Abstract
Objective
To investigate the feasibility of internal mammary sentinel lymph node biopsy as a method to refine and thereby improve nodal staging in breast cancer.
Summary Background Data
The internal mammary lymph node status is a major prognostic factor in breast cancer. If positive, prognosis is less favorable. However, staging this regional nodal basin is not performed routinely, thus discarding additional staging information.
Methods
In a consecutive series of 256 patients with primary breast cancer, sentinel node biopsy was performed based on lymphoscintigraphy, intraoperative gamma probe detection, and blue dye mapping using 10 mCi (370 MBq) 99mTc-nanocolloid injected peritumorally and 0.5 to 1.0 mL Patent Blue V injected intradermally. During surgery, whenever possible, both axillary and internal mammary sentinel nodes were sampled.
Results
Lymphoscintigraphy showed axillary sentinel nodes in 95% (243/256) and additional internal mammary sentinel nodes in 25.3% (65/256). The overall success rate of axillary sentinel node biopsy was 97% (249/256). Sampling the internal mammary basin, based on the results of lymphoscintigraphy, was successful in 63% (41/65). In three patients a small pleural lesion resulted from staging this basin. This technique revealed internal mammary metastases in 26.8% (11/41). In 7.3% (3/41), internal mammary nodes showed metastatic involvement without accompanying axillary metastases.
Conclusions
Internal mammary sentinel node biopsy is feasible without serious additional complications. It improves nodal staging in breast cancer by identifying higher-risk subgroups with internal mammary nodal metastases, which might benefit from altered adjuvant treatment regimens.
Accurate staging is of the utmost importance in planning breast cancer treatment. To date, the axillary lymph node status is considered the most important prognostic factor in breast cancer. Axillary staging is usually performed by axillary lymph node dissection (ALND). However, in patients thus classified as node-negative, long-term follow-up shows treatment failures in 15% to 30%. 1
The internal mammary lymph nodes represent a second regional basin of lymph drainage from the breast. This regional nodal basin was studied intensively in the 1950s and 1960s. 2–6 Because metastatic involvement of the internal mammary nodes is associated with a less favorable prognosis, the internal mammary nodal status is also considered a major prognostic factor in breast cancer. 6–12 Thus, sampling of the internal mammary nodes would be necessary to obtain complete staging. 6,13,14 However, this procedure is not routinely performed in contemporary surgery. 12
Sentinel node (SN) biopsy was recently introduced as a less invasive alternative to ALND in staging breast cancer. 13–19 This new technique has an equivalent accuracy to ALND, as is shown in numerous studies. 20–27 By using lymphoscintigraphy, the SN technique also visualizes SNs outside the axilla, mainly in the internal mammary basin. The purpose of this study is to investigate the feasibility of internal mammary SN biopsy as a minimally invasive procedure, refining nodal staging in breast cancer.
PATIENTS AND METHODS
From April 1997 to February 2000, after receiving approval of the local ethics committee and after obtaining informed consent, all consecutive patients with clinically node-negative operable primary breast cancer were included in a prospective study on SN biopsy. Except for pregnant women and those with T4 tumors, no patients were excluded. Our technique of SN biopsy has been described elsewhere. 27 After injection of 370 MBq (10 mCi) 99mTc-nanocolloid (Nanocoll, Nycomed Amersham Sorin, Saluggia, Italy) in 4 mL saline, either peritumorally or into the breast tissue adjacent to the cavity of the previous excisional biopsy, lymphoscintigraphy was performed after a mean interval of 16 hours (range 12–18). This “next-day procedure” is preferred because good visualization of hot spots in this way can be combined with the logistic advantage of easy operating room planning and improved radiation safety considerations for the surgeon, the operating room personnel, and the pathologist. Scintigraphic images were obtained in three standard positions: anterior, anterior oblique, and lateral. The location of axillary and nonaxillary SNs was marked on the skin. After induction of general anesthesia in the operating room, 10 to 15 minutes before the incision, 0.8 to 1.0 mL Patent Blue V (Laboratoire Guerbet, Aulnay-sous-Bois, France) was injected intradermally above the tumor or alongside the scar of the excisional biopsy. During surgery in all patients, attempts were made to harvest both axillary and nonaxillary SNs, as visualized on lymphoscintigraphy. If necessary, additional incisions (2.5–3 cm) were used to sample internal mammary SNs. Intraoperative identification of the SNs was based both on blue dye mapping and gamma probe detection (RMD 10 mm, Radiation Monitoring Devices, Inc., Watertown, MA). Interference from primary site radioactivity of medial tumors, impeding internal mammary SN identification, was managed by using an additionally collimated gamma probe and by narrowing the energy window of the probe, which decreases the influence of scattered radiation from the primary injection site. Despite these measures, interference occasionally led to failure in sampling a parasternal SN. A postoperative chest radiograph was obtained routinely after internal mammary node biopsy to exclude accidental pneumothorax.
