespread extrahepatic recurrence but good general condition. This policy of management for recurrence was consistently used over the 10-year study period.
Statistical Analysis
Preoperative clinical parameters, surgical data, postoperative course, pathologic data of resected specimens, and follow-up results have been prospectively collected in a computerized database since 1989. Hospital death was defined as death during the same hospital admission after surgery. Survival analysis was performed from the time of hepatic resection to the date of death or time of analysis (September 2000). All patients have been followed up for at least 20 months after surgery. Group 1 had been followed for a median of 98 months and group 2 for a median of 44 months by the time of analysis.
Continuous variables were expressed as mean ± standard deviation and compared between groups by the unpaired t test. Categorical variables were compared by the chi-square test. Survival curves were computed using the Kaplan-Meier method and compared between groups using the log-rank test. Hospital death was included in the analysis of overall survival results but was excluded from the analysis of disease-free survival results. To elucidate factors that could have contributed to improved survival results, a further analysis was carried out to identify the prognostic factors for disease-free survival. Univariate and then multivariate analyses by the Cox proportional hazards model were performed on 19 factors of potential prognostic significance, which were all categorized as binary variables. These included nine clinical factors (age younger than or older than 60 years, sex, hepatitis B surface antigen status, subclinical or symptomatic presentation, serum AFP level ≤ or >500 ng/mL, indocyanine green retention at 15 minutes ≤ or >14%, Child grade A or B, any preoperative TACE, any postoperative chemotherapy), four surgical factors (major or minor resection, resection margin ≤ or >1 cm, blood loss ≤ or >2 L, any perioperative blood transfusion), and six pathologic factors (cirrhotic or noncirrhotic liver, tumor size ≤ or >5 cm, histologic margin involvement, tumor encapsulation, venous invasion, pTNM stage I/II or IIIA/IVA 28). All statistical analyses were made using statistical software (SPSS, Inc., Chicago, IL). Statistical significance was defined as P < .05.
RESULTS
Clinicopathologic and Surgical Data
Table 1 shows the comparison of the clinical, surgical, and pathologic data between the two groups of patients. Patients in group 2 had a significantly higher proportion of subclinical HCC diagnosed by regular screening using ultrasound and AFP measurement in hepatitis B surface antigen carriers and patients with chronic hepatitis or cirrhosis when compared with group 1 (37% vs. 19%, P < .001). There were significantly higher proportions of small or early pTNM stage tumors in group 2. A further analysis revealed that subclinical HCCs had a significantly smaller average tumor size (4.6 ± 2.8 vs. 9.0 ± 4.5 cm, P < .001) and a higher frequency of early pTNM stage I or II tumors (68% vs. 39%, P < .001) compared with symptomatic HCCs.
Table 1. CLINICAL, SURGICAL, AND PATHOLOGIC DATA
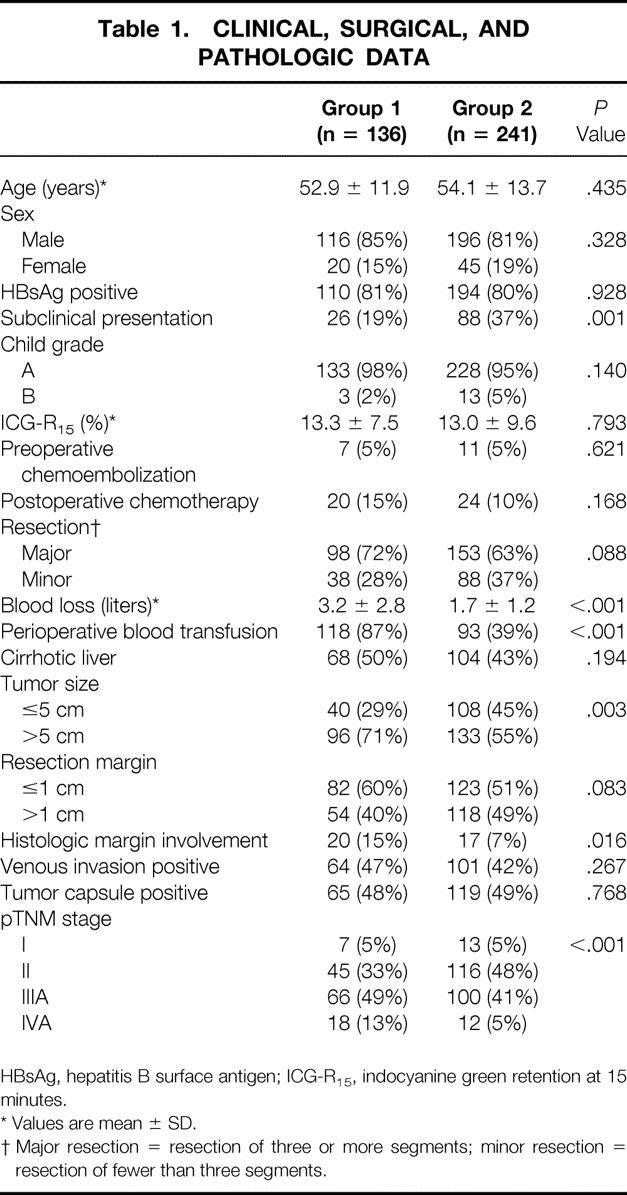
HBsAg, hepatitis B surface antigen; ICG-R15, indocyanine green retention at 15 minutes.
