Abstract
Objective
To define the protective effect of ischemic preconditioning on cold ischemia and reperfusion injury associated with intestinal transplantation, and the role of nitric oxide in this process.
Summary Background Data
Ischemia/reperfusion injury continues to be a significant obstacle in small bowel transplantation. Preconditioning is a mechanism that protects against this injury.
Methods
To study the capacity of preconditioning to prevent cold ischemia-associated injury and the inflammatory response associated with intestinal transplantation, the authors studied a control group of animals, cold ischemia groups with or without previous preconditioning and with or without previous administration of L-NAME or NONOS, and intestinal transplantation groups with or without previous preconditioning and with or without previous administration of L-NAME or NONOS.
Results
Histologic findings and the release of lactate dehydrogenase into the preservation solution showed that preconditioning protects against cold ischemic preservation-associated injury. Preconditioning also prevented the inflammatory response associated with intestinal transplantation, measured by the above parameters and by neutrophil recruitment in the intestine. Inhibition of nitric oxide eliminates the protective effect.
Conclusions
Preconditioning protects the intestinal grafts from cold preservation and reperfusion injury in the rat intestinal transplantation model. Nitric oxide is involved in this protection.
Intestinal transplantation has become an accepted therapy for intestinal disease in patients who require total parenteral nutrition. Although the results of small bowel transplantation have dramatically improved during the past few years, rejection, infection, preservation, and reperfusion injury resulting in primary nonfunction continues to be a major obstacle. 1
Preconditioning has been reported to be one of the mechanisms that may protect the organ from the ischemia/reperfusion syndrome (I/R). This phenomenon is defined as one or more brief periods of ischemia with intermittent reperfusion that protects against a subsequent and sustained ischemia/reperfusion. Our group was the first to describe this protective effect of preconditioning in the small intestine. 2 In that study, the massive epithelial lifting observed after sustained warm ischemia was prevented if preconditioning was carried out before the ischemia. We also reported that the preconditioning response might depend on the release of endogenous protective substances such as nitric oxide (NO). An increase in NO synthesis was detected after intestinal preconditioning, and the protection induced by this process could be partly inhibited by the addition of the NO synthesis inhibitor L-NAME. 2 Recently, our group presented a brief report of the possible application of these results to rat intestinal transplantation. 3 Altogether, the data available indicate that intestinal preconditioning is triggered by a small but rapid initial increase in NO synthesis.
As preconditioning is established as a pretreatment strategy, planned ischemic events are required. In transplantation surgery, the ischemic episode of the donor organ is always necessary, and so intestinal surgery is one possible setting for the application of the ischemic preconditioning technique. The beneficial effects of ischemic preconditioning have been previously described in transplantation procedures involving the heart, 4 lung, 5 and liver, 6 but its protective capacity against injury associated with cold preservation and its subsequent role in reperfusion have not been defined with regard to intestinal transplantation. Thus, the first objective of this study is to distinguish the protective role of preconditioning in cold ischemia from its role in cold ischemia followed by reperfusion (transplantation), and its relationship with NO in protecting intestinal grafts.
As far as the inflammatory response in the I/R process is concerned, it is known that neutrophil recruitment is one of the causes for vascular dysfunction. This is based on the observation that preventing neutrophil influx into tissues, either by depleting the numbers of circulating neutrophils or by preventing neutrophil adhesion, significantly reduced the microvascular dysfunction 7 and tissue injury 8 associated with I/R. It has also been clearly established that preconditioning previous to I/R reduces neutrophil adhesion and/or neutrophil rolling. 9,10 However, the role of preconditioning as a modulator of the inflammatory response associated with cold preservation and reperfusion in intestinal transplantation has not been described.
Because NO has been shown to have inhibitory effects on neutrophil recruitment 11 and also has been described as an endogenous modulator of the intestinal preconditioning response, the second objective of this study was to determine whether NO generation in preconditioning could regulate neutrophil recruitment and the inflammatory response associated with reperfusion in intestinal transplantation.
In short, by evaluating injury on the basis of lactate dehydrogenase (LDH) release and histology, this study aims to establish the ability of ischemic preconditioning to protect intestinal grafts against the inflammatory response associated with cold ischemic preservation and reperfusion injury in intestinal transplantation, and the role of NO in this process.
