Abstract
Objective
To evaluate the correlation between biliary-enteric surgical drainage and the late development of cholangiocarcinoma of the biliary tract.
Summary Background Data
In patients with biliary-enteric drainage, reflux of intestinal contents into the bile duct may occur and cause cholangitis, which is regarded as the most serious complication of these procedures. Lithiasis of the biliary tract and a previous biliary-enteric anastomosis have both been suggested to favor the late onset of cholangiocarcinoma.
Methods
Consecutive patients (n = 1,003) undergoing three different procedures of biliary-enteric anastomosis (transduodenal sphincteroplasty, choledochoduodenostomy, and hepaticojejunostomy) between 1967 and 1997 were included in this study. The postoperative clinical course and long-term outcome were evaluated by a retrospective review of the hospital records and follow-up. Mean follow-up was 129.6 months.
Results
Fifty-five (5.5%) cases of primary bile duct cancer were found among the 1,003 patients at intervals of 132 to 218 months from biliary-enteric anastomosis. The incidence of cholangiocarcinoma in the three groups was 5.8% in transduodenal sphincteroplasty patients, 7.6% in choledochoduodenostomy patients, and 1.9% in hepaticojejunostomy patients. The incidence of malignancy related to the different underlying diagnosis was 5.9%, 7.2%, and 1.9% in patients with choledocholithiasis, sphincter of Oddi stenosis, and postoperative benign stricture, respectively. Although only one patient who developed cholangiocarcinoma had previous concurrent lithiasis of the biliary tract, 40 patients had experienced mostly severe, recurrent cholangitis. No case of malignancy occurred in patients scored as having no cholangitis in the early and long-term postoperative outcome. Univariate and multivariate analyses have shown the presence of cholangitis as the only factor affecting the incidence of cholangiocarcinoma.
Conclusions
Chronic inflammatory changes consequent to biliary-enteric drainage should be closely monitored for the late development of biliary tract malignancies.
Choledochoduodenostomy and transduodenal sphincteroplasty (TS) are procedures currently used for treating benign distal common bile duct obstruction resulting from stones, inflammatory strictures, or motor dysfunction of the sphincter of Oddi. 1–6 Biliary reconstruction with a Roux-en-Y loop is, in turn, a surgical procedure widely used for the treatment of benign bile duct strictures. 7–9 The general perception has been that all these procedures are relatively innocuous, except for cholangitis. Reflux of intestinal contents, activated pancreatic juice, and bacterial flora have been regarded as the possible causes of this complication. 10,11 However, in recent years the number of reports of cholangiocarcinoma occurring in patients who many years previously had biliary-enteric drainage has been increasing, and cholangiocarcinoma has been suggested as a possible long-term complication of these surgical procedures. 12,13 This study involved a retrospective review of patients who had undergone biliary-enteric procedures 2 to 32 years previously at the First Department of Surgery of the University of Rome “La Sapienza” Medical School.
PATIENTS AND METHODS
Patients
The medical records of all patients treated with biliary-enteric anastomosis for benign diseases of the biliary tract between 1967 and 1997 were transferred into a database. Patients were divided into three groups according to the surgical procedure: group A, transduodenal sphincteroplasty (TS); group B, choledochoduodenostomy; and group C, Roux-en-Y hepaticojejunostomy. Initially, 1,162 patients were enrolled in this study; 62 were lost to follow-up and 97 died of disease other than malignancies of the liver and biliary tract. The number of patients finally entering the study was 1,003; 629 of them were women.
Outcome
Follow-up was obtained from medical records and primary physician and patient interviews or telephone communications. Long-term outcome was classified as excellent if no symptoms relating to cholangitis were referred, good if symptoms were transitory, fair if medical therapy was requested, and poor when patients experienced severe episodes of cholangitis. Cholangitis was considered present if three of the following signs were observed: right upper quadrant tenderness, fever, leukocytosis (white cell count > 12,000), bilirubin concentration exceeding 2 mg/dL, or alkaline phosphatases elevation more than 140 U/L.