* Values are mean ± SD.
† Major resection = resection of three or more segments; minor resection = resection of fewer than three segments.
Patients in group 2 also had a significantly lower frequency of histologic margin involvement (7% vs. 15%, P = .016). There was a higher proportion of wide resection margin (>1 cm) in group 2, but the difference between the two groups was not statistically significant (49% vs. 40%, P = .083). Another remarkable difference was the significantly reduced blood loss during surgery and hence blood transfusion rate (39 vs. 87%, P < .001) in the second half of the study. There were no significant differences in other clinicopathologic characteristics between the two groups.
Survival Results
The hospital death rate was reduced from 13.2% (18/136) in group 1 to 2.5% (6/241) in group 2 (P < .001). The overall survival results were significantly better in group 2 than group 1 (Fig. 1). The median overall survival was 59.4 months (95% confidence interval [CI] 45.2–74.5) in group 2 and 31.2 months (20.9–43.7) in group 1. The cumulative 1-, 3-, and 5-year overall survival rates were 82%, 62%, and 49%, respectively, for group 2 and 68%, 47%, and 36% for group 1.
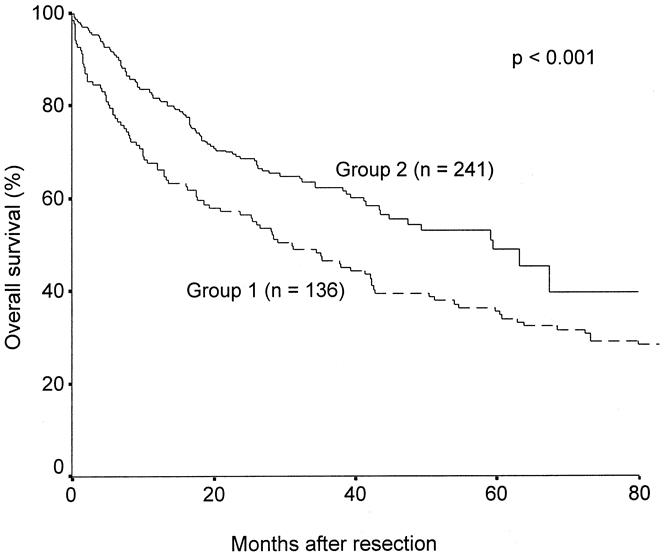
Figure 1. Cumulative overall survival curves of patients who underwent surgery before 1994 (group 1) and after 1994 (group 2).
The disease-free survival results after excluding hospital deaths were also significantly improved in group 2 compared with group 1 (Fig. 2). The median disease-free survival was 18.3 months (95% CI 11.7–24.8) in group 2 and 6.9 months (4.2–9.7) in group 1. The cumulative 1-, 3-, and 5-year disease-free survival rates were 60%, 38%, and 25%, respectively, for group 2 and 42%, 23%, and 16% for group 1. By the time of analysis, recurrence had developed in 100 patients in group 1 and 148 patients in group 2. There was no significant difference in the survival after recurrence between groups 1 and 2 (median 16.8 vs. 15.0 months, P = .168).