In phase 1 of this study (137 patients), SN biopsy was followed by completion axillary lymph node dissection in all patients. After validation of the SN technique in our institute (tumor-positive axilla in 40% [55/137], false-negative SN in 1.8% [1/55], sensitivity 98% [54/55]), in phase 2 completion axillary lymph node dissection was performed only in cases of a tumor-positive axillary SN, or after a doubtful or unsuccessful SN procedure. Histopathologic examination of the SNs consisted of routine hematoxylin and eosin (H&E) staining, followed by serial sectioning and immunohistochemical staining whenever routine H&E staining did not reveal metastases.
RESULTS
One pregnant patient and 12 patients with T4 tumors were excluded. All other 256 consecutive patients were included in this study, consisting of 119 T1 tumors (46.5%), 117 T2 tumors (45.7%), and 20 T3 tumors (7.8%). Preoperative diagnosis was documented by fine-needle aspiration or core biopsy in 160 patients (62.5%) and by previous excisional biopsy in 96 patients (37.5%). The primary tumor was located in the upper outer quadrant in 116 patients (45%), the lower outer quadrant in 36 patients (14%), centrally in 20 patients (8%), the upper inner quadrant in 58 patients (23%), and the lower inner quadrant in 26 patients (10%). Lymphoscintigraphy visualized axillary hot spots in 95% (243/256). In 65 patients (25.3%), additional hot spots were noted in the internal mammary chain, in 63% draining medial or central tumors, and in 37% draining lateral tumors. Exclusive drainage to the internal mammary nodes was absent in our series. The axillary SN biopsy was successful in 97% (249/256). The axillary basin was tumor-positive in 45% (115/256). In 53% (61/115), the axillary SN was the only metastatic lymph node present.
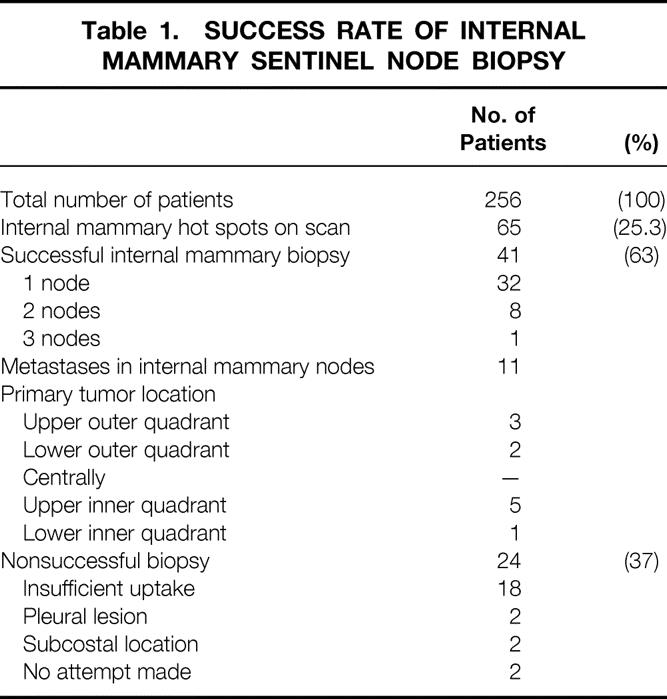
Internal mammary sentinel node biopsy proved successful in 63% (41/65) of patients, showing parasternal hot spots on the scan, and revealed metastatic involvement in 26.8% (11/41) (Table 1). Of these, 7.3% (3/41) had a negative axillary SN biopsy. In two, a completion ALND confirmed this result. One patient refused confirmation by ALND. Internal mammary metastases were associated with medial tumors in six patients and with lateral tumors in five patients.
Table 1. SUCCESS RATE OF INTERNAL MAMMARY SENTINEL NODE BIOPSY
In three cases a small pleural lesion resulted from the internal mammary biopsy. In all patients this was noted during surgery. Recovery was uneventful after simple vacuum drainage.
