Abstract
Objective
To assess the distribution and type of nerve fibers present in human peritoneal adhesions and to relate data on location and size of nerves with estimated age and with clinical parameters such as reports of chronic pelvic pain.
Summary Background Data
Peritoneal adhesions are implicated in the cause of chronic abdominopelvic pain, and many patients are relieved of their symptoms after adhesiolysis. Adhesions are thought to cause pain indirectly by restricting organ motion, thus stretching and pulling smooth muscle of adjacent viscera or the abdominal wall. However, in mapping studies using microlaparoscopic techniques, 80% of patients with pelvic adhesions reported tenderness when these structures were probed, an observation suggesting that adhesions themselves are capable of generating pain stimuli.
Methods
Human peritoneal adhesions were collected from 25 patients undergoing laparotomy, 20 of whom reported chronic pelvic pain. Tissue samples were prepared for histologic, immunohistochemical, and ultrastructural analysis. Nerve fibers were characterized using antibodies against several neuronal markers, including those expressed by sensory nerve fibers. In addition, the distribution of nerve fibers, their orientation, and their association with blood vessels were investigated by acetylcholinesterase histochemistry and dual immunolocalization.
Results
Nerve fibers, identified histologically, ultrastructurally, and immunohistochemically, were present in all the peritoneal adhesions examined. The location of the adhesion, its size, and its estimated age did not influence the type of nerve fibers found. Further, fibers expressing the sensory neuronal markers calcitonin gene-related protein and substance P were present in all adhesions irrespective of reports of chronic abdominopelvic pain. The nerves comprised both myelinated and nonmyelinated axons and were often, but not invariably, associated with blood vessels.
Conclusions
This study provides the first direct evidence for the presence of sensory nerve fibers in human peritoneal adhesions, suggesting that these structures may be capable of conducting pain after appropriate stimulation.
Peritoneal adhesions are bands of fibrous tissue that join abdominal organs to each other or the abdominal wall. Adhesions develop rapidly after damage to the peritoneum during surgery, infection, trauma, or irradiation. Postoperative adhesion formation occurs in 93% to 100% of patients undergoing laparotomy, 1 leading to complications such as intestinal obstruction and infertility in women. 2–4 Adhesions have also been implicated as a cause of chronic abdominopelvic pain, 5–8 and many patients have been relieved of their symptoms after adhesiolysis. 9–12 Chronic pelvic pain accounts for up to 25% of all gynecologic visits, 30% to 50% of all diagnostic laparoscopic procedures, and approximately 5% of hysterectomies. 13,14 In financial terms, the annual cost of resources for the diagnosis and treatment of women with chronic pelvic pain in the United Kingdom is approximately £600 million, 15 and therefore the role of adhesions in pain etiology should be addressed.
It has been proposed that peritoneal adhesions indirectly cause pain by restricting organ mobility or expansibility and thus stimulating stretch receptors in the smooth muscle of adjacent organs or the abdominal wall. 5,16,17 However, mapping studies of reported pain using microlaparoscopic techniques showed that 80% of patients with significant pelvic adhesions reported tenderness when these structures were probed, 18 suggesting that these structures can generate pain stimuli. The presence of nerve fibers in human pelvic and abdominal adhesions supports this theory. 19–21 The aim of the present study was to characterize the type of nerve fibers found in human peritoneal adhesions using histologic, ultrastructural, and immunohistochemical techniques and to relate the findings to the location, size, and estimated age of the adhesions and clinical parameters such as reports of chronic pelvic pain. Nerve fibers were identified using antibodies directed against synaptophysin, a protein of synaptic vesicles, and the neuropeptides calcitonin gene-related peptide (CGRP), expressed by sensory nerves, and substance P, present in pain-conducting fibers. 22,23 Fibers expressing vasoactive intestinal peptide (VIP), a marker for cholinergic sympathetic nerve fibers, and tyrosine hydroxylase (TH), an intermediary enzyme in catecholamine synthesis of adrenergic nerve fibers, were also identified.
METHODS
Patients
Twenty-five patients with abdominal or pelvic adhesions undergoing laparotomy for various conditions were included in this study. Ethical approval for this study was obtained from the Joint UCL/UCLH Human Research Ethics Committee, University College London, and informed consent was obtained from each patient. The age of the patients ranged from 18 to 81 years; 10 were men and 15 were women. The indications for surgery included colon cancer (five patients), ulcerative colitis (three patients), intestinal obstruction (two patients), fibroids (five patients), ovarian cyst (three patients), cervical/ovarian cancer (three patients), and persistent pelvic pain, appendectomy, cholecystectomy, and incisional hernia (one patient each). The age of the adhesions was estimated from the time of last surgical intervention and ranged from 1 to 24 years. Seven of the 25 patients had no previous history of abdominopelvic surgery but showed peritoneal adhesions after laparotomy. In these patients, adhesions were found between the ovary and uterus (patient with ovarian cyst), the uterus and bladder as well as the colon, fallopian tube, and lateral pelvic wall (four patients with fibroids), the duodenum and gall bladder (patient with chronic cholecystitis), and the ovary and lateral pelvic wall (patient with persistent pelvic pain). Twenty of the 25 patients had a history of abdominopelvic pain of more than 3 months’ duration.