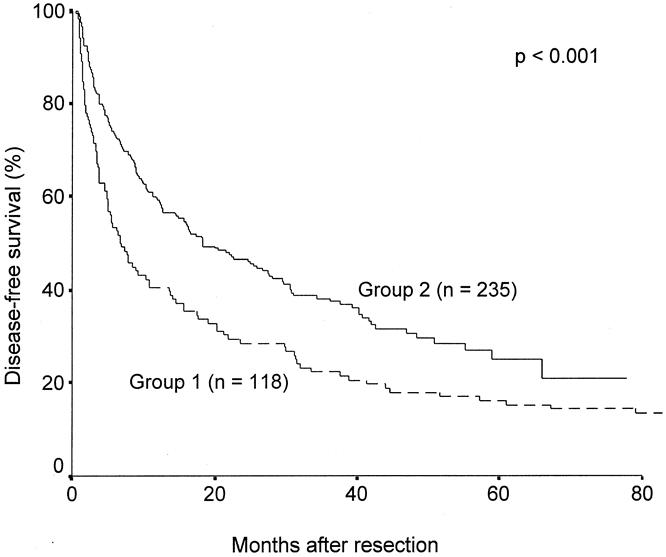
Figure 2. Cumulative disease-free survival curves of patients who underwent surgery before 1994 (group 1) and after 1994 (group 2).
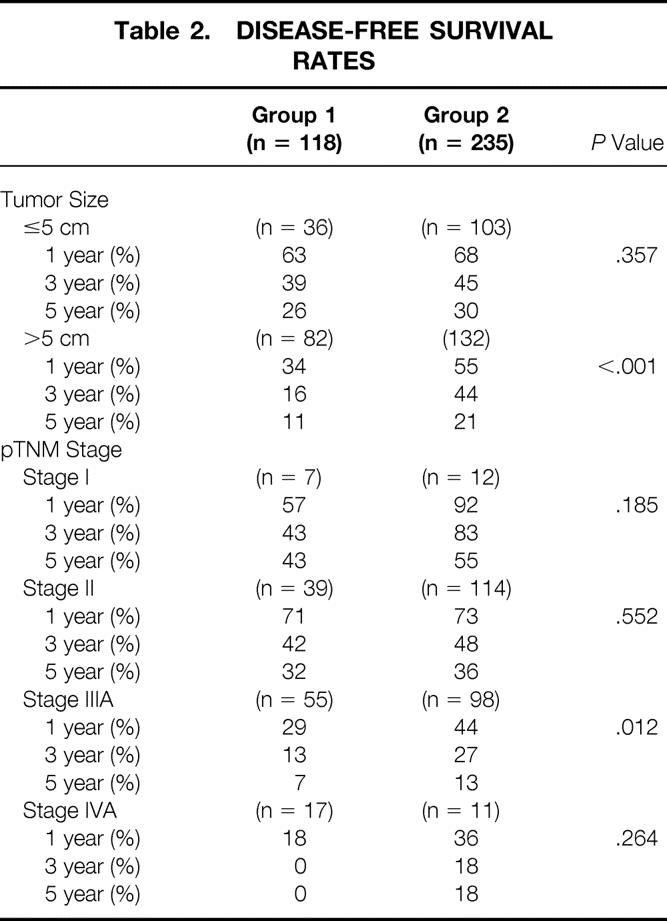
Because of the significant differences in the tumor size and pTNM stages between the two groups, a further comparison was made after stratifying patients in the two groups according to tumor size and pTNM stage to clarify whether the disease-free survival had improved for a specific tumor size or stage (Table 2). The average tumor size in the subcategory of small tumors (≤5 cm) was comparable between groups 1 and 2 (3.2 ± 1.2 vs. 3.3 ± 1.1 cm, P = .761), as was the average tumor size in the subcategory of large tumors (10.1 ± 3.7 vs. 9.9 ± 3.3 cm, P = .497). Significant improvement in the disease-free survival rates was observed in patients with large tumors, but not in those with small ones. The disease-free survival results appeared to have improved in all pTNM stages, but the difference was significant only for stage IIIA disease.