Diagnosis of cholangiocarcinoma was made either by histologic examination of the surgical specimen when secondary surgery had been done or by the pathologic findings at autopsy. The relationship between the age, gender, surgical procedure used, length of follow-up, and cholangitis with development of cholangiocarcinoma was examined by statistical analysis.
Statistical Analysis
Univariate comparisons were carried out using Fisher tests and the chi-square test for discrete variables, t tests and analysis of variance for continuous variables. Multivariate analysis was performed by the multiple regression test to isolate predictive factors.
RESULTS
Patient Population
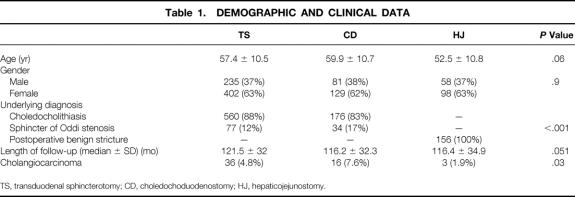
The mean age at the time of initial operation was 56.5 years (range 19–85, SD 10.7). The distribution of patients by type of procedure was 637 with TS, 210 with choledochoduodenostomy, and 156 with hepaticojejunostomy. The indication for biliary bypass was choledocholithiasis in 736 patients, sphincter of Oddi stenosis in 111 patients, and postoperative benign stricture in 156 patients. The mean follow-up period was 129.6 months (range 23–240, SD 37.9) from the initial operation. Demographic and clinical data are shown in Table 1.
Table 1. DEMOGRAPHIC AND CLINICAL DATA
TS, transduodenal sphincterotomy; CD, choledochoduodenostomy; HJ, hepaticojejunostomy.
Outcome
The long-term outcome of the different biliary-enteric procedures showed that the higher incidence of poor results occurred in the choledochoduodenostomy and TS groups; the rate of cholangitis was noticeably lower in the hepaticojejunostomy group (Table 2).
Table 2. LONG-TERM OUTCOME
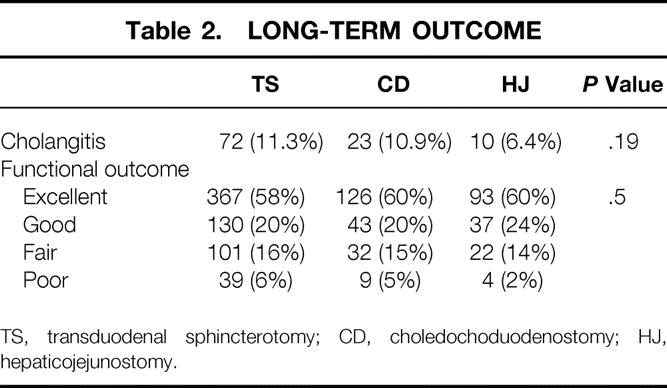
TS, transduodenal sphincterotomy; CD, choledochoduodenostomy; HJ, hepaticojejunostomy.
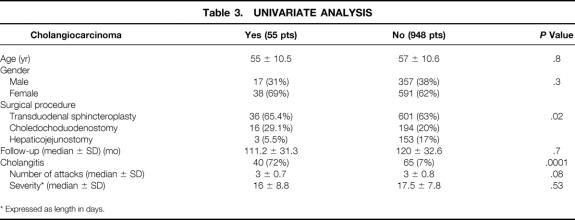
The overall incidence of bile duct carcinoma was 5.5%. The incidence in the three groups was 5.8% in TS patients, 7.6% in choledochoduodenostomy patients, and 1.9% in hepaticojejunostomy patients. The incidence of malignancy related to the different underlying diagnoses were 5.9%, 7.2%, and 1.9% (P = .09), respectively, in patients with choledocholithiasis, sphincter of Oddi stenosis, and postoperative benign stricture. No intrahepatic malignancy was observed. No malignancy occurred in patients scored as having excellent or good surgical results. The number and severity of attacks of cholangitis did not differ when comparing patients who developed cholangiocarcinoma and those who remained free from malignancy (Table 3). Recurrent biliary stones were diagnosed in 12 TS patients, in 2 choledochoduodenostomy patients, and in none of the hepaticojejunostomy patients; no malignancy of the biliary tract occurred in these patients except one in the choledochoduodenostomy group. The onset of cholangiocarcinoma occurred between 11 and 19 years from biliary-enteric anastomosis. At diagnosis of cancer, 34 patients were judged to have inoperable disease, and 9 were found to have unresectable disease at surgical exploration. Twelve patients undergoing curative resection died, all as a result of cancer recurrence within 9 months of the surgery.