Failure to harvest the internal mammary nodes was mostly (in 18 patients) due to insufficient radioactivity uptake in the internal mammary nodes (see Table 1). Usually (in 32 patients) one parasternal node was sampled per patient, either the most radioactive node or the node nearest the tumor. In eight patients two parasternal nodes and in one patient three parasternal nodes were harvested. In all 51 parasternal nodes were removed. Twelve nodes (23.5%) were located in the second interspace; 17 nodes (33.3%), 13 nodes (25.5%), and 7 nodes (13.7%) were found in the third, fourth, and fifth interspace, respectively. In two patients the site of the parasternal node was not documented.
Internal mammary metastases were mostly found in the third interspace (47%), with 20%, 27%, and 6% of internal mammary metastases in the second, fourth, and fifth interspace, respectively. Most of the internal mammary nodes were not blue-stained (69%). The median ex vivo activity of the internal mammary nodes was 345 counts/10 seconds (range 12–2,866) No significant difference was found between radioactive uptake in parasternal tumor-positive nodes (median 432 counts/10 seconds, range 101–1,546) and tumor-negative nodes (median 302 counts/10 seconds, range 12–2,866). Ex vivo ratios (i.e., ex vivo SN activity against background activity) are not useful in the parasternal basin because background activity is virtually nil after successful biopsy of a radioactive parasternal node. The median ex vivo activity of axillary SNs in patients with successful internal mammary biopsy was 943 counts/10 seconds (range 13–17,159, median ex vivo activity ratio 43.3). No significant differences were found in axillary lymphatic drainage as measured by the nodal ex vivo activity, when internal mammary node-positive patients (median axillary ex vivo activity 656 counts/10 seconds) were compared with internal mammary node-negative patients (median axillary ex vivo activity 1,034 counts/10 seconds) (two-sided Student t test, NS;P = .17).
DISCUSSION
Current nodal staging appears to be inadequate because distant metastases are known to occur in up to 30% of patients classified as node-negative by axillary staging. Therefore, other prognostic factors must be investigated to stratify high-risk subgroups among node-negative patients with breast cancer. The importance of bone marrow micrometastases as an (independent?) prognostic factor is under investigation 28,29 and is subject of a new research protocol of the American College of Surgeons Oncology Group (ACOSOG Z0010 and Z0011). The clinical relevance of this phenomenon is unknown and the results of these studies have to be awaited.
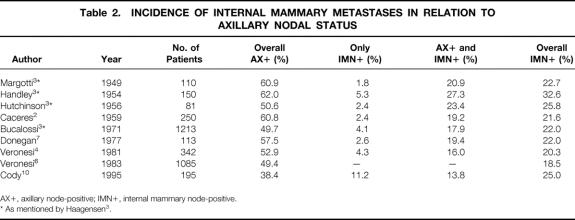
By visualizing lymphatic drainage patterns to the axilla as well as other nonaxillary locations, the recently introduced technique of SN biopsy renewed our interest in the internal mammary node status, historically recognized as a major prognostic factor in breast cancer. Besides the axillary drainage pattern, SN lymphoscintigraphy simultaneously reveals this additional drainage pathway to the internal mammary chain. Based on lymphatic anatomy and physiology studies, the importance of the internal mammary chain as a second lymph node basin in breast cancer is well established. 18,30–32 Various investigations have been published on sampling the internal mammary chain as part of the extended Halsted mastectomy to improve staging. 2–6,10 From these and other studies, the overall incidence of internal mammary metastases is well known (Table 2). Internal mammary nodal metastases are reported to be present in 18% to 33% (mean 23.4%) of patients with breast cancer, mostly concomitant with axillary metastases. Metastases exclusively situated in the internal mammary chain occur in 2% to 11% of patients.
Table 2. INCIDENCE OF INTERNAL MAMMARY METASTASES IN RELATION TO AXILLARY NODAL STATUS
AX+, axillary node-positive; IMN+, internal mammary node-positive.
* As mentioned by Haagensen 3.
