Abstract
Objective
To define the incidence and manifestations of and optimal therapy for children with intravascular extension of Wilms tumor.
Methods
Children on a collaborative study of Wilms tumor who had intravascular extension into the inferior vena cava (IVC) or atrium were identified. Surgical checklists and surgical and pathology reports were reviewed.
Results
One hundred sixty-five of 2,731 patients had intravascular extension of Wilms tumor. The level of extension was IVC in 134 and atrium in 31. Sixty-nine had received preoperative therapy (55 with IVC extension and 14 with atrial extension) for a median of 8 weeks. Complications during preoperative chemotherapy were seen in five patients (tumor embolism and tumor progression in one each, and three with adult respiratory distress syndrome, one of which was fatal). The intravascular extension of the tumor regressed in 39 of 49 children with comparable pre- and posttherapy radiographic studies, including 7 of 12 in whom the tumor regressed from an atrial location, thus obviating the need for cardiopulmonary bypass. Surgical complications occurred in 36.7% of the children in the atrial group and 17.2% in the IVC group. The frequency of surgical complications was 26% in the primary resection group versus 13.2% in children with preoperative therapy. When all the complications of therapy were considered, including those that occurred during the interval of preoperative chemotherapy (one of the five also had a surgical complication), the incidence of complications among those receiving preoperative therapy was not statistically different from the incidence among those who underwent primary resection. The difference in 3-year relapse-free survival (76.9% for 165 patients with intravascular extension, 80.3% for 1,622 patients with no extension) was not statistically significant whether or not it was adjusted for stage and histology.
Conclusions
Preoperative treatment of these children may facilitate resection by decreasing the extent of the tumor thrombus, but the overall frequency of complications is similar in both groups.
Most cases of Wilms tumor, the most common renal tumor in infants and children, can be cured by current multimodal therapy. The 2-year relapse-free survival rate of children in the fourth study of the National Wilms Tumor Study Group (NWTS-4) exceeded 91%. 1 There remain, however, a few children with advanced local disease or particularly aggressive tumors that fail to respond to current therapies. Intravascular extension of Wilms tumor, reported to occur in 4.1% of patients on NWTS-3, has been associated with an increased incidence of surgical complications. 2–4 Prior reports have suggested that preoperative treatment of children with intravascular extension may be of benefit. 5–17 We reviewed the case histories of children treated on the most recent NWTS to define the incidence and manifestations of and optimal therapy for those with intravascular extension of Wilms tumor.
METHODS
Children treated on NWTS-4 who had intravascular extension into the inferior vena cava (IVC) and in some from the IVC into the atrium were identified. The surgical checklists and surgical and pathology reports were reviewed and the pertinent data were abstracted. Patients were staged according to the criteria of NWTS-4. Patients in whom the entire thrombus was removed without adhesions to the vessel wall were classified as stage 2 unless they had additional findings compatible with stage 3 disease (lymph node involvement, residual nonhematogenous tumor confined to the abdomen, or diffuse peritoneal soilage during resection). For patients who received initial chemotherapy, the evaluation of the intravascular extension of the tumor was based on their pretherapy imaging studies; those with initial surgical resection had the extent of intravascular extension based on the findings at surgery.
Statistical Analysis
Percentages of patients remaining alive or alive and continuously free of disease at 3 years from diagnosis were estimated using actuarial techniques. Relapse-free survival rates for patients with and without intravascular extension were compared using the log-rank test. Only survival information was available for historical comparisons.
The relationship between the use of preoperative chemotherapy, the tumor site (right vs. left kidney), and complication rates was examined by computation of the odds ratio and the Fisher exact test. Subgroup analyses were performed to examine the relationship between the use of preoperative therapy and complication rates separately for patients who did or did not have atrial extension. The order of treatment was not randomized, and if a bias occurred, it would be expected that it would be to treat the larger lesions with preoperative chemotherapy.
