Abstract
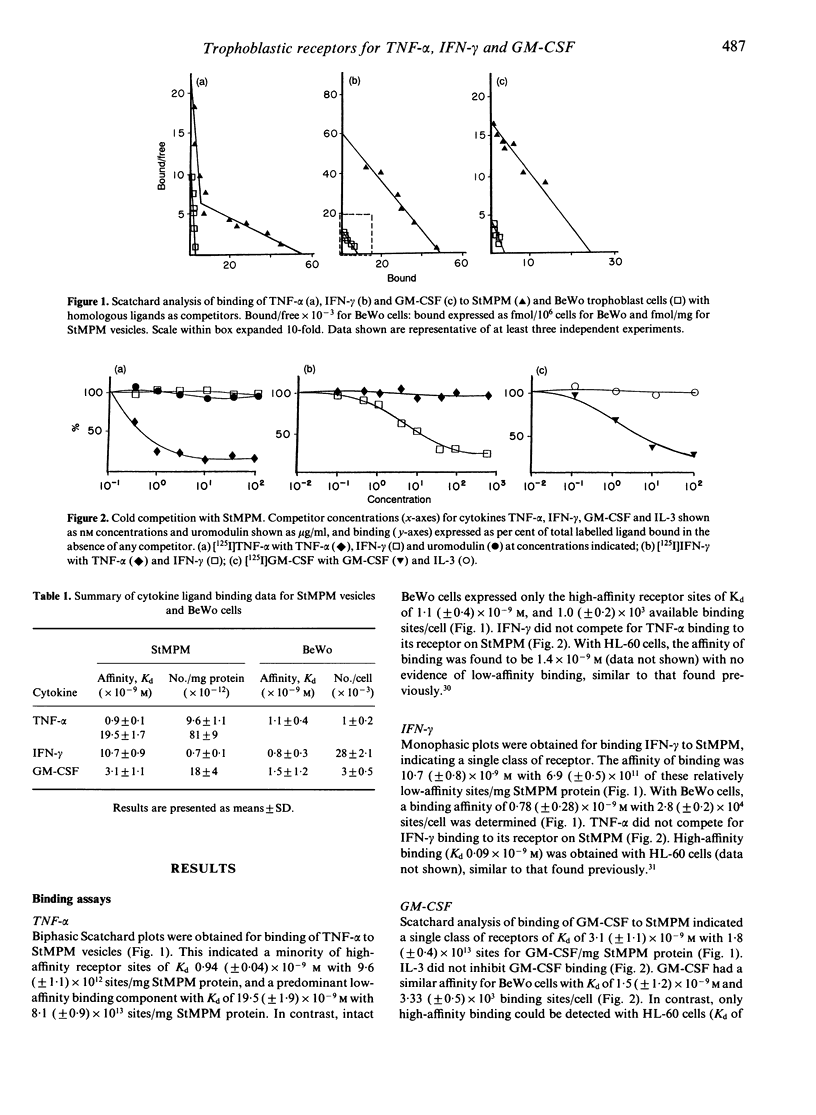
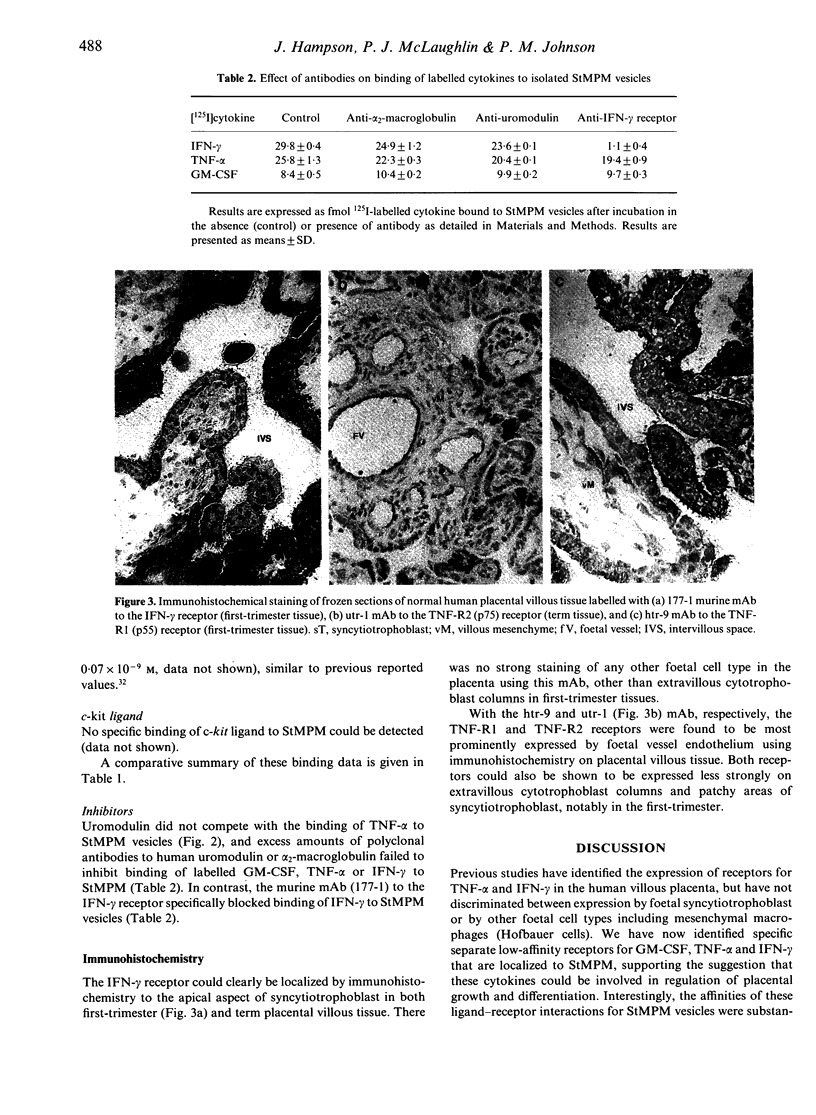
Scatchard binding analysis has been employed to characterize expression of low-affinity receptors for tumour necrosis factor-alpha (TNF-alpha), interferon-gamma (IFN-gamma) and granulocyte-macrophage colony-stimulating factor (GM-CSF) by isolated human placental syncytiotrophoblast microvillous plasma membrane (StMPM) vesicles. Trophoblastic receptors for the c-kit ligand (stem cell factor) could not be identified using the same methods. No high-affinity receptors could be detected for GM-CSF or IFN-gamma, but a minority of high-affinity TNF-alpha receptors were identified. Cross-inhibition studies indicated the low-affinity receptors to be specific for each cytokine rather than to be non-specific cytokine-binding factors. Only relatively high-affinity receptors for TNF-alpha and IFN-gamma could be detected on the BeWo human choriocarcinoma cytotrophoblast cell line, whereas receptor affinity for GM-CSF was similar to that on syncytiotrophoblast. Immunohistochemical staining has confirmed expression of IFN-gamma receptor by syncytiotrophoblast: in contrast, staining for the established TNF-R1 and TNF-R2 receptors was associated mainly with placental vascular endothelium. These low-affinity cytokine receptors could reflect unique biological responses of foetal syncytiotrophoblast in the presence of local concentrations of maternal cytokine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Eessalu T. E., Hass P. E. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985 Dec 19;318(6047):665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Aiyer R. A., Aggarwal B. B. Characterization of receptors for recombinant human tumor necrosis factor-alpha from human placental membranes. Lymphokine Res. 1990 Fall;9(3):333–344. [PubMed] [Google Scholar]
- Anderson D. J., Berkowitz R. S. Gamma-interferon enhances expression of Class I MHC antigens in the weakly HLA+ human choriocarcinoma cell line BeWo, but does not induce MHC expression in the HLA- choriocarcinoma cell line Jar. J Immunol. 1985 Oct;135(4):2498–2501. [PubMed] [Google Scholar]
- Arceci R. J., Shanahan F., Stanley E. R., Pollard J. W. Temporal expression and location of colony-stimulating factor 1 (CSF-1) and its receptor in the female reproductive tract are consistent with CSF-1-regulated placental development. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8818–8822. doi: 10.1073/pnas.86.22.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz R. S., Hill J. A., Kurtz C. B., Anderson D. J. Effects of products of activated leukocytes (lymphokines and monokines) on the growth of malignant trophoblast cells in vitro. Am J Obstet Gynecol. 1988 Jan;158(1):199–203. doi: 10.1016/0002-9378(88)90810-1. [DOI] [PubMed] [Google Scholar]