Table 3. UNIVARIATE ANALYSIS
* Expressed as length in days.
Statistical Analysis
No significant differences in demographic data were shown in the three treatment groups. Univariate analysis showed a significantly higher incidence of cholangiocarcinoma in the choledochoduodenostomy group (P = .02), and cholangitis was the only factor to influence the incidence of cholangiocarcinoma (see Table 3). Multivariate analysis confirmed cholangitis as the independent factor affecting the incidence of cholangiocarcinoma (P < .001, odds ratio 35.7, standard error 0.32, 95% confidence interval 18.7–68.3).
DISCUSSION
In Western populations, the incidence of cholangiocarcinoma is approximately 1 per 100,000 individuals, 14 strikingly lower than that found in our series. These tumors have been reported to be more common in patients with biliary stones, biliary stasis, and infection. 15,16 Since 1942, when two instances of intrahepatic cholangiocarcinoma associated with hepatolithiasis were reported, the relationship between lithiasis and carcinoma of the biliary tract has been debated. 17–20 Subsequently, in Eastern countries intrahepatic cholangiocarcinoma continued to be described most regularly associated with hepatolithiasis;18,19,21–23 however, the same has not occurred in the West, where this malignancy has been most frequently reported unrelated to biliary lithiasis. 13,20 In our series as well, cholangiocarcinoma of the biliary tract was associated with biliary stones in just one patient. Cholestasis and cholangitis, the next factors considered to have a possible influence on the onset of cholangiocarcinoma of the biliary tract, were detected in the clinical history of 39 of the 55 patients of our series who developed cholangiocarcinoma. A further specific factor associated with an increased incidence of cholangiocarcinoma is biliary cyst disease. After Kasai et al’s first report, 24 such an association has been confirmed in several large series, 25–28 and most recently cumulative data from 73 institutions in Japan have shown that the incidence of choledochal malignancies in biliary cyst disease is 17.5%. 27 However, although biliary cyst disease is only rarely associated with biliary stones, it is almost always associated with an anomalous arrangement of the pancreaticobiliary ductal system, 29 which has been suggested as a factor for carcinogenesis of the biliary tract. 30 Moreover, internal drainage procedures, conceivably enhancing such an anomalous arrangement, increase the incidence of cholangiocarcinoma to as high as 50% in these patients. 27 The sphincter of Oddi provides a barrier that prevents any reflux into the bile duct. By abolishing this barrier, biliary-intestinal drainage yields an anomalous arrangement of the biliary-digestive junction, making possible the backflow of unwanted substances into the biliary tract. Hyperplastic choledochal mucosa with metaplastic goblet cells and pyloric-like gland formation has been seen 31 in patients with a choledochoduodenostomy and consequent long-standing cholangitis. These changes, interpreted as an adaptation to the new environment, are the same as those observed in patients with biliary cyst disease and can be interpreted as premalignant. 32,33 Histologic examination of resected specimens, including cholangiocarcinoma of the biliary tract arising from long-standing cholangitis secondary to a bilioduodenal drainage, has shown, at sites distant from the malignancy, various degrees of hyperplastic lesions occurring in the area of cholangitis. 13
The results of experimental research further support the existence of a relationship between biliary-enteric drainage and malignancies of the biliary tract. Although ablation of the biliary lining and replacement by hyperplastic and atypical epithelium has been documented to follow experimental choledochojejunostomy, 34 administration of carcinogens in animals with bilioenteric anastomoses induced a significantly higher number of bile duct cancers than in sham-operated controls. 35,36 Although a direct connection between the intestinal tract and biliary tree and reflux of activated pancreatic juice and intestinal bacteria into the biliary tract are considered as the factors causing chronic relapsing cholangitis, the latter has been suspected as a predisposing factor for the late development of cholangiocarcinoma. 12,13,20,37,38
When considering different biliary-enteric procedures, the Roux-en-Y technique is the one expected to produce lower rates of cholangitis because it is immune to pancreatic reflux and less prone to intestinal bacterial backflow because of the protective action exerted by the interposed jejunal loop. 28,39,40 The remarkably lower rate of cholangitis found in the hepaticojejunostomy group of our series correlates favorably with the above proposition; however, the lower rate of cholangiocarcinoma detected in the hepaticojejunostomy group reinforces the suggestion of a connection between cholangitis and the development of malignancies of the biliary tract. A further confirmation of this correlation is seen by matching the rate of cholangiocarcinoma with that of cholangitis complicating the different procedures used in our series. The rate of cholangiocarcinoma was shown to relate to that of cholangitis occurring in the three groups.