RESULTS
There were 2,731 evaluable patients with central pathology review and diagnosis of Wilms tumor enrolled on the randomized or followed portions of NWTS-4 between July 1986 and August 1995. Intravascular extension was identified by pretherapy imaging or at surgery in 165 patients (6.0%). The level of extension was the IVC in 134 and the atrium in 31. Symptoms that might have been related to the intravascular extension were identified in six patients and included hepatomegaly in three (two with ascites), dilated abdominal wall venous collaterals in two, and a varicocele in one. Various radiographic studies were used to define the intravascular extension in these children, but they were not uniformly obtained in all patients, making comparisons of the sensitivity and specificity of the methods difficult. Studies showing intravascular extension included computed tomography (66 children), ultrasonography (56 children), echocardiography (16 children), magnetic resonance imaging (13 children), and vena cavography (5 children).
Sixty-nine of these children had preoperative therapy (55 with IVC extension and 14 with atrial extension) for a median of 8.0 weeks (interquartile range 6.0–10.0). No difference in the extent of the vascular extension could be detected between children receiving initial chemotherapy and those with primary resection: the proportion between atrial and IVC extension was comparable between the two groups. Pretreatment biopsy was incisional (33 children), percutaneous (21 children), or of unknown type (6 children). Nine children were treated without an initial biopsy. Twenty-two children received two agents (dactinomycin and vincristine), 45 received three agents (dactinomycin, vincristine, and doxorubicin), and two received four agents (dactinomycin, vincristine, doxorubicin, and cyclophosphamide). In only two children was the regimen changed because of poor tumor response: doxorubicin was added to initial two-drug therapy in one child, and a change was made from initial three-drug therapy to ifosfamide and etoposide in the second. Radiotherapy was administered before resection in five patients (1,200 cGy in four and 1,050 cGy in the fifth). Complications during preoperative chemotherapy were seen in five children: one tumor embolism, one case of tumor progression, and three cases of acute respiratory distress syndrome (one of which was fatal). Regression of the intravascular extension of the tumor was documented in 39 of 49 children with comparable pre- and posttherapy studies, including 7 of 12 in whom the tumor regressed from an atrial location, thus obviating the need for cardiopulmonary bypass.
Surgical resection was performed in 164 children. One patient died of acute respiratory distress syndrome during preoperative therapy. Ninety-six patients underwent tumor resections before receiving any adjuvant therapy (79 with IVC extension and 17 with atrial extension), and cardiopulmonary bypass was used in 19 of the 96 (19.8%). Fourteen of the 19 had atrial extension and five had only IVC extension. In two patients cardiopulmonary bypass was used for removal of the thrombus, and several days later (4 and 6 days) the nephrectomy was performed. Cardiopulmonary bypass was used in only nine of the preoperative therapy patients, five with atrial extension and four with caval extension.
Resection of the tumor in the 164 children included infrahepatic caval occlusion in 82 and suprahepatic occlusion in 21. An intraoperative bypass shunt was not used in any patient. In 120 patients all gross intravascular neoplastic tissue was removed. It was adherent to the vessel wall in 75 (43.7% incidence in the primary surgery group and 61.7% in the preoperative therapy group, P = .04). Eight children required vascular resection of a portion of the caval wall, and in two a graft replacement for part of the wall was used. En bloc resection of the tumor and thrombus was achieved in only 55 patients. In 18 the intravascular extension of the tumor was not resected. Nine of these patients had received preoperative therapy. In all patients but one viable tumor was found at resection. Despite leaving tumor behind, only two children had relapse, one local and one distant.
The caval or atrial thrombus was examined in 117 patients. Viable tumor was noted in 97; totally necrotic tumor only or fibrosis was found in 17. Two had no evidence of tumor, and in one child the findings were not noted. When considered by timing of resection, the tumor was viable when examined in all 75 children with initial resection. With preoperative therapy, viable tumor was seen in 22 of the 42 patients examined (52.4%), and 17 children (40.4%) had only necrotic or fibrotic tumor. Tumor was not seen or was not noted in the remaining three patients.