METHODS
The study was performed with male Sprague-Dawley rats (Ifa Credo, Barcelona, Spain) weighing 250 to 300 g. All animals were fasted for 12 hours before surgery, anesthetized with urethane 10% (10 mL/kg, given intraperitoneally), and placed in a supine position, keeping the body temperature at 36°C to 37°C. The experiment was conducted under the supervision of our institution’s Research Commission and followed EU guidelines for the handling and care of laboratory animals.
Effect of Preconditioning on the Cold Ischemia-Associated Injury
Animals were randomly assigned to the following study groups (n = 7 each). In group 1 (cold ischemia), after anesthesia the animals underwent a midline laparotomy. The entire small bowel was removed and preserved in cold Ringer’s lactate solution (4°C) for a maximum of 4 hours. In group 2 (preconditioned), animals underwent the same procedure as group 1 but with previous preconditioning (as described above) before cold ischemia. In group 3 (preconditioned + NAME), animals were treated as in group 2 with previous administration of L-NAME (10 mg/kg), given intravenously 5 minutes before the beginning of the preconditioning process. In group 4 (cold ischemia + NAME), animals were treated as in group 1 but with L-NAME. In group 5 (cold ischemia + spermine NONOate [NONOS]), animals underwent the same procedure as group 1 but with previous administration of intravenous NONOS (10 mg/kg resuspended in phosphate-buffered saline, pH 7.4, 30 minutes before the administration) by direct puncture in the inferior cava.
Effect of Preconditioning on the Inflammatory Response Associated With Intestinal Transplantation
The following groups of animals (n = 8 each) were tested. In group 6 (sham), animals were subjected to anesthesia and laparotomy for 150 minutes. The abdominal area was covered with gauze soaked in saline at 37°C and a plastic remnant to minimize dehydration and evaporative heat loss of exposed tissues. Animals in group 7 (intestinal transplantation group) underwent 90 minutes of cold ischemia in Ringer’s lactate at 4°C and posterior transplantation with 60 minutes of reperfusion. Animals in group 8 (preconditioned group) were treated as in group 7 (intestinal transplantation) but with previous preconditioning. Animals in group 9 (Preconditioning + NAME) were treated as in group 8 but with previous administration of L-NAME as described above. Animals in group 10 (intestinal transplantation group + NAME) were treated as in group 7 but with L-NAME administration. Animals in group 11 (intestinal transplantation group + NONOS) underwent the same procedure as in group 7 but with previous administration of NONOS, as described in group 5.
To assess the efficacy of preconditioning, plasma samples were obtained by cardiac puncture to evaluate LDH release. Tissues were obtained after reperfusion to evaluate myeloperoxidase (MPO) activity, reduced glutathione (GSH) and NO production, and histopathology. To prevent possible differences related to the area of the intestine selected, all intestinal samples were obtained from the medium zone of the jejunum.
To address when NO is enhanced in the preconditioned animals, we performed an additional experiment. We induced intestinal preconditioning (10 minutes of warm ischemia + 5 minutes of reperfusion) with or without L-NAME administration and removed the entire small bowel to evaluate NO generation.
Biochemical Analysis
LDH activity in blood and in the preservation solution was determined using a commercial kit purchased from Boehringer Mannheim (Mannheim, Germany). Myeloperoxidase activity was measured, as previously described, 13 spectrophotometrically at 630 nm using 3, 3′, 5, 5′-tetramethylbenzidine as a substrate. To assess reduced glutathione, intestinal samples were homogenized in KCl 1.15% solution. Proteins were precipitated with 1N perchloric acid. After centrifugation, samples were neutralized with K2CO3 10%. The amount of reduced glutathione was measured and monitored at 340 to 400 nm, using glutathione transferase and 1-chloro-2, 2-dinitrobenzene. 14 NO production in intestinal bowel was determined by tissue accumulation of nitrite and nitrate, using a modification of the method previously described 2 and using a commercial kit from Cayman Chemical (Ann Arbor, MI). Total protein concentration in homogenates was determined using a commercial kit from BioRad (Munich, Germany). For histopathologic analyses, samples were embedded in paraffin, cut into 4-μm sections, and stained with hematoxylin and eosin (×127). Evaluation was performed with light microscopy without knowledge of the study groups, using Park’s classification;15 assessment was carried out by five different expert observers.
Statistics
Data are expressed as means ± standard error of the mean. Means of different groups were compared using a one-way analysis of variance. The Student t test was performed for evaluation of significant differences between groups. Significant differences were assumed when P < .05.
In the histopathologic analysis, the means of different groups was compared using the Mann-Whitney test. Significant differences were assumed when P < .05.
