Abstract
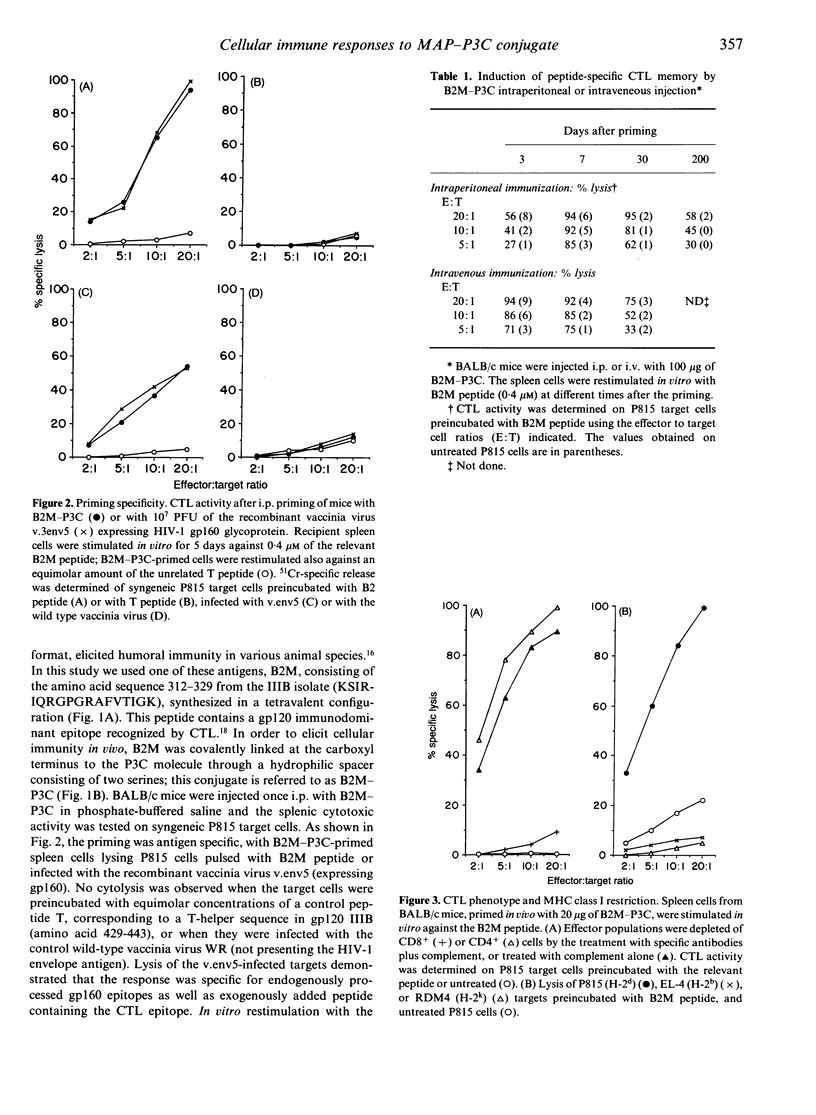
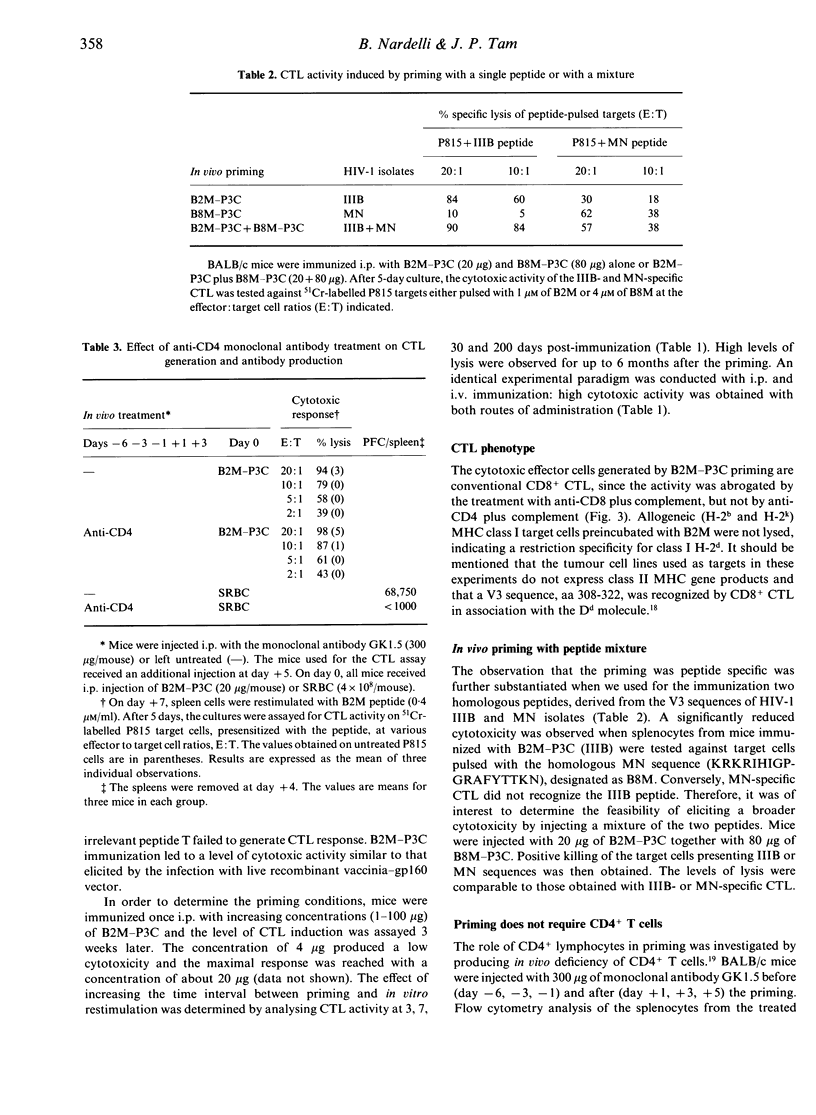
This report investigates the generation of cytotoxic T lymphocytes (CTL) by in vivo administration of a synthetic antigen linked to a lipid moiety, tripalmitoyl-S-glyceryl cysteine (P3C). The antigen consisted of a 17-mer peptide, derived from the gp120 envelope protein of the human immunodeficiency virus type-1 (HIV-1), in a tetravalent multiple antigenic peptide (MAP) configuration. A single injection of MAP-P3C elicited a long-lasting CTL response in mice. The route of administration was not a determining factor, since intravenous (i.v.) and intraperitoneal (i.p.) priming were both effective. The HIV strain-specific cytotoxic lymphocytes were of the CD8+ subset and class I restricted. A broad cytolytic activity could be achieved by priming with a mixture of homologous peptides from gp120 IIIB and MN strains. Following the administration of the monoclonal antibody GK1.5, resulting in the depletion of the CD4+ T-lymphocyte subpopulation, mice were able to mount a strong CTL response. This finding demonstrates that in priming with a peptide antigen covalently linked to a lipid, such as MAP-P3C, CD4+ cells are not required for the generation of CD8+ cytotoxicity. In contrast, the elimination of macrophages by the carrageenan pretreatment caused suppression of the T-cell lytic activity, suggesting a substantial contribution of the phagocytic cells in mounting CTL response. Taken together, these results may lead to new strategies in designing a human immunodeficiency virus type-1 (HIV-1) vaccine based on synthetic peptides.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braciale T. J., Braciale V. L. Antigen presentation: structural themes and functional variations. Immunol Today. 1991 Apr;12(4):124–129. doi: 10.1016/0167-5699(91)90096-C. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Holmes K. L., Hügin A., Frederickson T. N., Morse H. C., 3rd Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987 Jul 2;328(6125):77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984 Sep;51(3):682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone F. R., Bevan M. J. Class I-restricted processing and presentation of exogenous cell-associated antigen in vivo. J Exp Med. 1990 Feb 1;171(2):377–387. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D. S., Findlay K., Harding C. V. Processing of exogenous liposome-encapsulated antigens in vivo generates class I MHC-restricted T cell responses. J Immunol. 1992 Jun 1;148(11):3336–3341. [PubMed] [Google Scholar]
- Debrick J. E., Campbell P. A., Staerz U. D. Macrophages as accessory cells for class I MHC-restricted immune responses. J Immunol. 1991 Nov 1;147(9):2846–2851. [PubMed] [Google Scholar]
- Defoort J. P., Nardelli B., Huang W., Ho D. D., Tam J. P. Macromolecular assemblage in the design of a synthetic AIDS vaccine. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3879–3883. doi: 10.1073/pnas.89.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Fayolle C., Deriaud E., Leclerc C. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol. 1991 Dec 15;147(12):4069–4073. [PubMed] [Google Scholar]