Table 2. DISEASE-FREE SURVIVAL RATES
Prognostic Factors for Disease-Free Survival
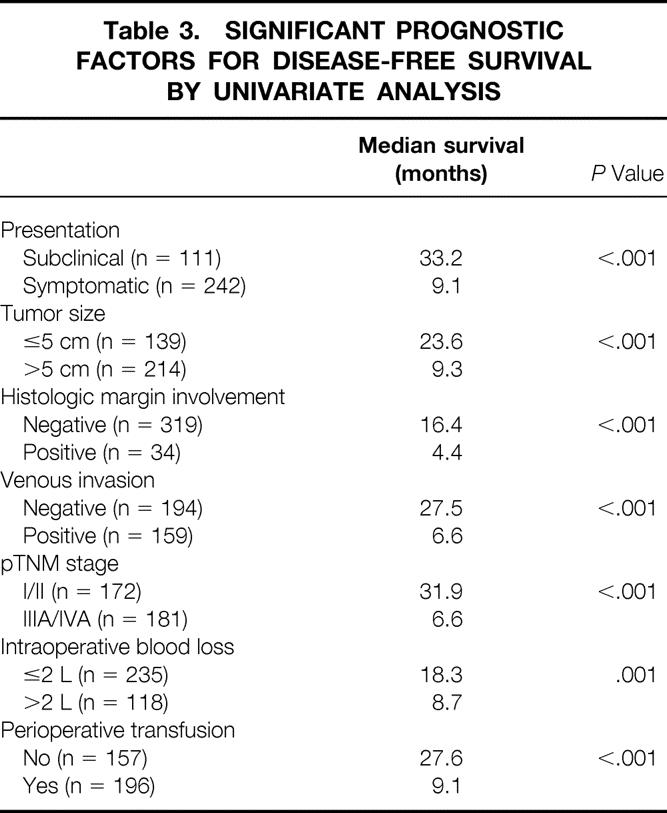
Univariate analysis of prognostic factors for disease-free survival for the whole group of patients over the 10-year period revealed that 7 of the 19 evaluated factors had a significant prognostic influence (Table 3). After multivariate analysis, only pTNM stage (IIIA/IVA vs. I/II, risk ratio [RR] 1.962, 95% CI 1.615–2.474, P < .001), mode of presentation (symptomatic vs. subclinical, RR 1.460, 95% CI 1.102–2.014, P = .012), and perioperative blood transfusion (transfused vs. not transfused, RR 1.326, 95% CI 1.054–1.618, P = .028) were the significant prognostic factors for disease-free survival.
Table 3. SIGNIFICANT PROGNOSTIC FACTORS FOR DISEASE-FREE SURVIVAL BY UNIVARIATE ANALYSIS
DISCUSSION
Hepatic resection for HCC is now a safe procedure with a low surgical death rate as a result of technologic advances during the past decade. Improving the survival results has become the next important target in the surgical management of HCC. A previous study from our institution found no improvement in disease-free survival comparing the periods before and after 1987, but the overall survival has been significantly prolonged in the late 1980s and early 1990s as a result of better management of patients with recurrent disease. 16 The current study shows for the first time that not only have the overall survival rates improved, but there was also improving disease-free survival in the past decade.
By Kaplan-Meier analysis, both the overall and disease-free survival curves of group 2 were significantly better than group 1, and the median overall and disease-free survival times in group 2 were significantly improved. The cumulative overall and disease-free survival rates also appeared to have improved substantially. However, the comparison of the long-term survival rates estimated by Kaplan-Meier analysis between the two groups must be interpreted with caution because the follow-up period for group 2 was shorter than for group 1. Whereas all patients in group 1 have either died or been followed up for more than 5 years, only a small proportion of the patients in group 2 had been followed up for 5 years or more by the time of analysis. With such a limitation, there is the potential for error in the comparison of 5-year survival rates.
The better overall survival outcomes in group 2 were due to both decreased early in-hospital deaths and increased long-term survival, particularly disease-free survival. With a policy of aggressive management of recurrent tumors in the recent 10 years, survival after recurrence has been substantially prolonged compared with a median survival of 3.5 months after recurrence in the 1980s, 16 but there was no significant difference in the survival after recurrence between the two groups in this study. Further improvement in this aspect requires more effective treatment for recurrent disease. An area to be studied for better management of intrahepatic recurrence is the combined use of percutaneous ethanol injection and TACE, which seems to produce better survival results than either treatment alone. 29 The improved survival observed during the 10-year period in this study was mainly due to increased disease-free survival.