RESULTS
Effect of Preconditioning on the Cold Ischemia-Associated Injury
According to Park’s classification and as shown in Figure 1 and Table 1, the histologic findings for all the groups at 0 minutes of each procedure were injury grade 0, corresponding to normal mucosa. Samples obtained after 90 minutes of cold ischemia were injury grade 1 to 2. When preconditioning was carried out, the histology study showed an injury grade between 0 and 1. In contrast, in the preconditioning + NAME + cold ischemia group (group 3) and in the cold ischemia + NAME group (group 4), histology showed an injury grade 1 to 2 with a pattern similar to the ischemic group. The addition of NO donors to the cold ischemia group had a protective effect such as when preconditioning was performed on the animals.

Figure 1. Histologic lesions of the rat small bowel corresponding to the different groups in this study. (A) Normal mucosa, 0 minutes of intestinal cold ischemia. (B) Extended subepithelial space along villi; representative of cold ischemia preconditioning + NAME + cold ischemia and cold ischemia + NAME (90 minutes of preservation) and preconditioning + transplant and transplant + NONOS (90 minutes of preservation followed by transplantation with 60 minutes of reperfusion). (C) Subepithelial space at the tip of some villi; preconditioning + cold ischemia and cold ischemia + NONOS (90 minutes of preservation). (D) Epithelial lifting along villus; cold ischemia and cold ischemia + NAME (4 hours of preservation) and transplant and transplant + NAME (90 minutes of preservation followed by a transplantation with 60 minutes of reperfusion). (E) Extended subepithelial space; preconditioning + cold ischemia and cold ischemia + NONOS (4 hours of preservation). (F) Denuded villi; preconditioning + NAME + cold ischemia (4 hours of preservation) and preconditioning + NAME + transplant (90 minutes of preservation followed by transplant with 60 minutes of reperfusion). Hematoxylin and eosin stain, ×127.
Table 1. MEANS OF HISTOLOGY OF COLD ISCHEMIC PERIODS

Prec, preconditioning; CI, cold ischemia.
Histology is evaluated according to Park’s classification. Values are compared using the Mann-Whitney test and expressed as means ± SD.
*P < .05 vs. time 0.
†P < .05 vs. Prec + CI.
After 4 hours of cold ischemia, histologic findings corresponded to injury grade 3. In the preconditioning group, histology indicated an injury grade 2, and when L-NAME was added, the injury grade was 4. Tissue cold preservation with previous administration of L-NAME, in the absence of preconditioning, followed a pattern similar to the cold ischemic group (injury grade 3), whereas NO administration was able to restore the preconditioned pattern (injury grade 2).
Figure 2 shows LDH release into the preservation solution at different preservation periods. LDH release into Ringer’s solution increased proportionally to the increase in the preservation time. In the animals subjected to cold ischemic conditions, the release of the enzyme was significantly greater in all the preservation conditions than at baseline (0 minutes). In contrast, when preconditioning was carried out before preservation (preconditioning + cold ischemia), no increases were found. However, the addition of L-NAME to preconditioned rats (preconditioning + NAME + cold ischemia, group 3) reversed the beneficial effects of preconditioning, showing an LDH profile similar to that obtained in the ischemic group without preconditioning. In cold ischemic conditions with L-NAME, the release had the same behavior as in the cold ischemic group. In contrast, the addition of NONOS presented a significant decrease at all the times studied.

Figure 2. Lactate dehydrogenase (LDH) release (U/mL) as a function of the preservation time. CI: cold ischemia (small bowel extracted and preserved in cold Ringer’s lactate solution at 4°C for a maximum of 4 hours). Prec + CI: preconditioning before the cold ischemia (small bowel clamped for 10 minutes, followed by 5 minutes of reperfusion before preservation in cold Ringer’s lactate solution at 4°C for a maximum of 4 hours). Prec + NAME + CI: preconditioning + NAME (as in preconditioning group but with prior addition of L-NAME). CI + NAME: cold ischemia with previous L-NAME administration. CI + NONOS: cold ischemia with previous NONOS administration. * P < .05 vs. time 0 of the corresponding group. +P < .05 vs. Prec + CI.