- Borth W., Luger T. A. Identification of alpha 2-macroglobulin as a cytokine binding plasma protein. Binding of interleukin-1 beta to "F" alpha 2-macroglobulin. J Biol Chem. 1989 Apr 5;264(10):5818–5825. [PubMed] [Google Scholar]
- Brockhaus M., Schoenfeld H. J., Schlaeger E. J., Hunziker W., Lesslauer W., Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3127–3131. doi: 10.1073/pnas.87.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer J. N., Morrison L., Johnson P. M., Meager A. Immunohistochemical localization of interferons in human placental tissues in normal, ectopic, and molar pregnancy. Am J Reprod Immunol. 1990 Mar-Apr;22(3-4):109–116. doi: 10.1111/j.1600-0897.1990.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Calderon J., Sheehan K. C., Chance C., Thomas M. L., Schreiber R. D. Purification and characterization of the human interferon-gamma receptor from placenta. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4837–4841. doi: 10.1073/pnas.85.13.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. L., Yang Y. P., Hu X. L., Yelavarthi K. K., Fishback J. L., Hunt J. S. Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol. 1991 Aug;139(2):327–335. [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Shibuya K., Piao Y. F., Tojo A., Sasaki N., Matsuki S., Miyagawa K., Miyazono K., Takaku F. Identification and cellular distribution of distinct proteins forming human GM-CSF receptor. Cell Regul. 1990 Mar;1(4):327–335. doi: 10.1091/mbc.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielpour D., Sporn M. B. Differential inhibition of transforming growth factor beta 1 and beta 2 activity by alpha 2-macroglobulin. J Biol Chem. 1990 Apr 25;265(12):6973–6977. [PubMed] [Google Scholar]
- DiPersio J., Billing P., Kaufman S., Eghtesady P., Williams R. E., Gasson J. C. Characterization of the human granulocyte-macrophage colony-stimulating factor receptor. J Biol Chem. 1988 Feb 5;263(4):1834–1841. [PubMed] [Google Scholar]
- Eades D. K., Cornelius P., Pekala P. H. Characterization of the tumour necrosis factor receptor in human placenta. Placenta. 1988 May-Jun;9(3):247–251. doi: 10.1016/0143-4004(88)90032-x. [DOI] [PubMed] [Google Scholar]
- Fulop V., Steller M. A., Berkowitz R. S., Anderson D. J. Interferon-gamma receptors on human gestational choriocarcinoma cell lines: quantitative and functional studies. Am J Obstet Gynecol. 1992 Aug;167(2):524–530. doi: 10.1016/s0002-9378(11)91448-3. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesner T. G., Mufson R. A., Norton C. R., Turner K. J., Yang Y. C., Clark S. C. Specific binding, internalization, and degradation of human recombinant interleukin-3 by cells of the acute myelogenous, leukemia line, KG-1. J Cell Physiol. 1988 Sep;136(3):493–499. doi: 10.1002/jcp.1041360314. [DOI] [PubMed] [Google Scholar]