Current results, although confirming the existence of a correlation between cholangiocarcinoma and previous long-term bilioenteric drainage, suggest that the incidence of cholangiocarcinoma relates not so much to the anomalous arrangement of the biliary-intestinal junction but more to the incidence and seriousness of cholangitis complicating the outcome of the different surgical procedures used. Bacterial infection of the biliary epithelium, the accepted main risk factor for the development of cholangiocarcinoma in patients with biliary cyst disease, 20,23,25,41 was the only factor among those analyzed that was present in each of the study group patients.
Whether cholangitis secondary to biliary-enteric drainage does promote malignancy of the biliary tract, it is quite likely that more reports of this association would be present in the literature. However, an unforeseeable event and the discrepancy between the length of the time from surgery to the diagnosis of cholangiocarcinoma and the usual duration of follow-up may have been responsible for the current underreporting of this correlation.
Three fourths of our patients were judged to have inoperable disease at the time of diagnosis of carcinoma, and nine had unresectable tumors at surgery. Twelve patients who underwent curative surgery died of cancer recurrence within 9 months of surgery. This unfavorable prognosis is probably due to a delay in diagnosis because several symptoms, after biliary-enteric drainage, usually tend to be treated conservatively based on the incorrect diagnosis of ascending cholangitis alone rather than considering the development of a carcinoma. Accordingly, all the patients of our series who did not develop bile duct cancer at present follow-up have been enrolled in a program of long-term close follow-up to analyze the association between biliary-enteric drainage and cholangiocarcinoma and to ascertain whether earlier detection of the malignancy improves the prognosis.
Although larger retrospective studies and prospective randomized trials are needed to establish the definitive risk for late biliary carcinogenesis after biliointestinal drainage, our recommendation is that any patient treated with these procedures and experiencing relapsing cholangitis should be monitored for the late development of bile duct cancer.
Footnotes
Correspondence: Adriano Tocchi, MD, FACS, Via Bruno Bruni 94, Rome 00189, Italy.
E-mail: adraino.tocchi@uniroma1.it
Accepted for publication January 8, 2001.