Effect of Preconditioning on the Inflammatory Response Associated With Intestinal Transplantation
Figure 3 shows LDH activity in plasma in the transplanted groups. Intestinal transplantation induced a significant increase in plasma LDH release compared with the sham group. This increase was attenuated when ischemic preconditioning was carried out before transplantation (preconditioning + transplant, group 8). When the generation of NO was inhibited before preconditioning (preconditioning + NAME + transplant, group 9), LDH increased again. In contrast, L-NAME administration to the transplanted group (transplant + NAME, group 10) did not modify the LDH release previously detected in transplanted animals without NAME. These increases found in the transplant and the transplant +NAME groups were also significant compared with preconditioned animals. Administration of NO to transplanted group (transplant + NONOS, group 11) had a beneficial effect, showing an LDH release similar to that in group 8 (preconditioning + transplant).
Figure 3. Lactate dehydrogenase (LDH) release (U/mL) in blood in the following groups. Sham; Trp: 90 minutes of cold ischemia followed by a transplantation with 60 minutes of reperfusion; Prec + Trp: as in transplanted group but with preconditioning before cold ischemia; Prec + NAME + Trp: as in ischemic preconditioning group but with previous administration of L-NAME before preconditioning; Trp + NAME: as Trp but with NAME administration; Trp + NONOS: as Trp but with previous NONOS administration. * P < .05 vs. sham. +P < .05 vs. Prec + Trp.
The pattern of myeloperoxidase activity was similar. As shown in Figure 4, myeloperoxidase activity in intestinal tissue (used here as a marker of neutrophil recruitment) was significantly higher after intestinal transplantation than in the sham group. When ischemic preconditioning was carried out before transplantation (preconditioning + transplant, group 8), this significant increase was not found. Inhibition of NO synthesis before preconditioning (preconditioning + NAME + transplant, group 9) reversed the myeloperoxidase decrease. These values did not differ significantly from those observed in the transplanted group, but significant differences were found compared with the preconditioning + transplant group (group 8). Again, NO administration induced a decrease in myeloperoxidase activity similar to that in the preconditioned group, whereas NAME administration to the transplanted group had no effect on these animals.
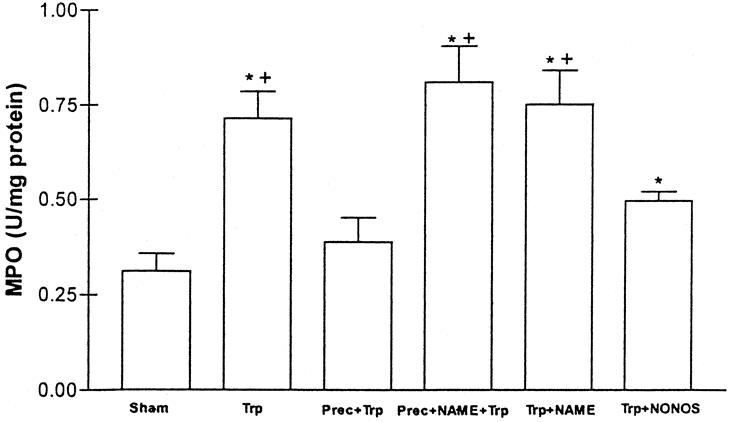
Figure 4. Myeloperoxidase (MPO) activity (U/mg protein) in the following groups. Sham; Trp: intestinal transplantation; Prec + Trp: ischemic preconditioning; Prec + NAME + Trp: preconditioning + NAME; Trp + NAME: intestinal transplantation + NAME; Trp + NONOS: intestinal transplantation + NONOS. * P < .05 vs. sham. +P < .05 vs. Prec + Trp.
When we measured reduced glutathione as a marker of free radical production and oxidative stress in the intestine (Fig. 5), we also observed a significant difference compared with the preconditioned group. In this case, a significant decrease in the levels of reduced glutathione in the transplanted and L-NAME treated groups (preconditioning + NAME + transplant and transplant + NAME) was found compared with the sham and preconditioned groups. No significant differences were found between these three last groups. NO administration was able to reverse this situation.

Figure 5. Reduced glutathione (GSH; nmol/μg protein) in the following groups. Sham; Trp: intestinal transplantation; Prec + Trp: ischemic preconditioning; Prec + NAME + Trp: preconditioning + NAME; Trp + NAME: intestinal transplantation + NAME; Trp + NONOS: intestinal transplantation + NONOS. * P < .05 vs. sham. +P < .05 vs. Prec + Trp.
