Abstract
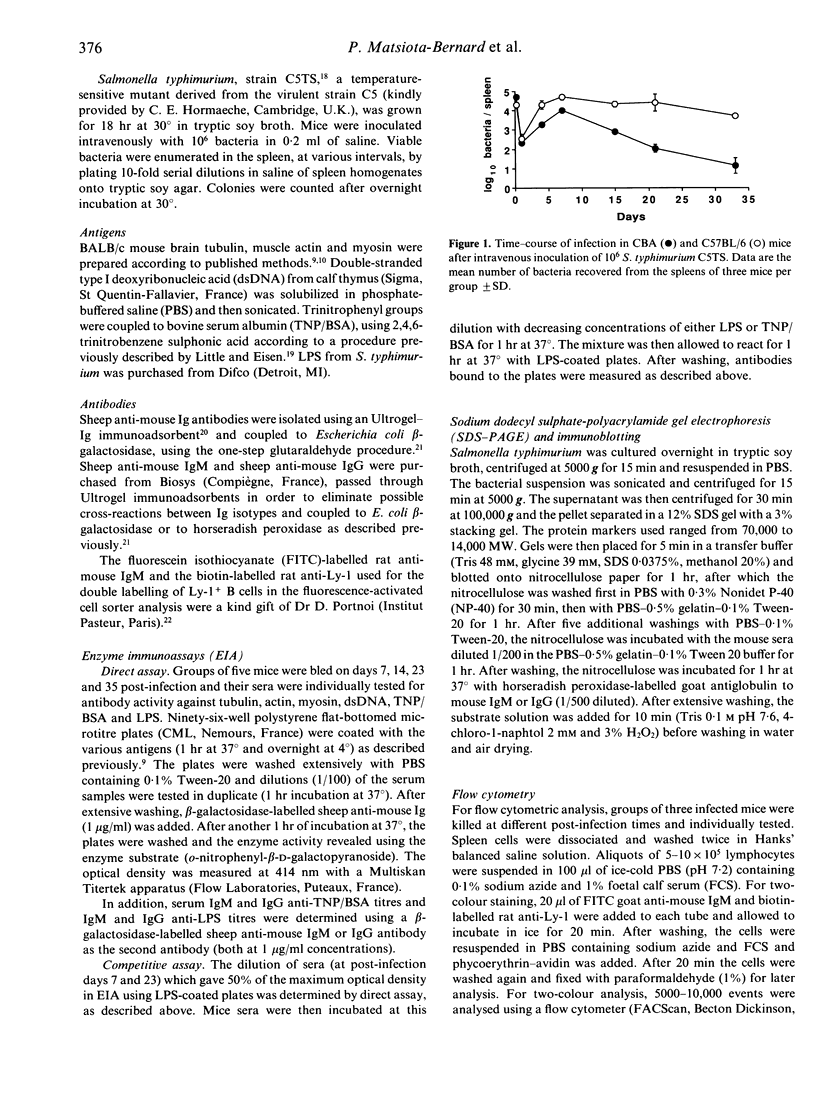
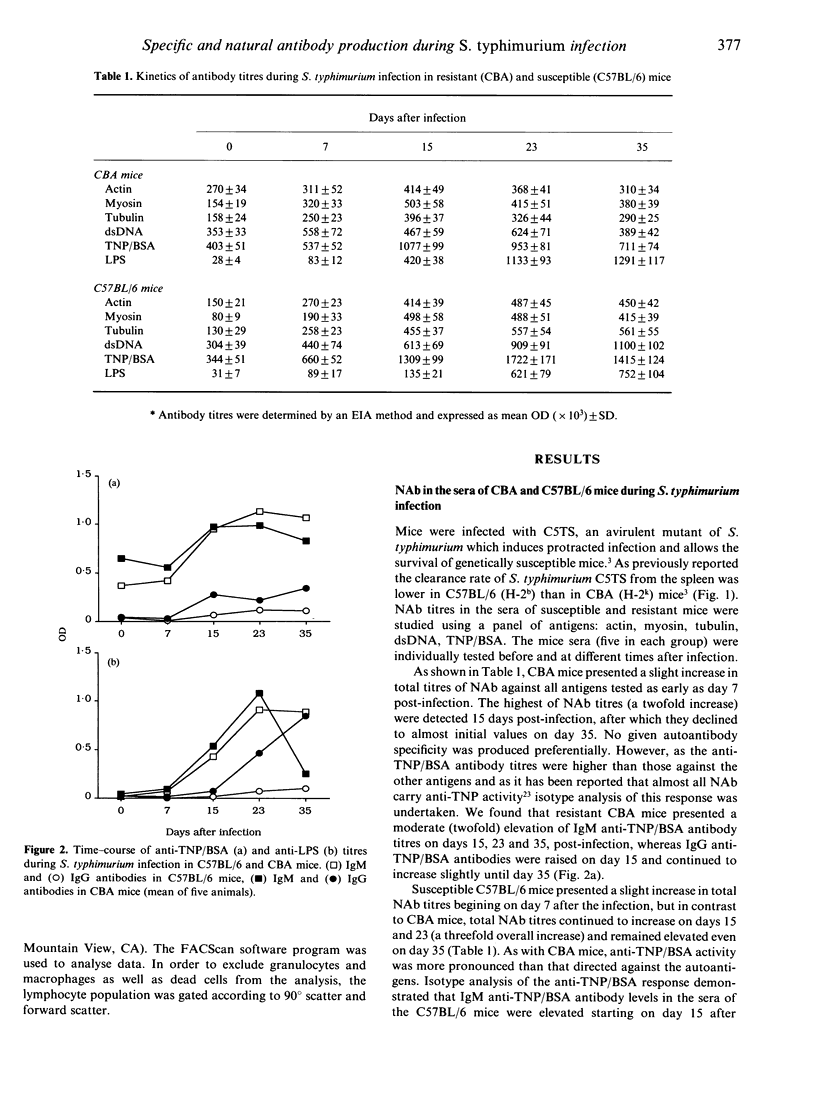
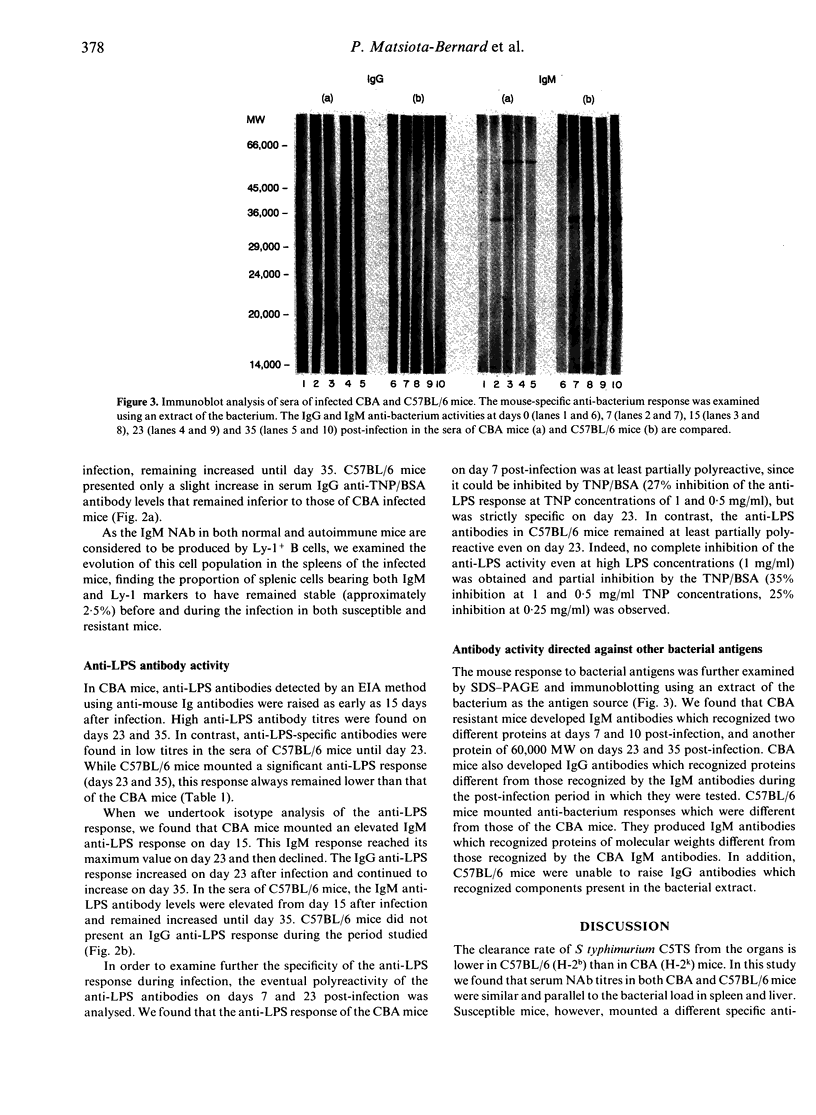
Genetically susceptible (C57BL/6) and resistant (CBA) mice were infected with an avirulent strain of Salmonella typhimurium and studied over a 35-day period for the production of antibodies directed against bacterial antigens including lipopolysaccharide (LPS) (specific antibodies) and antibodies directed against self antigens [natural antibodies (NAb)]. Antibodies directed against LPS and self antigens were detected by enzyme immunoassay (EIA) and those directed against other bacterial antigens by immunoblotting. We found that serum natural antibody titres in C57BL/6 and CBA mice were similar and correlated with the bacterial load in the spleen and liver. In C57BL/6 mice, anti-LPS antibodies remained polyreactive and of the IgM isotype. In contrast, CBA mice, after an early increase in polyreactive IgM anti-LPS antibodies, mounted a specific anti-LPS IgG antibody response. The immunoblotting results demonstrated that the IgM polyreactive antibodies in the resistant and susceptible mice recognized bacterial antigens of different molecular weights and that CBA, but not C57BL/6 mice, were able to produce IgG antibodies recognizing bacterial components. Our results suggest that the synthesis of antibodies directed against bacterial antigens and natural antibodies follow, at least partially, distinct pathways, but they do not allow us to determine whether these two antibody populations are produced by the same or distinct B-cell subpopulations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Natural autoantibodies: from 'horror autotoxicus' to 'gnothi seauton'. Immunol Today. 