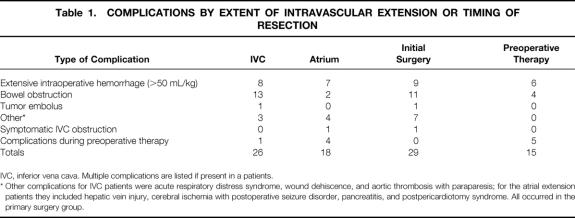
Surgical complications occurred in 23 of 134 patients (17.2%) with IVC extension and in 11 of 30 (36.7%) patients with atrial extension (P = .025). The complications are shown in Table 1. Postoperative IVC occlusion was identified by radiographic study in 14 patients (6 with primary resection and 8 with preoperative therapy), but it was symptomatic in only 1 child, who had pitting edema of the lower extremities that spontaneously resolved. There were no cases of cardiac arrest, extensive postoperative hemorrhage, or death.
Table 1. COMPLICATIONS BY EXTENT OF INTRAVASCULAR EXTENSION OR TIMING OF RESECTION
IVC, inferior vena cava. Multiple complications are listed if present in a patients.
* Other complications for IVC patients were acute respiratory distress syndrome, wound dehiscence, and aortic thrombosis with paraparesis; for the atrial extension patients they included hepatic vein injury, cerebral ischemia with postoperative seizure disorder, pancreatitis, and postpericardiotomy syndrome. All occurred in the primary surgery group.
The frequency of complications was analyzed by the timing of surgery. Complications occurred in 25 of 96 (26.0%) children with initial surgical resection and in 9 of 68 (13.2%) with preoperative chemotherapy (see Table 1). This difference was at the margins of statistical significance (P = .053). However, when the five complications that occurred during the interval of preoperative chemotherapy were included to assess all complications of therapy, the difference between 26% (25/96) for those with preoperative therapy versus 18.8% (13/69) for those with primary resection was no longer significant (odds ratio 0.66, P = .35). Subgroup analysis showed that use of preoperative therapy did not significantly decrease the frequency of complications for either the IVC group (odds ratio 0.53, 95% confidence interval 0.20–1.39, P = .20) or the atrial group (odds ratio 0.84, 95% confidence interval 0.20–3.25, P = .82). The only death occurred in a patient receiving preoperative therapy. The other major complications occurred in the primary resection group (wound dehiscence, aortic thrombosis with paraparesis, and cerebral ischemia with a postoperative seizure disorder).
Preoperative tumor rupture was noted in seven patients with IVC extension. Local spill occurred in 65 patients with IVC extension and 17 with atrial extension, and diffuse spill occurred in 12 and 7 children, respectively.
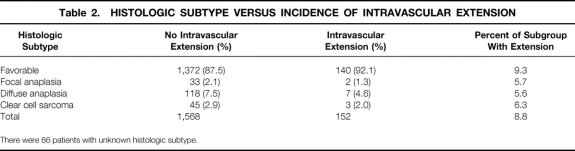
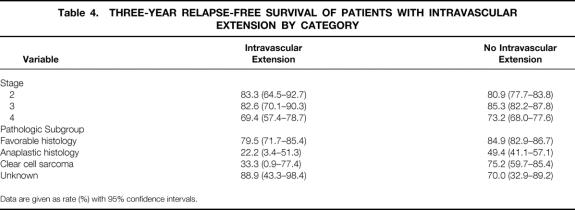
The histologic subtypes of the Wilms tumor and the incidence of intravascular extension are shown in Table 2. The frequency among patients with unfavorable histology was no greater than in patients with favorable histology (P = .12). Outcome of treatment for patients with intravascular extension, all of whom were stage 2 to 4, was compared with that of 1,621 patients without intravascular extension who had comparable stage. There was no adverse effect on the 3-year relapse-free survival rate of these 165 patients treated according to current staging criteria based on the presence of intravascular extension when considered in aggregate (Table 3 and Fig. 1; log-rank P = .32) or by stage or pathologic subgroup (Table 4). The 3-year survival rate for children with favorable histology was 90%; the rate for those with unfavorable histology was 41.7%. Analyzed by stage, the survival rates were l00%, 94.7%, and 75.3% for stages 2, 3, and 4, respectively.