- Hart M. K., Weinhold K. J., Scearce R. M., Washburn E. M., Clark C. A., Palker T. J., Haynes B. F. Priming of anti-human immunodeficiency virus (HIV) CD8+ cytotoxic T cells in vivo by carrier-free HIV synthetic peptides. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9448–9452. doi: 10.1073/pnas.88.21.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschildt S., Hoffmann P., Beuscher H. U., Dufhues G., Heinrich P., Wiesmüller K. H., Jung G., Bessler W. G. Activation of bone marrow-derived mouse macrophages by bacterial lipopeptide: cytokine production, phagocytosis and Ia expression. Eur J Immunol. 1990 Jan;20(1):63–68. doi: 10.1002/eji.1830200110. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Henry C., Nordin A. A., Fuji H., Koros A. M., Lefkovits I. Plaque forming cells: methodology and theory. Transplant Rev. 1974;18:130–191. doi: 10.1111/j.1600-065x.1974.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Kabanov A. V., Levashov A. V., Alakhov VYu Lipid modification of proteins and their membrane transport. Protein Eng. 1989 Oct;3(1):39–42. doi: 10.1093/protein/3.1.39. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Roux L., Curren J., Blom H. J., Voordouw A. C., Meloen R. H., Kolakofsky D., Melief C. J. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2283–2287. doi: 10.1073/pnas.88.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuochi T., Hügin A. W., Morse H. C., 3rd, Singer A., Buller R. M. Role of lymphokine-secreting CD8+ T cells in cytotoxic T lymphocyte responses against vaccinia virus. J Immunol. 1989 Jan 1;142(1):270–273. [PubMed] [Google Scholar]
- Moldwin R. L., Lancki D. W., Herold K. C., Fitch F. W. An antigen receptor-driven, interleukin 2-independent pathway for proliferation of murine cytolytic T lymphocyte clones. J Exp Med. 1986 Jun 1;163(6):1566–1582. doi: 10.1084/jem.163.6.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli B., Defoort J. P., Huang W., Tam J. P. Design of a complete synthetic peptide-based AIDS vaccine with a built-in adjuvant. AIDS Res Hum Retroviruses. 1992 Aug;8(8):1405–1407. doi: 10.1089/aid.1992.8.1405. [DOI] [PubMed] [Google Scholar]
- Nardelli B., Lu Y. A., Shiu D. R., Delpierre-Defoort C., Profy A. T., Tam J. P. A chemically defined synthetic vaccine model for HIV-1. J Immunol. 1992 Feb 1;148(3):914–920. [PubMed] [Google Scholar]
- Raychaudhuri S., Tonks M., Carbone F., Ryskamp T., Morrow W. J., Hanna N. Induction of antigen-specific class I-restricted cytotoxic T cells by soluble proteins in vivo. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8308–8312. doi: 10.1073/pnas.89.17.8308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitermann A., Metzger J., Wiesmüller K. H., Jung G., Bessler W. G. Lipopeptide derivatives of bacterial lipoprotein constitute potent immune adjuvants combined with or covalently coupled to antigen or hapten. Biol Chem Hoppe Seyler. 1989 Apr;370(4):343–352. doi: 10.1515/bchm3.1989.370.1.343. [DOI] [PubMed] [Google Scholar]