1991 May;12(5):154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- Barbouche R., Forveille M., Fischer A., Avrameas S., Durandy A. Spontaneous IgM autoantibody production in vitro by B lymphocytes of normal human neonates. Scand J Immunol. 1992 Jun;35(6):659–667. doi: 10.1111/j.1365-3083.1992.tb02972.x. [DOI] [PubMed] [Google Scholar]
- Boyden S. V. Natural antibodies and the immune response. Adv Immunol. 1966;5:1–28. doi: 10.1016/s0065-2776(08)60271-0. [DOI] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. CD5+ B lymphocytes, polyreactive antibodies and the human B-cell repertoire. Immunol Today. 1989 Nov;10(11):364–368. doi: 10.1016/0167-5699(89)90268-5. [DOI] [PubMed] [Google Scholar]
- Deschenes M., Guenounou M., Ronco E., Vacheron F., Nauciel C. Impairment of lymphocyte proliferative responses and interleukin-2 production in susceptible (C57BL/6) mice infected with Salmonella typhimurium. Immunology. 1986 Jun;58(2):225–230. [PMC free article] [PubMed] [Google Scholar]
- Deschênes M., Guenounou M., Nauciel C. Suppression of primary antibody response in genetically susceptible mice infected with Salmonella typhimurium: restoration by catalase. Res Immunol. 1989 Jan;140(1):55–65. doi: 10.1016/0923-2494(89)90006-0. [DOI] [PubMed] [Google Scholar]