Table 2. HISTOLOGIC SUBTYPE VERSUS INCIDENCE OF INTRAVASCULAR EXTENSION
There were 66 patients with unknown histologic subtype.
Table 3. THREE-YEAR RELAPSE-FREE SURVIVAL (CENSORING TOXIC DEATHS) BY INCIDENCE OF INTRAVASCULAR EXTENSION
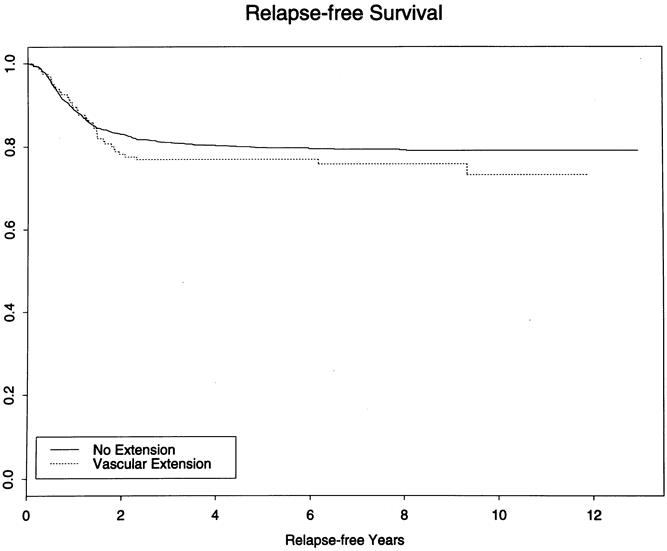
Figure 1. Kaplan-Meier relapse-free survival curves show no difference between children with or without vascular extension (P = .32).
Table 4. THREE-YEAR RELAPSE-FREE SURVIVAL OF PATIENTS WITH INTRAVASCULAR EXTENSION BY CATEGORY
Data are given as rate (%) with 95% confidence intervals.
DISCUSSION
Intravascular extension must be considered in all children with Wilms tumor because it occurs in a small but not insignificant number of children. It was seen in 4.1% and 6.0% of children treated on NWTS-3 and NWTS-4, respectively. 3 It is frequently asymptomatic, as was noted in the review of the NWTS-3 series and confirmed in this study. Current imaging modalities, particularly ultrasonography, make it possible to identify before surgery most if not all patients who have intravascular tumor extension. Magnetic resonance imaging can be used to confirm these findings if needed and to define further the extent of the tumor extension. The high incidence (63%) of intraoperative or postoperative discovery of intravascular extension reported by Nakayama et al 2 in the first three studies of the NWTS can now be avoided, along with the associated severe complications they reported.
The outcome of children with intravascular extension has been favorable. Ritchey et al 3 reported an excellent survival rate in the 77 patients with intravascular extension who were treated on NWTS-3 and found no significant difference in survival outcomes in comparison with patients without intravascular extension when patients were matched by stage and histologic type. The 3-year actuarial survival rate for children with favorable histology tumors was 86%; for unfavorable histology tumors, it was 35%. Analyzed by stage it was 88%, 89%, and 62% for stages 2, 3, and 4, respectively. This survival rate was comparable to that seen in NWTS-4, in which the 3-year survival was 90.0% for children with favorable histology tumors and 4.17% for those with unfavorable tumors. Analyzed by stage the survival was 100%, 94.7%, and 75.3% for stages 2, 3, and 4, respectively. Because the survival rate is favorable, it is critical in these patients to minimize surgical complications if possible.