- Riddell S. R., Watanabe K. S., Goodrich J. M., Li C. R., Agha M. E., Greenberg P. D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992 Jul 10;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Gamble S., Rothstein L. Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science. 1990 Aug 24;249(4971):918–921. doi: 10.1126/science.2392683. [DOI] [PubMed] [Google Scholar]
- Schulz M., Zinkernagel R. M., Hengartner H. Peptide-induced antiviral protection by cytotoxic T cells. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):991–993. doi: 10.1073/pnas.88.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek P. N., Lukovich S. The role of macrophages in promoting the antibody response mediated by liposome-associated protein antigens. Immunol Lett. 1982;5(6):305–309. doi: 10.1016/0165-2478(82)90118-3. [DOI] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Capacity of purified Lyt-2+ T cells to mount primary proliferative and cytotoxic responses to Ia- tumour cells. Nature. 1986 Aug 7;322(6079):541–544. doi: 10.1038/322541a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Cohen J., Hosmalin A., Cease K. B., Houghten R., Cornette J. L., DeLisi C., Moss B., Germain R. N., Berzofsky J. A. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3105–3109. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Germain R. N., Moss B., Berzofsky J. A. An immunodominant class I-restricted cytotoxic T lymphocyte determinant of human immunodeficiency virus type 1 induces CD4 class II-restricted help for itself. J Exp Med. 1990 Feb 1;171(2):571–576. doi: 10.1084/jem.171.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Takeshita T., Morein B., Putney S., Germain R. N., Berzofsky J. A. Induction of CD8+ cytotoxic T cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature. 1990 Apr 26;344(6269):873–875. doi: 10.1038/344873a0. [DOI] [PubMed] [Google Scholar]
- Taylor P. M., Askonas B. A. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986 Jul;58(3):417–420. [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Tsubota H., Lord C. I., Watkins D. I., Morimoto C., Letvin N. L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989 Apr 1;169(4):1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl B., Wolf B., Schwinde A., Metzger J., Jung G., Bessler W. G., Hauschildt S. Intracellular localization of a lipopeptide macrophage activator: immunocytochemical investigations and EELS analysis on ultrathin cryosections of bone marrow-derived macrophages. J Leukoc Biol. 1991 Jul;50(1):10–18. doi: 10.1002/jlb.50.1.10. [DOI] [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. M., Herscowitz H. B. Immune activation by T-independent antigens: lack of effect of macrophage depletion on the immune response to TNP-LPS, PVP and dextran. Immunology. 1979 Aug;37(4):765–775. [PMC free article] [PubMed] [Google Scholar]