Important factors in determining the likelihood of internal mammary metastases are the axillary nodal status and the number of axillary nodes involved, 2,3,5–7,10 the site of the primary tumor, whether it is medial/central or lateral, 2,3,5,8,10,12,24 the size of the primary tumor, 3,5,6 and the patient’s age, with younger patients having a higher risk. 6
The extended radical mastectomy was abandoned in the 1970s both as a staging procedure, because of a low rate of internal mammary metastases in the absence of axillary metastases, and as a therapeutic procedure, because removal of all internal mammary lymph nodes (in earlier days without adjuvant therapy) did not improve the prognosis. 3–5,7,10 However, these early studies clearly show that internal mammary metastases are associated with more advanced disease and have a unfavorable prognosis. 3,5–8,10–12
A combination of both axillary and internal mammary metastases is associated with poor prognosis, showing a 10-year overall survival rate of only 37%. 6 Because axillary node-positive patients generally receive adjuvant systemic therapy, the therapeutic consequence for patients in this subgroup with identified internal mammary metastases, although controversial, would be radiotherapy on the internal mammary chain. The effect of radiotherapy on the internal mammary chain on survival is the subject of an ongoing trial (EORTC 22922). If proven beneficial, patient selection for adjuvant radiotherapy on the internal mammary chain could be based on internal mammary SN biopsy.
The 10-year overall survival for patients with metastases exclusively located in the internal mammary nodes is approximately the same as that for axillary-positive and internal mammary-negative patients, averaging 60%. 6,10,11 Thus, patients staged as axillary-negative and internal mammary-positive are a higher-risk subgroup with (hidden) internal mammary metastases. As a result of internal mammary SN biopsy, this subgroup might benefit from this refinement of nodal staging by receiving adjuvant systemic therapy and radiotherapy. 7,8,10
In this study, lymphoscintigraphy revealed internal mammary SNs in 25.3% of patients by using peritumoral injections of 370 MBq radiocolloid followed by a mean interval of 16 hours. These scintigraphic results are related to the tracer type, the injection site, the interval, and the tracer dose. After peritumoral injection of the usual dose of 40 to 60 MBq 99mTc-nanocolloid, lymphoscintigraphy generally reveals parasternal hot spots in only 9% to 16%. 11,21,24 When a higher tracer dose is used, as in this study, the scintigraphic identification rate of internal mammary hot spots increases. Moreover, it results in higher nodal uptake of radioactive tracer, facilitating intraoperative identification and harvesting of the SN in both the internal mammary and the axillary basin.
The value of blue dye mapping in internal mammary SN biopsy is limited. When routinely used as a complementary mapping technique in axillary SN biopsy, we prefer intradermal blue dye injection instead of peritumoral injection, because this results in more frequent blue staining of axillary SNs. 27 However, intradermal or subdermal radiocolloid and/or blue dye injection will not map internal mammary nodes because the skin of the breast does not drain to the internal mammary basin. 33–36 A technique using peritumoral blue dye only would involve blind dissection of the internal mammary basin without knowing to which interspace lymphatic drainage has occurred, if any. Therefore, when using a technique involving blue dye only, the surgeon should refrain from internal mammary SN biopsy.
The actual internal mammary biopsy can usually be performed using the mastectomy incision. In breast-conserving therapy, a small additional horizontal incision over the desired interspace is made, after which the biopsy technique described by Haagensen 3 proved useful. Internal mammary lymph nodes can be small and in this study were found mostly lateral to the internal mammary vessels.
Internal mammary SN biopsy in our series was successful in 63% (41/65) of patients with visualized internal mammary SNs, revealing overall internal mammary nodal metastases in 26.8% (11/41). This rate of metastatic involvement in the internal mammary nodes is in accordance with literature findings concerning internal mammary involvement in extended radical mastectomy, as is the percentage of patients with metastases exclusively located in the internal mammary chain, as found in our series (see Table 2).
When metastases were found both in the axillary and internal mammary basin (eight patients), the extent of axillary involvement was less than four positive nodes in five patients and more than four positive nodes in the remaining three patients.
The finding that 5 of 11 patients with internal mammary metastases had their primary tumor in the lateral quadrants of the breast (see Table 1) corroborates the assumption that all quadrants of the breast can drain into the internal mammary chain.
Additional complications of internal mammary biopsy were limited to small incisions over the intercostal space in relevant patients, with good cosmetic results, and pleural lesions in three patients, with uneventful recovery in all.
All 11 patients with internal mammary metastases received adjuvant therapy as a direct consequence of internal mammary biopsy: eight patients with positive axillary and internal mammary nodes received additional radiotherapy, and three patients with negative axillary nodes but positive internal mammary nodes received both chemotherapy and radiotherapy.