- Dighiero G., Guilbert B., Fermand J. P., Lymberi P., Danon F., Avrameas S. Thirty-six human monoclonal immunoglobulins with antibody activity against cytoskeleton proteins, thyroglobulin, and native DNA: immunologic studies and clinical correlations. Blood. 1983 Aug;62(2):264–270. [PubMed] [Google Scholar]
- Eisenstein T. K., Sultzer B. M. Immunity to Salmonella infection. Adv Exp Med Biol. 1983;162:261–296. doi: 10.1007/978-1-4684-4481-0_26. [DOI] [PubMed] [Google Scholar]
- Guilbert B., Dighiero G., Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982 Jun;128(6):2779–2787. [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E., Pettifor R. A., Brock J. The fate of temperature-sensitive salmonella mutants in vivo in naturally resistant and susceptible mice. Immunology. 1981 Apr;42(4):569–576. [PMC free article] [PubMed] [Google Scholar]
- Mackenzie L. E., Mageed R. A., Youinou P. Y., Yuksel B., Jefferis R., Lydyard P. M. Repertoire of CD5+ and CD5- cord blood B cells: specificity and expression of VH I and VH III associated idiotopes. Clin Exp Immunol. 1992 Apr;88(1):107–111. doi: 10.1111/j.1365-2249.1992.tb03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsiota P., Druet P., Dosquet P., Guilbert B., Avrameas S. Natural autoantibodies in systemic lupus erythematosus. Clin Exp Immunol. 1987 Jul;69(1):79–88. [PMC free article] [PubMed] [Google Scholar]
- Nauciel C., Ronco E., Guenet J. L. Genetic control of Salmonella typhimurium-induced depression of delayed-type hypersensitivity to sheep erythrocytes in mice. Infect Immun. 