Tissue Processing
Peritoneal adhesion specimens were collected during surgery from between loops of small bowel, small bowel and colon, omentum and small bowel, uterus and colon, small bowel and anterior abdominal wall and ranged in size from 0.5 to 5 cm. The adhesions were excised from serosal surfaces, washed briefly in phosphate-buffered saline (pH 7.4; PBS), and fixed according to the required protocol. For histologic assessment, tissue was immediately placed in 4% paraformaldehyde in PBS, fixed overnight at 4°C, and subsequently processed for wax embedding. Tissue was sectioned at 5 μm and stained with hematoxylin and eosin and Masson’s trichrome.
Immunocytochemistry
Tissue was fixed in 4% paraformaldehyde in PBS for 1 hour at room temperature, washed in PBS for 30 minutes, transferred to 7% sucrose with 0.1% sodium azide (NaN3) in PBS (pH 7.4), and stored overnight at 4°C. The specimens were embedded in optimal cutting compound (OCT; Miles Inc., Elkhart, IN) and frozen over liquid nitrogen. Serial frozen sections (10 μm) were prepared from each adhesion and incubated overnight at 4°C with a 1:1,000 dilution of polyclonal antibodies to synaptophysin (rabbit anti-human synaptophysin; Dako, Bucks, Denmark), CGRP (rabbit anti-rat calcitonin gene-related peptide; Affiniti, Exeter, UK), substance P (rabbit anti-human substance P; Chemicon International, Harrow, UK), TH (rabbit anti-tyrosine hydroxylase; Chemicon International, UK), and VIP (rabbit anti-vasoactive intestinal peptide; Sigma, Poole, UK). Control sections were incubated with either normal blocking serum or rabbit IgG1 instead of primary antisera. After washing with PBS, all sections were incubated with a 1:250 dilution of biotinylated donkey anti-rabbit IgG antiserum (Amersham, Bucks, UK) for 1 hour at room temperature. Sections were washed, incubated with a 1:100 dilution of streptavidin-fluorescein conjugate (Amersham) for 1 hour at room temperature, washed, mounted with Citifluor (Canterbury, UK) and examined by fluorescent microscopy (Axiophot microscope; Zeiss, Germany). For dual immunolocalization, sections were initially immunostained for neuronal markers as previously described. The same sections were then stained with a 1:1,000 dilution of primary antisera against an endothelial cell marker, von Willebrand factor (sheep anti-human vWF; Chemicon International) for 1 hour, followed by incubation with a 1:500 dilution of rhodamine-conjugated secondary antisera (anti-sheep IgG-rhodamine; Chemicon International) for 1 hour before mounting as described.
Acetylcholinesterase Histochemistry
The excised adhesion specimens were pinned onto a slab of sylgard (Dow Corning, Wiesbaden, Germany) as a whole-mount preparation and fixed in 10% buffered formalin (pH 7.2) at 4°C for 1 hour. The specimens were then washed in 100 mL PBS (pH 7.2) containing 1,280 units hyaluronidase (Sigma) and 10-4 mol/L tetraisopropylpyrophosphoramide (iso-OMPA; Sigma) overnight at 4°C. The tissue was then incubated with acetylcholine incubating solution (5 mg acetylcholine iodide, 6.5 mL of 0.1 mol/L acetate buffer with 1.5% Triton X-100, 0.5 mL of 0.15 mol/L sodium citrate, 1 mL of 30 mmol/L copper sulfate, 10 mL of 1 mmol/L iso-OMPA, and 1 mL of 5 mmol/L potassium ferric cyanide) for 24 hours with at least four solution changes. The specimen was regularly viewed under a stereomicroscope, and when staining was sufficiently intense, the reaction was stopped by immersing the specimen in 10% buffered formalin for 1 hour, followed by washing in PBS and mounting in 70% glycerol between glass slides. The tissue was viewed and photographed through a stereo-dissecting microscope under epiillumination.