Our results show the technique and feasibility of internal mammary biopsy. Internal mammary nodal staging reveals otherwise hidden parasternal metastases, thereby stratifying higher-risk groups, however small, in both axillary node-positive and axillary node-negative patients. The clinical relevance of this additional staging information is based on the worse prognosis associated with the presence of internal mammary metastases and the high incidence of breast cancer; thus, an improvement in the outcome of treatment, even in limited subgroups, may result in a substantial contribution to breast cancer therapy. Combining this improved staging information with survival rates of ongoing and future adjuvant treatment protocols might lead to improvements in not only nodal staging but also in breast cancer treatment.
One might argue that internal mammary SN staging does not really alter the prognosis but merely results in stage migration. However, without internal mammary sampling, staging is incomplete, ignoring the internal mammary status as the second major important prognostic factor in breast cancer. Internal mammary biopsy, by refinement, leads to more accurate staging and offers the possibility of a more rational use of adjuvant systemic therapies in patients with proven node-positive breast cancer. Therefore, we believe that internal mammary staging, as a minimally invasive procedure, is a useful and promising adjunct.
CONCLUSIONS
We conclude from the results of this study that internal mammary SN biopsy in breast cancer is technically feasible and improves nodal staging. This technique helps to identify a higher-risk subgroup of patients with internal mammary metastases who could benefit from adjuvant therapy. As a consequence of internal mammary biopsy, patients staged as axillary-positive and internal mammary-positive could receive additional radiotherapy, and patients staged as axillary negative and internal mammary-positive could receive adjuvant chemotherapy as well as locoregional radiotherapy. In turn, more accurate staging might help to identify patients who are truly node-negative, and they may be spared the complications of additional chemotherapy.
Footnotes
Correspondence: Fred W. C. van der Ent, MD, PhD, Department of Surgery, Maaslandziekenhuis, Postbus 5500, 6130 MB Sittard, The Netherlands.
E-mail: f.vanderent@orbisconcern.nl
Accepted for publication November 17, 2000.
References
- 1.Rosen PP, Groshen S, Saigo PE, et al. A long-term follow-up study of survival in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma. J Clin Oncol 1989; 7: 355–366. [DOI] [PubMed] [Google Scholar]
- 2.Caceres E. Incidence of metastasis in the internal mammary chain in operable cancer of the breast. Surg Gynaecol Obstet 1959; 108: 715–720. [PubMed] [Google Scholar]
- 3.Haagensen CD. The natural history of breast carcinoma. In: Diseases of the breast. Philadelphia: WB Saunders; 1986: 635–718.
- 4.Veronesi U, Valagussa P. Inefficacy of internal mammary nodes dissection in breast cancer surgery. Cancer 1981; 47: 170–175. [DOI] [PubMed] [Google Scholar]
- 5.Lacour J, Le M, Caceres E, et al. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Ten-year results of an international cooperative trial in breast cancer. Cancer 1983; 51: 1941–1943. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, Cascinelli N, Bufalino R, et al. Risk of internal mammary lymph node metastases and its relevance on prognosis of breast cancer patients. Ann Surg 1983; 198: 681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donegan WL. The influence of untreated internal mammary metastases upon the course of mammary cancer. Cancer 1977; 39: 533–538. [DOI] [PubMed] [Google Scholar]
- 8.Morrow M, Foster RS, Jr. Staging of breast cancer: a new rationale for internal mammary node biopsy. Arch Surg 1981; 116: 748–751. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi M, Koyasaki N, Ohta N, et al. Internal mammary nodal status is a more reliable prognostic factor than DNA ploidy and c-erb B-2 expression in patients with breast cancer. Arch Surg 1993; 128: 242–246. [DOI] [PubMed] [Google Scholar]
- 10.Cody HS, Urban JA. Internal mammary node status: a major prognosticator in axillary node-negative breast cancer. Ann Surg Oncol 1995; 2: 32–37. [DOI] [PubMed] [Google Scholar]
- 11.Krag DN. Minimal access surgery for staging regional lymph nodes: the sentinel node concept. Curr Probl Surg 1998; 35: 951–1016. [DOI] [PubMed] [Google Scholar]