- Hayakawa M., Hori T., Shibamoto S., Tsujimoto M., Oku N., Ito F. Solubilization of human placental tumor necrosis factor receptors as a complex with a guanine nucleotide-binding protein. Arch Biochem Biophys. 1991 May 1;286(2):323–329. doi: 10.1016/0003-9861(91)90047-m. [DOI] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. A., Haimovici F., Anderson D. J. Products of activated lymphocytes and macrophages inhibit mouse embryo development in vitro. J Immunol. 1987 Oct 1;139(7):2250–2254. [PubMed] [Google Scholar]
- Hohmann H. P., Brockhaus M., Baeuerle P. A., Remy R., Kolbeck R., van Loon A. P. Expression of the types A and B tumor necrosis factor (TNF) receptors is independently regulated, and both receptors mediate activation of the transcription factor NF-kappa B. TNF alpha is not needed for induction of a biological effect via TNF receptors. J Biol Chem. 1990 Dec 25;265(36):22409–22417. [PubMed] [Google Scholar]
- Hunt J. S. Current topic: the role of macrophages in the uterine response to pregnancy. Placenta. 1990 Nov-Dec;11(6):467–475. doi: 10.1016/s0143-4004(05)80192-4. [DOI] [PubMed] [Google Scholar]
- Johnson P. M., Arnaud P., Werner P., Galbraith R. M. Native alpha 2-macroglobulin binds to a surface component of human placental trophoblast. Placenta. 1985 Jul-Aug;6(4):323–328. doi: 10.1016/s0143-4004(85)80041-2. [DOI] [PubMed] [Google Scholar]
- Johnson P. M., Ogbimi A. O., Brown P. J., Shah L. C. Antigens of the human syncytiotrophoblast microvillus plasma membrane. Am J Reprod Immunol. 1981;1(2):83–87. doi: 10.1111/j.1600-0897.1981.tb00022.x. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Zsebo K. M., Hogan B. L. Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature. 1990 Oct 18;347(6294):667–669. doi: 10.1038/347667a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin P. J., Aikawa A., Davies H. M., Ward R. G., Bakran A., Sells R. A., Johnson P. M. Uromodulin levels are decreased in urine during acute tubular necrosis but not during immune rejection after renal transplantation. Clin Sci (Lond) 1993 Feb;84(2):243–246. doi: 10.1042/cs0840243. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A., Gearing D. P., Gough N. M. Low-affinity placenta-derived receptors for human granulocyte-macrophage colony-stimulating factor can deliver a proliferative signal to murine hemopoietic cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4670–4674. doi: 10.1073/pnas.87.12.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motro B., van der Kooy D., Rossant J., Reith A., Bernstein A. Contiguous patterns of c-kit and steel expression: analysis of mutations at the W and Sl loci. Development. 1991 Dec;113(4):1207–1221. doi: 10.1242/dev.113.4.1207. [DOI] [PubMed] [Google Scholar]
- Munker R., DiPersio J., Koeffler H. P. Tumor necrosis factor: receptors on hematopoietic cells. Blood. 1987 Dec;70(6):1730–1734. [PubMed] [Google Scholar]