The improved disease-free survival results in group 2 were, to a large extent, attributable to the higher frequency of early-stage tumors in this group. The pTNM stage of the tumor, which incorporates pathologic parameters such as tumor size and venous invasion, 28 was found to be the most significant prognostic factor for disease-free survival. Other authors have shown that the pTNM stage was an accurate determinant of long-term overall or disease-free survival after resection of HCC. 12,14,30 The availability of better imaging modalities in recent years has certainly led to an earlier diagnosis of HCC. Both helical CT and magnetic resonance imaging are useful in detecting small HCCs, particularly in a background of cirrhosis, because they can differentiate small HCCs from regenerative or dysplastic nodules. 31,32
In group 2, there was a higher proportion of subclinical HCCs as a result of more widespread application of screening for hepatitis B carriers in our community, and thus increased referral of such patients to our center in recent years. Subclinical HCCs had a smaller size and earlier pTNM stage compared with symptomatic HCCs. However, subclinical presentation was found to be an independent favorable prognostic factor in addition to pTNM stage, which is surprising. The prognostic influence of subclinical presentation on the survival results after resection of HCC was seldom investigated in previous studies. Although the explanation is unknown, our finding might suggest that early tumors detected by regular screening in high-risk patients probably have a less malignant biologic behavior compared with tumors of similar size or pTNM stage that are detected after symptomatic presentation. Studies on the natural history of HCC have shown that it often has a long subclinical incubation period, during which it may grow slowly as a solitary mass before it turns into a more aggressive form with exponential growth. 33–35 A study has shown that the growth rate measured by tumor volume doubling time is a determining factor for survival after resection of small HCCs (<5 cm). 36 The growth rate of the tumor is a biologic factor that is not encompassed in the pTNM stage, and the slower growth rate of subclinical HCC may account for its independent prognostic influence. The efficacy of screening for subclinical HCC has been controversial. Some studies showed that regular screening of high-risk patients by serum AFP level and ultrasonography resulted in the detection of early HCC associated with better prognosis, 4,5,36,37 whereas others failed to show an improved survival outcome with screening. 38,39 This may be related to different patient populations and different regimens of screening in various studies. Our study clearly showed improved survival outcomes in patients with subclinical HCCs compared with those with symptomatic HCCs. Screening for subclinical HCCs in chronic hepatitis B carriers appears to be an effective strategy to improve the prognosis of HCC, although its cost-effectiveness requires further evaluation.
Early diagnosis of HCC was a major but not the only factor that has accounted for the improved survival results in the latter half of the study period, because analysis after stratifying patients according to tumor size or pTNM stage also revealed improved disease-free survival rates. Significant improvement was observed in patients with large tumors or stage IIIA disease. The results in Table 2 suggest that group 2 had some early survival advantage in terms of 1- and 3-year disease-free survival rates, whereas the differences in the 5-year rates were less remarkable. Reduced perioperative transfusion, which was an independent prognostic factor for disease-free survival, appeared to be a factor that might have contributed to the improved early disease-free survival results. Other authors have shown a deleterious effect of blood transfusion on disease-free survival, presumably as a result of suppression of the host immune mechanism that results in enhanced early postoperative recurrence from intrahepatic or extrahepatic metastasis. 40,41 The effect of perioperative transfusion is likely to be more prominent after resection of large or advanced-stage tumors, which are more prone to early recurrence from intrahepatic or extrahepatic spread. 42 The markedly reduced intraoperative blood loss and transfusion rate in the latter half of the study period was the result of both technologic advances and the increased experience of the surgical team. Use of the ultrasonic dissector has been shown to reduce blood loss compared with the finger-fracture technique in our experience. 43 Further improvement in the disease-free survival may be expected with the continuous evolution of surgical techniques toward “bloodless” hepatectomy for HCC, with the ultimate target of a zero transfusion rate.