References
- 1.Anderson TM, Pitt HA, Longmire WP. Experience with sphincteroplasty and sphincterotomy in pancreaticobiliary surgery. Ann Surg 1985; 201: 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moody FG, Beeker JN, Potts JR. Transduodenal sphincteroplasty and transampullary septectomy for post-cholecystectomy pain. Ann Surg 1983; 197: 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warren KW, Mountain JC, Midell AJ. Management of strictures of the biliary tract. Surg Clin North Am 1971; 51: 711–731. [DOI] [PubMed] [Google Scholar]
- 4.Way LW, Admirand WH, Dunphy JE. Management of choledocholithiasis. Ann Surg 1972; 176: 347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallooan AN, Pasrichs PJ. Therapy of sphincter of Oddi dysfunction. Gastrointest Endosc Clin North Am 1996; 6: 117–125. [PubMed] [Google Scholar]
- 6.Trovaras Y, Rowlands BJ. Diagnosis and treatment of sphincter of Oddi dysfunction. Br J Surg 1998; 85: 588–595. [DOI] [PubMed] [Google Scholar]
- 7.Tocchi A, Costa G, Lepre L, et al. The long-term outcome of hepaticojejunostomy in the treatment of benign bile duct strictures. Ann Surg 1996; 224: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothlin MA, Lopfe M, Schlumpt R, et al. Long-term results of hepaticojejunostomy for benign lesions of the bile ducts. Am J Surg 1998; 175: 22–24. [DOI] [PubMed] [Google Scholar]
- 9.Bismuth H, Franco D, Corlette MB, et al. Long-term results of Roux en Y hepatico-jejunostomy. Surg Gynecol Obstet 1978; 146: 161–167. [PubMed] [Google Scholar]
- 10.Sun JY, Leung JWC, Shaffer EA, et al. Ascending infection of the biliary tract after surgical sphincterotomy and biliary stenting. J Gastroenterol Hepatol 1992; 7: 240–244. [DOI] [PubMed] [Google Scholar]
- 11.De Almeida AM, Cruz AG, Aldeia FJ. Side-to-side choledochoduodenostomy in the management of choledocholithiasis and associated diseases. Facts and fiction. Am J Surg 1984; 147: 253–259. [DOI] [PubMed] [Google Scholar]
- 12.Strong RW. Late bile duct cancer complicating biliary-enteric anastomosis for benign disease. Am J Surg 1999; 177: 472–474. [DOI] [PubMed] [Google Scholar]
- 13.Hakamada K, Sasaki M, Endoh M, et al. Late development of bile duct cancer after sphincteroplasty: a ten- to twenty-two-year follow-up study. Surgery 1997; 121: 488–492. [DOI] [PubMed] [Google Scholar]
- 14.Pitt HA, Dooley WC, Yeo CJ, et al. Malignancies of the biliary tree. Curr Probl Surg 1995; 32: 1–90. [DOI] [PubMed] [Google Scholar]
- 15.Lenriot JP, Gigot JF, Ségol P, et al. Bile duct cysts in adults. A multi-institutional retrospective study. Ann Surg 1998; 228: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuchida Y, Ishida M. Dilatation of the intrahepatic bile ducts in congenital cystic dilatation of the common bile duct. Surgery 1971; 69: 776–781. [PubMed] [Google Scholar]
- 17.Sanes S, MacCallum JD. Primary carcinoma of the liver. Cholangioma in hepatolithiasis. Am J Pathol 1942; 18: 675–683. [PMC free article] [PubMed] [Google Scholar]
- 18.Koga A, Ichimiya H, Yamaguchi K, et al. Hepatolithiasis associated with cholangiocarcinoma: possible etiologic significance. Cancer 1985; 55: 2826–2829. [DOI] [PubMed] [Google Scholar]
- 19.Sheen-Chen SM, Chou FF, Eng HL. Intrahepatic cholangiocarcinoma in hepatolithiasis: a frequently overlooked disease. J Surg Oncol 1991; 147: 131–135. [DOI] [PubMed] [Google Scholar]
- 20.Chijiiwa K, Ichimiya H, Kuroki S, et al. Late development of cholangiocarcinoma after the treatment of hepatolithiasis. Surg Gynecol Obstet 1993; 177: 279–282. [PubMed] [Google Scholar]
- 21.Falchuk KR, Lesser PB, Galdabini JJ, et al. Cholangiocarcinoma as related to chronic intrahepatic cholangitis and hepatolithiasis. Am J Gastroenterol 1976; 66: 57–61. [PubMed] [Google Scholar]