As for the histologic findings (Table 2), the lesion observed in the transplanted group consisted of epithelial villus lifting affecting only the villus tip. A slight mixed inflammatory infiltrate was present in the lamina propria (grade 2–3). L-NAME administration to this group had no effect on histologic findings. In contrast, the preconditioned group (preconditioning + transplant) showed subepithelial space along the villi (grade 1–2). NONOS administration to the transplant group was able to diminish the injury in the transplanted group and showed a grade 2 injury. Finally, the preconditioned and L-NAME group (preconditioning + NAME + transplant) had an injury grade 3 to 4 and a moderate to intense mixed inflammatory infiltrate in the lamina propria.
Table 2. MEANS OF HISTOLOGY OF TRANSPLANTED PERIODS

Trp, transplant; Prec; preconditioning. Histology is evaluated according to Park’s classification. Values are compared using the Mann-Whitney test and expressed as means ± SD.
*P < .05 vs. time 0.
†P < 0.5 vs. Prec + CI.
Figure 6 shows the intestinal generation of NO, evaluated here as nitrite and nitrate production. NO production increased significantly after the preconditioned period. L-NAME administration reversed it. Nitrite and nitrate production returned to basal levels after 5 minutes of ischemia. No differences were observed between the cold ischemic, preconditioned (preconditioning + cold ischemia) or preconditioning + NAME + cold ischemia groups during any period studied. This was also observed when the reperfusion is carried out (transplantation situation); no differences were observed between the groups.
Figure 6. Nitrate and nitrite tissue production (μmol/mg protein) in the intestine in the following groups. (A) Sham; preconditioning (10 minutes of ischemia followed by 5 minutes of reperfusion); Prec + NAME: preconditioning group plus L-NAME; CI: cold ischemia; Prec + CI; preconditioning before cold ischemia; Prec + NAME + CI: preconditioning followed by sustained ischemia with prior addition of L-NAME. (B) Sham; Trp: intestinal transplantation (90 minutes of cold ischemia followed by transplant with 60 minutes of reperfusion); Prec + Trp: ischemic preconditioning (as in transplanted group but with preconditioning before cold ischemia); Prec + NAME + Trp: as in ischemic preconditioning group but with previous administration of L-NAME before preconditioning. * P < .05 vs. sham.
DISCUSSION
Ischemic preconditioning is a process in which a brief I/R episode confers a state of protection against subsequent long-term I/R injury. The role of ischemic preconditioning in reducing ischemic injury has been widely documented in a range of tissues. 2,16–18 However, the underlying mechanism of ischemic preconditioning remains unclear, and the results in experimental animals have only slowly been integrated into clinical practice. In the case of the small intestine, several studies have shown that preconditioning can reduce the damage associated with warm I/R injury, preventing the expression of P-selectin and leukocyte adhesion 19 and reducing LDH release in the intestinal lumen. 2,3 Moreover, this protective effect of preconditioning can be eliminated by blocking NO synthesis in preconditioned rats, indicating that endogenous NO is one of the inflammatory mediators able to regulate intestinal preconditioning.
Compared with other solid organ transplants, the transfer of the results of intestinal transplantation at the experimental level to clinical practice has been slow. The effectiveness of preconditioning in reducing I/R injury has been described in lung, liver, and heart transplantation 4–6 but to our knowledge has not been assessed with relation to intestinal transplantation. The results of the present study show that preconditioning is effective in attenuating the intestinal damage associated with cold ischemia preservation and the subsequent reperfusion injury in transplantation.
Multiple organ injury is frequently assessed by the release of LDH activity to the extracellular medium and into the plasma. 20–22 LDH is commonly used as an indicator of intestinal injury in the perfusate as well as in serum samples. 23–26 As a result of the difficulty of the ischemic small intestine in having one specific injury marker, we decided to measure release of this enzyme into the preservation solution as an index of tissue injury in cold preservation conditions, and circulating LDH serum levels in the case of tissue transplantation (warm reperfusion). As shown in Figure 2, preconditioning reduced the LDH release into the preservation solution. In contrast, addition of L-NAME eliminated the protective effect. This indicates that NO is involved in the protective response to cold preservation ischemic injury.
This NO does not have to be continuously produced to exert its beneficial effect. Indeed, under our experimental conditions, nitrate and nitrite production increased only at the end of the preconditioning process. This brief and reversible NO production during the preconditioning period is enough to initiate a cascade that leads to the protection.