Another factor that could affect the disease-free survival after resection of HCC is the surgical margin. In group 2, there was a significantly lower frequency of histologic margin involvement, which was an adverse prognostic factor for disease-free survival by univariate analysis. Histologic involvement of margin is more likely when the resection margin is narrow. 44 Resection of HCC in group 2 was associated with a higher proportion of resection margins of more than 1 cm, although the difference compared with group 1 was not significant. The change of transection technique from finger-fracture to ultrasonic dissector enables a more precise transection plane and hence a wider resection margin. 43 However, it is unlikely that surgical margin has made a major contribution to the improved survival outcomes because the number of patients with histologic margin involvement in each group was small, and histologic margin involvement did not have an independent prognostic influence in multivariate analysis. Other studies also found that the resection margin was not an independent prognostic factor for resection of HCC. 14,45,46 A previous study from our institution has shown that a resection margin of 1 cm could not ensure disease clearance, because microsatellite or venous permeation is frequently present beyond 1 cm of the tumor. 47
Although multivariate analysis has helped to identify the clinicopathologic factors that might have contributed to the improved survival results, the exact significance of each of the three prognostic factors remains uncertain. The true significance of each factor is difficult to assess when interrelated factors are entered into the analysis, and one must bear in mind the limitation of this methodology when interpreting the results. To prove the relevance of the factors that were found to have significant influence on the disease-free survival, each factor must be further evaluated in a prospective manner. It is also difficult to assess how much of the improved survival results can be ascribed to improved surgical techniques. Although our analysis suggested that reduced blood transfusion as a result of improved surgical techniques was a contributory factor for the improved outcomes, much of the improvement in survival results was due to the better prognosis of earlier disease detected by improved diagnostic imaging studies and screening for HCC in the latter 5-year period.
Adjuvant therapy to reduce recurrence is also a potentially useful approach in prolonging the disease-free survival after resection of HCC, but it had little impact on the survival results in this study. Neither preoperative chemoembolization nor postoperative transarterial chemotherapy has been found to be of prognostic significance, although the number of patients who received these treatments was small. The efficacy of adjuvant therapy remains uncertain despite substantial research efforts in the past decade. A few retrospective studies have found that preoperative chemoembolization or postoperative transarterial chemotherapy might improve long-term results after resection of HCC. 48–50 However, a limited number of prospective randomized trials, including one from our institution, have failed to show a survival benefit with either approach. 25,51–54 Before the efficacy of these adjuvant therapies is fully established, they cannot be recommended for routine use.
In conclusion, this study has shown a significant improvement in both overall and disease-free survival results in the past decade as a result of advances in the diagnosis and surgical management of HCC. Earlier diagnosis of HCC by better imaging modalities, increased detection of subclinical HCCs by screening programs in high-risk patients, and a reduced perioperative transfusion rate as a result of improved surgical techniques were considered to be the main factors contributing to the improved survival outcomes. Continuous advances along the direction of these strategies may enhance the prognosis. Further improvement in the long-term survival after resection of HCC also depends on research efforts to develop an effective adjuvant therapy in the coming decade.
Footnotes
Supported by the Croucher Foundation and Development Fund for Area of Excellence, The University of Hong Kong.
Correspondence: Sheung-Tat Fan, MS, MD, FRCS, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, 102 Pokfulam Rd., Hong Kong. E-mail: hrmsfst@hkucc.hku.hk
Accepted for publication December 27, 2000.
References