Intravascular extension of Wilms tumor is associated with an increased frequency of surgical complications, with an odds ratio of 2.2 by multivariate analysis. 4 Primary nephrectomy has been recommended for patients with Wilms tumor by the NWTS, and the surgical technique for management of intravascular extension is well established. 18,19 In contrast, initial chemotherapy has been used extensively by members of the International Society of Pediatric Oncology. A decrease in the size of the intravascular extension has been reported by several authors as a result of preoperative therapy, albeit based on limited numbers of patients, 5–17 and it has been further suggested that preoperative therapy may decrease the risk of surgical complications. 3,4,11 The potential benefit of preoperative therapy must be balanced against the disadvantages, including loss of accurate staging information. In this review complications were more frequent in the children with atrial extension than in those with extension limited to the IVC. The overall incidence of complications in children with intravascular extension treated with primary surgery in this study was 26%, compared with 43% in children treated with primary resection on NWTS-3. 2 In a review of the first three NWTS studies, the surgical complication rate in children with atrial extension with primary surgery was 73%, so the frequency of surgical complications is progressively declining over time, although we cannot prove that it is due to the use of preoperative therapy. 3 The frequency of complications in the preoperative therapy patients in the current study was almost identical to that of the smaller cohort of preoperative therapy patients reported by Ritchey et al. 11 Resolution or decrease in the extent of intravascular extension was frequently seen in our cohort of patients, as was reported by Ritchey et al 11 and Mushtaq et al. 15 Postoperative occlusion of the IVC has been previously documented. 3 It is of note, however, that although postoperative occlusion of the IVC was documented in 14 children, only 1 had symptoms of lower extremity edema, and this resolved over time. This suggests that adequate retroperitoneal collaterals develop in a child to avoid significant complications.
Concern regarding dense adherence of a tumor thrombus to the cava resulting from preoperative therapy was expressed by deLorimier 18 during the early use of preoperative therapy. In this review, fibrosis of the thrombus was not seen in pathologic examination of the specimens from patients who underwent primary nephrectomy, compared with a 43% incidence in those treated with initial chemotherapy. Thrombus adherence to the vessel wall was noted in 43.7% of the primary surgery patients versus 61.7% of those treated with preoperative therapy. Removal of the residual thrombus after treatment would seem to be important, however, in that 52% of children receiving initial therapy still had viable tumor, although there was no increased incidence of relapse in the 18 patients in whom tumor thrombus remained after resection.
We have reviewed the complications of treatment among patients with intravascular extension of tumor. The differences in the rate of complications after surgery between the primary resection versus preoperative chemotherapy groups were at the margins of statistical significance. When all complications that occurred during therapy were included, there was no evidence that complications were less frequent among those who received preoperative chemotherapy, regardless of the level of intravascular extension. This issue remains complex. Although the severity of the complications in the primary nephrectomy group was of greater magnitude, the only death occurred in the preoperative chemotherapy group. In children with extensive vascular invasion, particularly into the atrium, and with large primary tumors, the best management may be primary chemotherapy and delayed resection to minimize the risk of surgical complications. Improvements in the clinical care of these children over the duration of the NWTS investigations have resulted in a progressive decline in the frequency of surgical complications. One such innovation may be the inclusion of preoperative chemotherapy in the care of selected groups of these children.
Acknowledgments
The authors thank the investigators of the Pediatric Oncology Group and the Children’s Cancer Group and the many pathologists, surgeons, pediatricians, radiation oncologists, and other health professionals who managed the children entered on the National Wilms Tumor Studies.
Footnotes
Supported in part by USPHS Grant CA-42326.
Correspondence: Robert C. Shamberger, MD, Department of Surgery, Children’s Hospital, 300 Longwood Ave., Boston, MA 02115.
E-mail: shamberger@a1.tch.harvard.edu
Accepted for publication November 21, 2000.