These results are correlated with the histologic analysis (see Table 1 and Fig. 1) in this first part of the study, in which preconditioning conferred protection to the intestinal grafts preserved for 90 minutes. At this preservation time, preconditioning maintained a histologic injury similar to that obtained at time 0 of cold ischemia. Like LDH release in the preservation solution, L-NAME administration eliminated the histologic protection and NONOS conferred it again. In the samples removed after 4 hours of cold ischemia preservation, the histology followed a similar pattern. This histology corresponding to 4 hours of cold ischemia was completely representative of that obtained at 3 hours of cold storage.
The present study was undertaken to delineate the beneficial role of preconditioning in protecting against cold ischemia preservation and/or after warm reperfusion (intestinal transplantation) by itself. For this reason and to avoid the confounding effect of other protective agents, Ringer’s lactate was used instead of other apparently more suitable solutions. This allowed us to show a more profound effect of ischemic preconditioning without the assistance of other protective substances. Our intention was not to determine the optimal solution for small bowel preservation but rather to assess a “surgical” mechanism reported elsewhere to be protective in other tissue transplantations, which could by itself provide the tissue with endogenous protection against the I/R injury that occurs after or during the transplant.
For the second set of experiments, the intestinal transplant underwent 90 minutes of cold ischemia followed by 60 minutes of warm reperfusion. These times are long enough to provoke injury but not long enough to initiate the recovery of the injury under our experimental conditions. We were thus able to identify the primary protective effects that were due to the preconditioning and not to the recovery of the epithelium. The gut shows a remarkable rate of turnover under physiologic conditions, and a rapid regeneration of the epithelial layer has also been reported after intestinal hypoxia/reperfusion. 27 These times were selected to prevent the recovery of the mucosal epithelium.
With these premises, we found that preconditioning also protects the intestine from the injury associated with warm reperfusion in transplanted intestines. The preconditioned group released less LDH in circulating blood than the nonpreconditioned one (see Fig. 3). This could be a consequence of the protection established previously during cold ischemic preservation, or a direct consequence of protection from reperfusion damage.
Although the protective effect of ischemic preconditioning in transplantation procedures has been described in other organs, the role of inflammatory mediators in preconditioning has not been clearly defined. Among the mechanisms involved in the inflammatory response, the generation of NO by preconditioned tissue has received attention. 2,17 NO is effective in protecting the intestine from injury associated with I/R, 2,3 and it also can attenuate the increased albumin leakage from mesenteric venules exposed to I/R. 28 Our experimental results indicate that NO is one of the mediators involved in the preconditioning response to preservation reperfusion injury. The addition of L-NAME to preconditioned rats was able to reverse the beneficial effects of preconditioning (see Fig. 3). The fact that NO donor administration to transplanted groups also has a beneficial effect confirms the protective role of NO in this process.
Intestinal I/R injury results from reactive oxygen metabolites generated by the xanthine oxidase system and activated neutrophils. 19 Reduced glutathione is an essential component of the cellular defense mechanisms against radical-mediated tissue injury and can be used as indicator of oxidative stress induced by oxygen free radicals during the process of I/R. Using this marker (see Fig. 5), we found that preconditioning seems to afford protection against oxygen free radical-mediated tissue injury when carried out before transplantation.
As far as neutrophil recruitment is concerned, it has been shown that removal of neutrophils from the reperfusate in human small intestine before transplantation decreases the severity of injury. 28 Previous studies have found that preconditioning prevented the I/R-induced neutrophil rolling, adhesion, and microvascular dysfunction in rat mesentery. 10 In this way, any mechanism that reduces neutrophil infiltration during the period of surgery could account for the protection from I/R injury associated with transplantation procedures. Neutrophil recruitment is the first step in the development of the inflammatory response associated with I/R, leading to alterations in the microvascular barrier function. 19 As shown in Figure 4, neutrophil accumulation in the intestine (evaluated as myeloperoxidase activity) was reduced if preconditioning was carried out or if NO donors were given before transplantation. Administration of L-NAME to preconditioned rats was able to reverse the beneficial effects of preconditioning in myeloperoxidase activity, indicating that NO can regulate neutrophil accumulation in intestinal tissue. This indicates that preconditioning attenuates one of the first steps that trigger the inflammatory response after reperfusion of the transplanted intestine.
Despite these results, the exact mechanism by which NO exerts its protective effect has not been established in the present study. Possible mechanisms of action of NO include upregulation of cGMP (cyclic guanosine monophosphate), 29,30 transient inhibition of the glycolytic enzyme GAPDH with a concomitant reduction in lactate accumulation, 31 or inhibition of xanthine oxidase activity by NO, 32 among others.