Transmission Electron Microscopy
Adhesion specimens were fixed in a solution of 1% paraformaldehyde and 5% glutaraldehyde in 100 mmol/L sodium cacodylate buffer (pH 7.4) at room temperature overnight, with postfixing in 1% osmium tetroxide in 0.1 mol/L sodium cacodylate buffer (pH 7.4) for 1 hour. Specimens were washed, stained with an aqueous saturated solution of uranyl acetate for 1 hour, dehydrated, and embedded in araldite epoxy resin. Semithin sections (0.5–1 μm) were cut on a Reichert microtome (Reichert, Austria), stained with 1% toluidine blue, and examined under a Zeiss Axiophot light microscope equipped with phase-contrast optics. Ultrathin sections (70–100 nm) were collected on copper grids, stained with uranyl acetate and lead citrate, and viewed in a Philips 400 transmission electron microscope (Eindhoven, Holland).
RESULTS
Peritoneal adhesions were heterogenous in appearance but consisted mainly of vascularized collagenous bands lined by adipose tissue. Some adhesions from the upper abdominal cavity showed clear zones of demarcation between the fatty and fibrous tissues, whereas pelvic adhesions appeared more fibrous (Fig. 1).
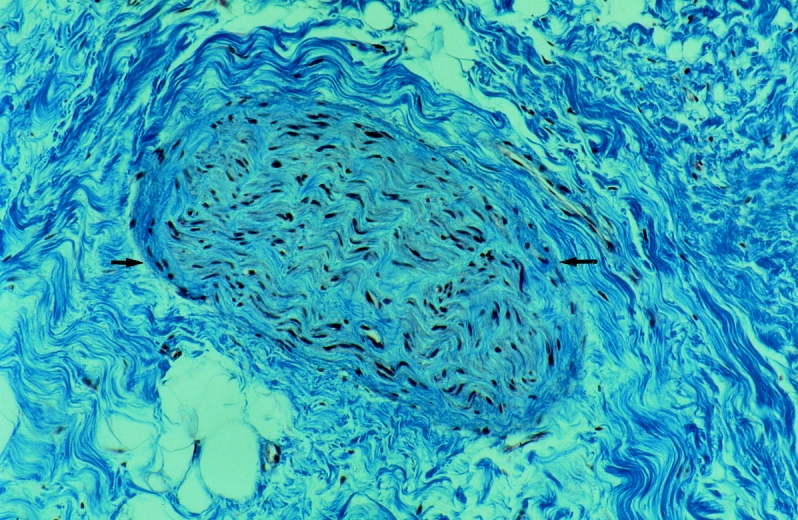
Figure 1. A nerve (arrows) in a human peritoneal adhesion. Adhesions were highly vascularized and contained dense collagen bundles (Masson’s trichrome, ×430).
Nerve fibers were clearly identified in all peritoneal adhesions examined immunohistochemically and appeared more abundant in the abdominal adhesions versus the pelvic adhesions. Fibers were immunoreactive for all the neuronal markers examined (synaptophysin, CGRP, substance P, VIP, TH), irrespective of site, size, and estimated age of the adhesion or the surgical history of the patient. Adhesions from the five patients without a history of chronic abdominopelvic pain also displayed the sensory nerve markers CGRP and substance P. Small bundles of axons, as well as single axons with a distinct beaded or varicose appearance, were identified (Fig. 2).
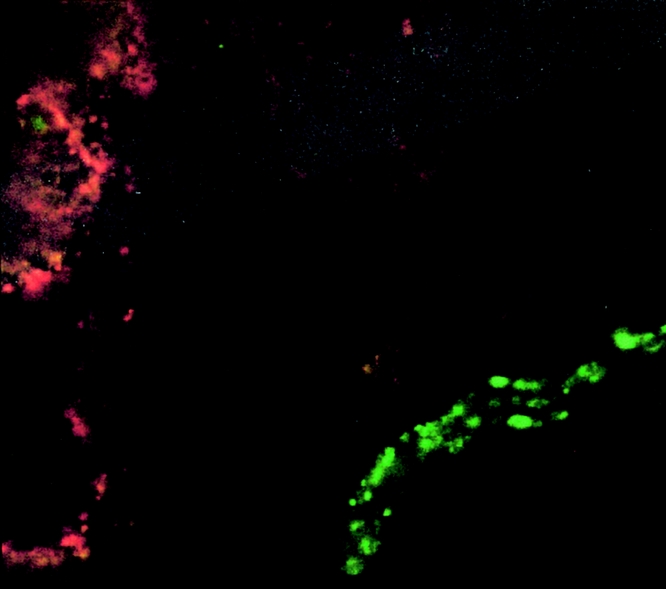
Figure 2. Calcitonin gene-related peptide-immunoreactive nerve fibers in human adhesions. The nerve fiber (green) shows typical varicosities in parallel or a beaded appearance. Blood vessels were immunoreactive for von Willebrand factor (red) (×1,500).