- 12.Glass EC, Essner R, Giuliano AE. Sentinel node localization in breast cancer. Semin Nucl Med 1999; 29: 57–68. [DOI] [PubMed] [Google Scholar]
- 13.Albertini JJ, Lyman GH, Cox C, et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA 1996; 276: 1818–1822. [PubMed] [Google Scholar]
- 14.McMasters KM, Giuliano AE, Ross MI, et al. Sentinel lymph node biopsy for breast cancer–not yet the standard of care. N Engl J Med 1998; 339: 990–995. [DOI] [PubMed] [Google Scholar]
- 15.Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol 1993; 2: 335–339. [DOI] [PubMed] [Google Scholar]
- 16.Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 1994; 220: 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg 1995; 222: 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uren RF, Howman-Giles RB, Thompson JF, et al. Mammary lymphoscintigraphy in breast cancer. J Nucl Med 1995; 36: 1775–1780. [PubMed] [Google Scholar]
- 19.Giuliano AE. Sentinel lymphadenectomy in primary breast carcinoma: an alternative to routine axillary dissection. J Surg Oncol 1996; 62: 75–77. [DOI] [PubMed] [Google Scholar]
- 20.Giuliano AE, Jones RC, Brennan M, Statman R. Sentinel lymphadenectomy in breast cancer. J Clin Oncol 1997; 15: 2345–2350. [DOI] [PubMed] [Google Scholar]
- 21.Roumen RMH, Valkenburg JGM, Geuskens LM. Lymphoscintigraphy and feasibility of sentinel node biopsy in 83 patients with primary breast cancer. Eur J Surg Oncol 1997; 23: 495–502. [DOI] [PubMed] [Google Scholar]
- 22.Veronesi U, Paganelli G, Galimberti V, et al. Sentinel node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph nodes. Lancet 1997; 349: 1864–1867. [DOI] [PubMed] [Google Scholar]
- 23.Turner RR, Ollila DW, Krasne DL, Giuliano AE. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg 1997; 226: 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borgstein PJ, Pijpers R, Comans EF, et al. Sentinel lymph node biopsy in breast cancer: guidelines and pitfalls of lymphoscintigraphy and gamma probe detection. J Am Coll Surg 1998; 186: 275–283. [DOI] [PubMed] [Google Scholar]
- 25.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer–a multicenter validation study. N Engl J Med 1998; 339: 941–946. [DOI] [PubMed] [Google Scholar]
- 26.Cox CE, Pendas S, Cox JM, et al. Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Ann Surg 1998; 227: 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Ent FWC, Kengen RAM, van der Pol HAG, Hoofwijk AGM. Sentinel node biopsy in 70 unselected patients with breast cancer: increased feasibility by using 10 mCi radiocolloid in combination with a blue dye tracer. Eur J Surg Oncol 1999; 25: 24–29. [DOI] [PubMed] [Google Scholar]
- 28.Mansi JL, Gogas H, Bliss JM, et al. Outcome of primary breast cancer patients with micrometastases: a long-term follow-up study. Lancet 1999; 354: 197–202. [DOI] [PubMed] [Google Scholar]
- 29.Braun S, Pantel K, Muller P, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 2000; 342: 525–533. [DOI] [PubMed] [Google Scholar]
- 30.Hultborn KA, Larsson LG, Ragnhult I. The lymph drainage from the breast to the axillary and parasternal lymph nodes, studied with the aid of colloidal AU198. Acta Radiol 1955; 43: 52–64. [DOI] [PubMed] [Google Scholar]
- 31.Turner-Warwick RT. The lymphatics of the breast. Br J Surg 1959; 46: 574–582. [DOI] [PubMed] [Google Scholar]
- 32.Vendrell-Torné E, Setoain-Quinquer J, Doménech-Torné FM. Study of normal mammary lymphatic drainage using radioactive isotopes. J Nucl Med 1972; 13: 801–805. [PubMed] [Google Scholar]
- 33.Klimberg VS, Rubio IT, Henry R, et al. Subareolar versus peritumoral injection for location of the sentinel lymph node. Ann Surg 1999; 229: 860–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieweg OE, Jansen L, Valdes OR, et al. Lymphatic mapping and sentinel lymph node biopsy in breast cancer. Eur J Nucl Med 1999; 26: S11–S16. [DOI] [PubMed] [Google Scholar]
- 35.Alazraki NP, Styblo T, Grant SF, et al. Sentinel node staging of early breast cancer using lymphoscintigraphy and the intraoperative gamma-detecting probe. Semin Nucl Med 2000; 30: 56–64. [DOI] [PubMed] [Google Scholar]
- 36.Borgstein PJ, Meijer S, Pijpers RJ, Van Diest PJ. Functional lymphatic anatomy for sentinel node biopsy in breast cancer: echoes from the past and the periareolar blue method. Ann Surg 2000; 232: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]