All these biochemical findings correlate well with the histopathologic results (see Table 2 and Fig. 1), which show the protection of preconditioning in the transplantation procedure.
The main objective of this study was to show the effectiveness of preconditioning in intestinal transplantation. Over a period of many years, sophisticated preservation solutions have been developed to minimize cellular damage during cold storage of solid organs. Although significant progress has been achieved, improvements of the response of the transplanted organ would be welcome. This is the case with preconditioning. Simple, surgical, and short I/R performed before cold ischemia storage improves the transplantation and is therefore a promising technique. It is important that the preconditioning is compatible with other technical improvements. In short, our aim is to complement and improve the existing situation.
In summary, our results show that ischemic preconditioning before the cold ischemic period protects intestinal grafts from cold preservation and reperfusion injury in the rat intestinal transplantation model. The fact that the protective role of ischemic preconditioning can be eliminated by the inhibition of NO synthesis also suggests that NO is involved in this protection.
Footnotes
Supported by FIS 98/002901. Anna Sola was supported by a grant from Institut d’Investigacions Biomèdiques August Pi i Sunyer.
Correspondence: Dr. Georgina Hotter, PhD, Department of Medical Bioanalysis, IIBB-CSIC-IDIBAPS, C/Roselló, 161, 7a planta, 08036 Barcelona, Spain.
E-mail: ghcbam@iibb.csic.es
Accepted for publication November 16, 2000.
References
- 1.Frezza EE, Tzakis A, Fung JJ, et al. Small bowel transplantation: current progress and clinical application. Hepato-Gastroenterology 1996; 43: 363–376. [PubMed] [Google Scholar]
- 2.Hotter G, Closa D, Prados M, et al. Intestinal preconditioning is mediated by a transient increase in nitric oxide. Biochem Biophys Res Commun 1996; 222: 27–32. [DOI] [PubMed] [Google Scholar]
- 3.De Oca J, Hotter G, Sola A, et al. Role of nitric oxide in preconditioning for intestinal transplantation. Transplant Proc 1999; 31: 2573. [DOI] [PubMed] [Google Scholar]
- 4.Karck M, Rahmanian P, Haverich A. Ischemic preconditioning enhances donor heart preservation. Transplantation 1996; 62: 17–22. [DOI] [PubMed] [Google Scholar]
- 5.Du ZY, Hicks M, Winlaw D, et al. Ischemic preconditioning enhances donor lung preservation in the rat. J. Heart Lung Transplant 1996; 15: 1258–1267. [PubMed] [Google Scholar]
- 6.Yin DP, Sankary HN, Chong ASF, et al. Protective effect of ischemic preconditioning on liver preservation reperfusion injury in rats. Transplantation 1998; 6: 152–157. [DOI] [PubMed] [Google Scholar]
- 7.Hernández LA, Grisham MB, Twohig B, et al. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol 1987; 253: H699–703. [DOI] [PubMed] [Google Scholar]
- 8.Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am. J. Physiol 1986; 250: G749–753. [DOI] [PubMed] [Google Scholar]
- 9.Akimitsu TD, Gute C, Korthuis RJ. Ischemic preconditioning attenuates postischemic leukocyte adhesion and emigration. Am J Physiol 1996; 271: H2052–2059. [DOI] [PubMed] [Google Scholar]
- 10.Kubes P, Payne D, Ostrovsky L. Preconditioning and adenosine in I/R-induced leukocyte-endothelial cell interactions. Am J Physiol 1998; 274: H1230–1238. [DOI] [PubMed] [Google Scholar]
- 11.Lefer AM, Siegfried MR, Ma X. Protection of ischemia-reperfusion injury by sydonimine NO donors via inhibition of neutrophil-endothelium interaction. J Cardiol Pharm 1992; 22: S27–31. [PubMed] [Google Scholar]
- 12.Monchik CJ, Russell PS. Transplantation of small bowel in the rat: technical and immunological considerations. Surgery 1971; 70: 693–702. [PubMed] [Google Scholar]