- Novick D., Fischer D. G., Reiter Z., Eshhar Z., Rubinstein M. Monoclonal antibodies to the human interferon-gamma receptor: blocking of the biological activities of interferon-gamma and purification of the receptor. J Interferon Res. 1989 Jun;9(3):315–328. doi: 10.1089/jir.1989.9.315. [DOI] [PubMed] [Google Scholar]
- Ogbimi A. O., Johnson P. M., Brown P. J., Fox H. Characterisation of the soluble fraction of human syncytiotrophoblast microvillous plasma membrane-associated proteins. J Reprod Immunol. 1979 Jul;1(2):127–140. doi: 10.1016/0165-0378(79)90013-5. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Sacca R., Furman W. L., Roussel M. F., Holt J. T., Nienhuis A. W., Stanley E. R., Sherr C. J. Expression of the human c-fms proto-oncogene product (colony-stimulating factor-1 receptor) on peripheral blood mononuclear cells and choriocarcinoma cell lines. J Clin Invest. 1986 Jun;77(6):1740–1746. doi: 10.1172/JCI112496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. M., Taylor C. T., Melling G. C., Kingsland C. R., Johnson P. M. Expression of the CD46 antigen, and absence of class I MHC antigen, on the human oocyte and preimplantation blastocyst. Immunology. 1992 Jan;75(1):202–205. [PMC free article] [PubMed] [Google Scholar]
- Robertson S. A., Mayrhofer G., Seamark R. F. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol Reprod. 1992 Jun;46(6):1069–1079. doi: 10.1095/biolreprod46.6.1069. [DOI] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Sullivan S. A., Williamson B. D., Carswell E. A., Old L. J. High affinity binding of 125I-labeled human tumor necrosis factor (LuKII) to specific cell surface receptors. J Exp Med. 1985 Sep 1;162(3):1099–1104. doi: 10.1084/jem.162.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherblom A. P., Sathyamoorthy N., Decker J. M., Muchmore A. V. IL-2, a lectin with specificity for high mannose glycopeptides. J Immunol. 1989 Aug 1;143(3):939–944. [PubMed] [Google Scholar]
- Tabibzadeh S. Ubiquitous expression of TNF-alpha/cachectin immunoreactivity in human endometrium. Am J Reprod Immunol. 1991 Aug;26(1):1–4. doi: 10.1111/j.1600-0897.1991.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Uzumaki H., Okabe T., Sasaki N., Hagiwara K., Takaku F., Tobita M., Yasukawa K., Ito S., Umezawa Y. Identification and characterization of receptors for granulocyte colony-stimulating factor on human placenta and trophoblastic cells. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9323–9326. doi: 10.1073/pnas.86.23.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann T. G., Athanassakis I., Guilbert L., Branch D., Dy M., Menu E., Chaouat G. The role of M-CSF and GM-CSF in fostering placental growth, fetal growth, and fetal survival. Transplant Proc. 1989 Feb;21(1 Pt 1):566–568. [PubMed] [Google Scholar]