- 1.Mor E, Kaspa RT, Sheiner P, Schwartz M. Treatment of hepatocellular carcinoma associated with cirrhosis in the era of liver transplantation. Ann Intern Med 1998; 129: 643–653. [DOI] [PubMed] [Google Scholar]
- 2.Ota K, Teraoka S, Kawai T. Donor difficulties in Japan and Asian countries. Transplant Proc 1995; 27: 83–86. [PubMed] [Google Scholar]
- 3.Mazziotti A, Grazi GL, Cavallari A. Surgical treatment of hepatocellular carcinoma on cirrhosis: a Western experience. Hepato-Gastroenterology 1998; 45 (suppl 3): 1281–1287. [PubMed] [Google Scholar]
- 4.Zoli M, Magalotti D, Bianchi G, et al. Efficacy of a surveillance program for early detection of hepatocellular carcinoma. Cancer 1996; 78: 977–985. [DOI] [PubMed] [Google Scholar]
- 5.Mima S, Sekiya C, Kanagawa H, et al. Mass screening for hepatocellular carcinoma: experience in Hokkaido, Japan. J Gastroenterol Hepatol 1994; 9: 361–365. [DOI] [PubMed] [Google Scholar]
- 6.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg 1999; 229: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torzilli G, Makuuchi M, Inoue K, et al. No-mortality liver resection for hepatocellular carcinoma in cirrhotic and noncirrhotic patients: is there a way? A prospective analysis of our approach. Arch Surg 1999; 134: 984–992. [DOI] [PubMed] [Google Scholar]
- 8.Belghiti J, Panis Y, Farges O, et al. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg 1991; 214: 114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugioka A, Tsuzuki T, Kanai T. Postresection prognosis of patients with hepatocellular carcinoma. Surgery 1993; 113: 612–618. [PubMed] [Google Scholar]
- 10.Chen MF, Hwang TL, Jeng LB, et al. Postoperative recurrence of hepatocellular carcinoma. Two hundred five consecutive patients who underwent hepatic resection in 15 years. Arch Surg 1994; 129: 738–742. [DOI] [PubMed] [Google Scholar]
- 11.Ballsells J, Charco R, Lazaro JL, et al. Resection of hepatocellular carcinoma in patients with cirrhosis. Br J Surg 1996; 83: 758–761. [DOI] [PubMed] [Google Scholar]
- 12.Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg 1991; 15: 270–285. [DOI] [PubMed] [Google Scholar]
- 13.Nagasue N, Kohno H, Chang YC, et al. Liver resection for hepatocellular carcinoma. Results of 229 consecutive patients during 11 years. Ann Surg 1993; 217: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosuge T, Makuuchi M, Takayama T, et al. Long-term results after resection of hepatocellular carcinoma: experience of 480 cases. Hepato-Gastroenterology 1993; 40: 328–332. [PubMed] [Google Scholar]
- 15.Segawa T, Tsuchiya R, Furui J, et al. Operative results in 143 patients with hepatocellular carcinoma. World J Surg 1993; 17: 663–668. [DOI] [PubMed] [Google Scholar]
- 16.Lai ECS, Fan ST, Lo CM, et al. Hepatic resection for hepatocellular carcinoma. An audit of 343 patients. Ann Surg 1995; 221: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bismuth H, Chiche L, Castaing D. Surgical treatment of hepatocellular carcinomas in noncirrhotic liver: experience with 68 liver resections. World J Surg 1995; 19: 35–41. [DOI] [PubMed] [Google Scholar]
- 18.Vauthey JN, Klimstra D, Franceschi D, et al. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg 1995; 169: 28–35. [DOI] [PubMed] [Google Scholar]
- 19.Lise M, Bacchetti S, Da Pian P, et al. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer 1998; 82: 1028–1036. [DOI] [PubMed] [Google Scholar]
- 20.Lee NW, Wong J, Ong GB. The surgical management of primary carcinoma of the liver. World J Surg 1982; 6: 66–75. [DOI] [PubMed] [Google Scholar]
- 21.Thompson HH, Tompkins RK, Longmire WP Jr. Major hepatic resection. A 25-year experience. Ann Surg 1983; 197: 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwatsuki S, Starzl TE. Personal experience with 411 hepatic resections. Ann Surg 1988; 208: 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau H, Man K, Fan ST, et al. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg 1997; 84: 1255–1259. [PubMed] [Google Scholar]
- 24.Couinaud C, Le Foi. Etudes anatomiques et chirugicales. Paris: Masson Publishers; 1957: 400–409.
- 25.Lai ECS, Lo CM, Fan ST, et al. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg 1998; 133: 183–188. [DOI] [PubMed] [Google Scholar]
- 26.Poon RTP, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 1999; 229: 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo CM, Lai ECS, Fan ST, et al. Resection for extrahepatic recurrence of hepatocellular carcinoma. Br J Surg 1994; 81: 1019–1021. [DOI] [PubMed] [Google Scholar]
- 28.Sobin LH, Whitekind Ch. TNM classification of malignant tumors, 5th ed. New York: John Wiley; 1997.