- 22.Tsunoda T. Intrahepatic stones associated with cholangiocarcinoma. Jpn J Gastroenterol Surg 1990, 23: 118–121. [PubMed] [Google Scholar]
- 23.Nakamuna Y, Terada T, Tanaka Y, Ohta G. Are hepatolithiasis and cholangiocarcinoma etiologically related? Virchows Arch A 1985; 406: 45–48. [DOI] [PubMed] [Google Scholar]
- 24.Kasai M, Asakura Y, Taira Y. Surgical treatment of choledochal cyst. Ann Surg 1970; 172: 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Todani T, Watanabe Y, Toki A, Urushihara N. Carcinoma related to choledochal cysts with internal drainage operations. Surg Gynecol Obstet 1987; 164: 61–64. [PubMed] [Google Scholar]
- 26.Irvin ST, Morison JE. Congenital cyst of common bile-duct containing stones and undergoing cancerous change. Br J Surg 1944; 32: 319–321. [Google Scholar]
- 27.Flanigan DP. Biliary cysts. Ann Surg 1975; 182: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuchiya R, Harada N, Ito T, et al. Malignant tumors in choledochal cysts. Ann Surg 1977; 186: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todani T, Watanabe Y, Fujii T, et al. Anomalous arrangement of the pancreatobiliary ductal system in patients with a choledochal cyst. Am J Surg 1984; 147: 672–676. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita H, Nagata E, Hirohashi K, et al. Carcinoma of the gallbladder with an anomalous connection between the choledochus and pancreatic duct. Report of 10 cases and review of the literature in Japan. Cancer 1984; 54: 762–769. [DOI] [PubMed] [Google Scholar]
- 31.Bleftheriadis E, Tzioufa V, Kotzampassi K, et al. Common bile duct mucosa in choledochoduodenostomy patients: histological and histochemical study. HPB Surg 1988; 1: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura Y, Ohra G, Nagakawa T, et al. Pathological findings of hepatolithiasis. Jpn J Gastroenterol 1981; 78: 874–882. [Google Scholar]
- 33.Komi N, Tamura T, Tsuge S, et al. Relation of patient age to premalignant alterations in choledochal cyst epithelium. J Pediatr Surg 1986; 21: 430–433. [DOI] [PubMed] [Google Scholar]
- 34.Kurumado K, Nagai T, Kondo Y, et al. Long-term observations on morphological changes of choledochal epithelium after choledochoenterostomy in rats. Dig Dis Sci 1994; 39: 809–820. [DOI] [PubMed] [Google Scholar]
- 35.Tajiima Y, Eto T, Tsunodo T, et al. Induction of extrahepatic biliary carcinoma by N-nitrosobis(2-oxopropyl) amine in hamsters given cholecystoduodenostomy with dissection of the common duct. Jpn J Cancer Res 1994; 85: 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farber E. Chemical carcinogenesis. Am J Pathol 1982; 106: 271–292. [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto J, Shimamura Y, Ohtani I, et al. Bile duct carcinoma arising from the anastomotic site of hepaticojejunostomy after the excision of congenital biliary dilatation: a case report. Surgery 1996; 119: 476–479. [DOI] [PubMed] [Google Scholar]
- 38.Schumacher G, Bechstein WO, Kling N, et al. Bile duct carcinoma in an adenoma in the anastomotic area after hepaticojejunostomy: a case report. Z Gastroenterol 1997; 35: 1081–1086. [PubMed] [Google Scholar]
- 39.Csendes A, Diaz C, Burdiles P, et al. Indications and results of hepaticojejunostomy in benign strictures of the biliary tract. Hepato-Gastroenterology 1992; 39: 333–336. [PubMed] [Google Scholar]
- 40.Blumgart LH, Kelley CJ, Benjamin IS. Benign bile duct stricture following cholecystectomy: critical factors in management. Br J Surg 1984; 71: 836–843. [DOI] [PubMed] [Google Scholar]
- 41.Ohta T, Nagakawa T, Ueda N, et al. Mucosal dysplasia of the liver and the intraductal variant of peripheral cholangiocarcinoma in hepatolithiasis. Cancer 1991; 68: 2217–2223. [DOI] [PubMed] [Google Scholar]