References
- 1.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol 1998; 16: 237–245. [DOI] [PubMed] [Google Scholar]
- 2.Nakayama DK, DeLorimier AA, O’Neill JA Jr, et al. Intracardiac extension of Wilms’ tumor: a report of the National Wilms’ Tumor Study. Ann Surg 1986; 204: 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchey ML, Kelalis PP, Breslow N, et al. Intracaval and atrial involvement with nephroblastoma: review of National Wilms’ Tumor Study 3. J Urol 1988; 140: 1113–1118. [DOI] [PubMed] [Google Scholar]
- 4.Ritchey ML, Kelalis PP, Breslow N, et al. Surgical complications after nephrectomy for Wilms’ tumor. Surg Gynecol Obstet 1992; 175: 507–514. [PubMed] [Google Scholar]
- 5.Wagget J, Koop CE. Wilms’ tumor: preoperative radiotherapy and chemotherapy in the management of massive tumors. Cancer 1970; 26: 338–340. [DOI] [PubMed] [Google Scholar]
- 6.Bracken RB, Sutow WW, Jaffe N, et al. Preoperative chemotherapy for Wilms tumor. Urology 1982; 19: 55–60. [DOI] [PubMed] [Google Scholar]
- 7.Bray GL, Pendergrass TW, Schaller RT Jr, et al. Preoperative chemotherapy in the treatment of Wilms’ tumor diagnosed with the aid of fine needle aspiration biopsy. Am J Pediatr Hematol Oncol 1986; 8: 75–78. [PubMed] [Google Scholar]
- 8.Kogan SJ, Marans H, Santorineau M, et al. Successful treatment of renal vein and vena caval extension of nephroblastoma by preoperative chemotherapy. J Urol 1986; 136: 312–317. [DOI] [PubMed] [Google Scholar]
- 9.Oberholzer HF, Falkson G, DeJager LC. Successful management of inferior vena cava and right atrial nephroblastoma tumor thrombus with preoperative chemotherapy. Med Pediatr Oncol 1992; 20: 61–63. [DOI] [PubMed] [Google Scholar]
- 10.Thompson WR, Newman K, Seibel N, et al. A strategy for resection of Wilms’ tumor with vena cava or atrial extension. J Pediatr Surg 1992; 27: 912–915. [DOI] [PubMed] [Google Scholar]
- 11.Ritchey ML, Kelalis PP, Haase GM, et al. Preoperative therapy for intracaval and atrial extension of Wilms tumor. Cancer 1993; 71: 4104–4110. [DOI] [PubMed] [Google Scholar]
- 12.Habib F, McLorie A, McKenna PH, et al. Effectiveness of preoperative chemotherapy in the treatment of Wilms tumor with vena caval and intracardiac extension. J Urol 1993; 150: 933–935. [DOI] [PubMed] [Google Scholar]
- 13.Lee ACW, Saing H, Leung MP, et al. Wilms’ tumor with intracardiac extension: chemotherapy before surgery. Pediatr Hematol Oncol 1994; 11: 535–540. [DOI] [PubMed] [Google Scholar]
- 14.Crombleholme TM, Jacir NN, Rosenfield CG, et al. Preoperativechemotherapy in the management of intracaval extension of Wilms’ tumor. J Pediatr Surg 1994; 29: 229–231. [DOI] [PubMed] [Google Scholar]
- 15.Mushtaq I, Carachi R, Roy G, et al. Childhood renal tumours with intravascular extension. Br J Urol 1996; 78: 772–776. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Ibanez V, Sanchez de Toledo J, De Diego M, et al. Wilms’ tumours with intracaval involvement. Med Pediatr Oncol 1996; 26: 268–271. [DOI] [PubMed] [Google Scholar]
- 17.Berberoglu S, Akyuz C, Buyukpamukcu M. Successful treatment of intracaval and atrial extension of Wilms’ tumour by chemotherapy. Postgrad Med J 1996; 72: 749–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLorimier AA. Surgical treatment of Wilms’ tumor. In: Pochedly C, Miller D, eds. Wilms’ tumor. New York: John Wiley & Sons; 1976: 167–188.
- 19.Grosfeld JL, Weber TR. Surgical considerations in the treatment of Wilms’ tumor. In: Gonzalez-Crussi F, ed. Wilms’ tumor (nephroblastoma) and related renal neoplasms of childhood. Boca Raton, FL: CRC Press, Inc; 1984: 263–283.