- 13.Hotter G, Closa D, Prats N, et al. Free radical enhancement promotes leukocyte recruitment trough a PAF and LTB4-dependent mechanism. Free Radical Biol Med 1997; 22: 947–954. [DOI] [PubMed] [Google Scholar]
- 14.Briguelius R, Muckel C, Akerboom TPM, Sies H. Identification and quantification of glutathione in hepatic protein mixed disulfides and its relationship to glutathione disulfide. Biochem Pharmacol 1983; 32: 2529–2534. [DOI] [PubMed] [Google Scholar]
- 15.Park PO, Wallander J, Tufveson G, Hadlung V. Cold ischemia and re-perfusion in a model of small bowel transplantation in the rat. Eur Surg Res 1991; 23: 18–23. [DOI] [PubMed] [Google Scholar]
- 16.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986; 74: 1124. [DOI] [PubMed] [Google Scholar]
- 17.Peralta C, Hotter G, Closa D, Gelpí E, et al. Protective effect of preconditioning on the injury associated t hepatic ischemia reperfusion in the rat: role of nitric oxide and adenosine. Hepatology 1997; 25: 934–937. [DOI] [PubMed] [Google Scholar]
- 18.Gidday JM, Shah AR, Maceren RG, et al. Nitric oxide mediates cerebral ischemic tolerance in a neonatal rat model of hypoxia preconditioning. J Cereb Blood Flow Metab 1999; 19: 331–40. [DOI] [PubMed] [Google Scholar]
- 19.Ishida T, Yarimuzu K, Gute DC, Korthuis RJ. Mechanisms of ischemic preconditioning. Shock 1997; 8: 86–94. [DOI] [PubMed] [Google Scholar]
- 20.Liu W, Pitcher DE, Morris SL, et al. Differential effects of heparin on the earl and late phases of hepatic ischemia and reperfusion injury in the pig. Shock 1999; 12: 134–138. [DOI] [PubMed] [Google Scholar]
- 21.Strasser A, Stanimirovic D, Kawai N, et al. Hypoxia modulates free radical formation in brain microvascular endothelium. Acta Neurochir Suppl 1997; 70: 8–11. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen VG, Tan S, Brix AE, et al. Hextend (hetastarch solution) decreases multiple organ injury and xanthine oxidase release after hepatoenteric ischemia-reperfusion in rabbits. Crit Care Med 1997; 25: 1565–1574. [DOI] [PubMed] [Google Scholar]
- 23.Minor T, Klauke H, Isselhard W. Assesment of intestinal integrity after ischemic preservation by luminal and vascular perfusion in vitro. Eur Surg Res 1997; 29: 246–253. [DOI] [PubMed] [Google Scholar]
- 24.Porcellini M, Renda A, Selvetella L, et al. Intestinal ischemia after aortic surgery. Int Surg 1996; 81: 195–199. [PubMed] [Google Scholar]
- 25.Thompson SS, Bragg LE, West WW. Serum enzyme levels during intestinal ischemia. Ann Surg 1990; 211: 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs DS, Robinson RH, Clarck GM, Tucker JM. Clinical significance of the isomorphic pattern on the isoenzymes of serum lactate dehydrogenase. Ann Clin Lab Sci 1997; 7: 411–421. [PubMed] [Google Scholar]
- 27.Menge H, Robinson JWL. Early phase of jejunal regeneration after short ischemia in the rat. Lab Invest 1979; 40: 25–30. [PubMed] [Google Scholar]
- 28.Sisley AC, Desai T, Harig JM, Gewertz BL. Neutrophil depletion attenuates human intestinal reperfusion injury. J Surg Res 1994; 57: 192–196. [DOI] [PubMed] [Google Scholar]
- 29.Linas SL, Repine JE. Endothelial cells regulate proximal tubule epithelial cell sodium transport. Kidney Int 1999; 55: 1251–1258. [DOI] [PubMed] [Google Scholar]
- 30.Parrat JR, Vegh A, Papp JG. Bradykinin as an endogenous myocardial protective substance with particular reference to ischemic preconditioning: a brief review of the evidence. Can J Physiol Pharmacol 1995; 73: 837–842. [DOI] [PubMed] [Google Scholar]
- 31.Sola A, Roselló-Catafau J, Alfaro V, et al. Modification of glyceraldehyde-3-phosphate dehydrogenase in response to nitric oxide in intestinal preconditioning. Transplantation 1999; 67: 1446–1452. [DOI] [PubMed] [Google Scholar]
- 32.Hassoun PM, Yu FS, Zulueta JJ, et al. Effect of nitric oxide and cell redox status on the regulation of endothelial cell xanthine dehydrogenase. Am J Physiol 1995; 268: L809–814. [DOI] [PubMed] [Google Scholar]