1988 Feb;56(2):310–313. doi: 10.1128/iai.56.2.310-313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C., Ronco E., Guenet J. L., Pla M. Role of H-2 and non-H-2 genes in control of bacterial clearance from the spleen in Salmonella typhimurium-infected mice. Infect Immun. 1988 Sep;56(9):2407–2411. doi: 10.1128/iai.56.9.2407-2411.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navin T. R., Krug E. C., Pearson R. D. Effect of immunoglobulin M from normal human serum on Leishmania donovani promastigote agglutination, complement-mediated killing, and phagocytosis by human monocytes. Infect Immun. 1989 Apr;57(4):1343–1346. doi: 10.1128/iai.57.4.1343-1346.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D. Influence of host genes on resistance of inbred mice to lethal infection with Salmonella typhimurium. Curr Top Microbiol Immunol. 1986;124:37–48. [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Thomas-Vaslin V., Coutinho A., Huetz F. Origin of CD5+ B cells and natural IgM-secreting cells: reconstitution potential of adult bone marrow, spleen and peritoneal cells. Eur J Immunol. 1992 May;22(5):1243–1251. doi: 10.1002/eji.1830220520. [DOI] [PubMed] [Google Scholar]
- al-Ramadi B. K., Brodkin M. A., Mosser D. M., Eisenstein T. K. Immunosuppression induced by attenuated Salmonella. Evidence for mediation by macrophage precursors. J Immunol. 1991 Apr 15;146(8):2737–2746. [PubMed] [Google Scholar]
- al-Ramadi B. K., Chen Y. W., Meissler J. J., Jr, Eisenstein T. K. Immunosuppression induced by attenuated Salmonella. Reversal by IL-4. J Immunol. 1991 Sep 15;147(6):1954–1961. [PubMed] [Google Scholar]