- 29.Ishii H, Okada S, Sato T, et al. Effect of percutaneous ethanol injection for postoperative recurrence of hepatocellular carcinoma in combination with transcatheter arterial embolization. Hepato-Gastroenterology 1996; 43: 644–650. [PubMed] [Google Scholar]
- 30.Nonami T, Harada A, Kurokawa T, et al. Hepatic resection for hepatocellular carcinoma. Am J Surg 1997; 173: 288–291. [DOI] [PubMed] [Google Scholar]
- 31.Baron RL, Oliver JH 3rd, Dodd GD 3rd, et al. Hepatocellular carcinoma: evaluation with biphasic, contrast-enhanced, helical CT. Radiology 1996; 199: 505–511. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita Y, Mitsuzaki K, Yi T, et al. Small hepatocellular carcinoma in patients with chronic liver damage: prospective comparison of detection with dynamic MR imaging and helical CT of the whole liver. Radiology 1996; 200: 79–84. [DOI] [PubMed] [Google Scholar]
- 33.Sheu JC, Sung JL, Chen DS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology 1985; 89: 259–266. [DOI] [PubMed] [Google Scholar]
- 34.Cottone M, Virdone R, Fusco G, et al. Asymptomatic hepatocellular carcinoma in Child’s A cirrhosis. A comparison of natural history and surgical treatment. Gastroenterology 1989; 96: 1566–1571. [DOI] [PubMed] [Google Scholar]
- 35.Colombo M. The natural history of hepatocellular carcinoma in Western countries. Hepato-Gastroenterology 1998; 45 (suppl 3): 1221–1225. [PubMed] [Google Scholar]
- 36.Okazaki N, Yoshino M, Yoshida T, et al. Evaluation of the prognosis for small hepatocellular carcinoma based on tumor volume doubling time. A preliminary report. Cancer 1989; 63: 2207–2210. [DOI] [PubMed] [Google Scholar]
- 37.Tang ZY, Yu YQ, Zhou XD, et al. Subclinical hepatocellular carcinoma: an analysis of 391 patients. J Surg Oncol 1993; 3 (suppl): 55–58. [DOI] [PubMed] [Google Scholar]
- 38.Colombo M, de Franchis R, Del-Ninno E, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med 1991; 325: 675–680. [DOI] [PubMed] [Google Scholar]
- 39.Izzo F, Cremona F, Ruffolo F, et al. Outcome of 67 patients with hepatocellular cancer detected during screening of 1125 patients with chronic hepatitis. Ann Surg 1998; 227: 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumata T, Ikeda Y, Hayashi H, et al. The association between transfusion and cancer-free survival after curative resection for hepatocellular carcinoma. Cancer 1993; 72: 1866–1871. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto J, Kosuge T, Takayama T, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery 1994; 115: 303–309. [PubMed] [Google Scholar]
- 42.Lauwers GY, Vauthey JN. Pathological aspects of hepatocellular carcinoma: a critical review of prognostic factors. Hepato-Gastroenterology 1998; 45 (suppl 3): 1197–1202. [PubMed] [Google Scholar]
- 43.Fan ST, Lai ECS, Lo CM, et al. Hepatectomy with an ultrasonic dissector for hepatocellular carcinoma. Br J Surg 1996; 83: 117–120. [DOI] [PubMed] [Google Scholar]
- 44.Lai ECS, Ng IOL, You KT, et al. Hepatectomy for large hepatocellular carcinoma: the optimal resection margin. World J Surg 1991; 15: 141–145. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida Y, Kanematsu T, Matsumata T, et al. Surgical margin and recurrence after resection of hepatocellular carcinoma in patients with cirrhosis. Further evaluation of limited hepatic resection. Ann Surg 1989; 209: 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ouchi K, Matsubara S, Fukuhara K, et al. Recurrence of hepatocellular carcinoma in the liver remnant after hepatic resection. Am J Surg 1993; 166: 270–273. [DOI] [PubMed] [Google Scholar]
- 47.Lai ECS, You KT, Ng IOL, Shek WH. The pathological basis of resection margin for hepatocellular carcinoma. World J Surg 1993; 17: 786–791. [DOI] [PubMed] [Google Scholar]
- 48.Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg 1997; 226: 688–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takenaka K, Yoshida K, Nishizaki T, et al. Postoperative prophylactic lipiodolization reduces the intrahepatic recurrence of hepatocellular carcinoma. Am J Surg 1995; 169: 400–405. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka K, Shimada H, Togo S, et al. Use of transcatheter arterial infusion of anticancer agents with lipiodol to prevent recurrence of hepatocellular carcinoma after hepatic resection. Hepato-Gastroenterology 1999; 46: 1083–1088. [PubMed] [Google Scholar]
- 51.Wu CC, Ho YZ, Ho WL, et al. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg 1995; 82: 122–126. [DOI] [PubMed] [Google Scholar]
- 52.Yamasaki S, Hasegawa H, Kinoshita H, et al. A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res 1996; 87: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical hepatic resection for hepatocellular carcinoma (HCC). Hepato-Gastroenterology 1996; 43: 1405–1409. [PubMed] [Google Scholar]
- 54.Ono T, Nagasue N, Kohno H, et al. Adjuvant chemotherapy with epirubicin and carmofur after radical resection of hepatocellular carcinoma: a prospective randomized study. Semin Oncol 1997; 24 (suppl 6): 18–25. [PubMed] [Google Scholar]