Most nerve fibers colocalized with blood vessels, as shown by dual staining (Fig. 3), and were generally arranged parallel to the longitudinal axis of the adhesion. Some nerve fibers were found in the interstitium and in the adventitia of the blood vessels (Fig. 4). This pattern of staining was similar for all the neuronal markers, except that TH-immunoreactive axons were fewer in the interstitium but more abundant in the adventitia of blood vessels. Some fibers branched from the longitudinal fibers and traversed the adhesions independent of the blood vessels. This finding was confirmed by acetylcholinesterase histochemistry (Fig. 5). On ultrastructural examination, nerves were composed of both myelinated and thinner nonmyelinated fibers with accompanying Schwann cells (Fig. 6). The nerves had a random orientation with respect to the peritoneal surface.
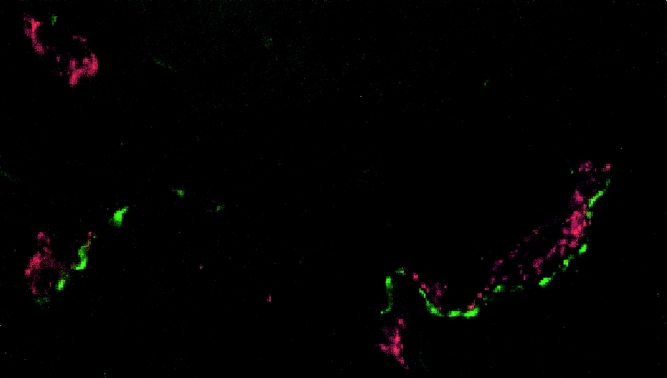
Figure 3. Colocalization of nerve fibers with blood vessels. Substance P-immunoreactive nerve fibers (green) were found associated with blood vessels immunoreactive for von Willebrand factor (red) in peritoneal adhesions. In addition, some fibers were independent of vessels (×1,500).
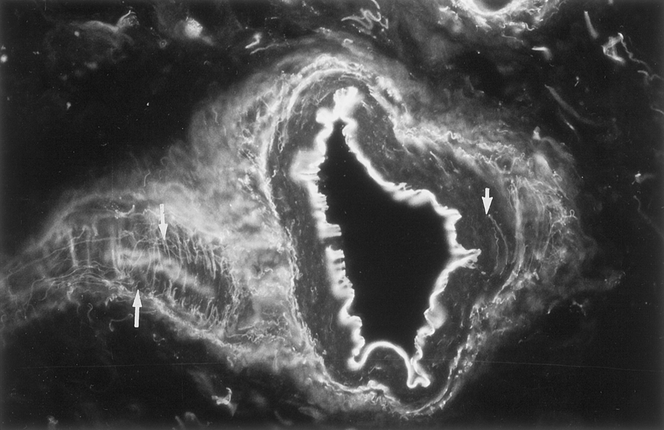
Figure 4. Extensive nerve meshwork within a blood vessel wall in human peritoneal adhesions (arrows). Note the circumferential and horizontal network of nerve fibers immunoreactive to Calcitonin gene-related peptide within the media (thick arrow) and adventitial layer (thin arrows) (×280).
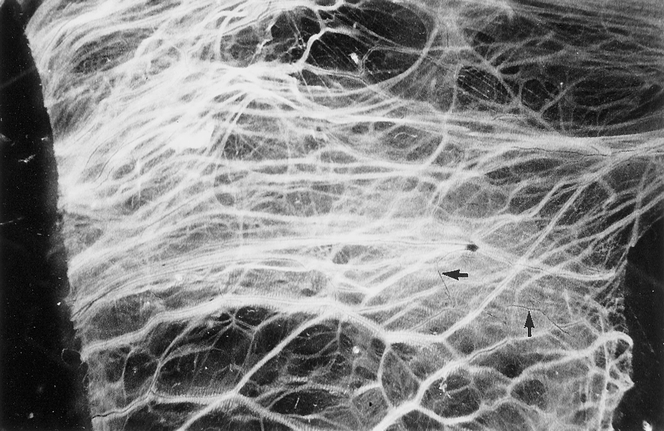
Figure 5. Distribution of blood vessels and nerve fibers in human peritoneal adhesions demonstrated in whole-mount preparations by acetylcholinesterase histochemistry. Generally, nerve fibers (black) accompanied blood vessels (white) arranged parallel to the long axis of the adhesion, but some nerve fibers branched independently (arrows) (×18).
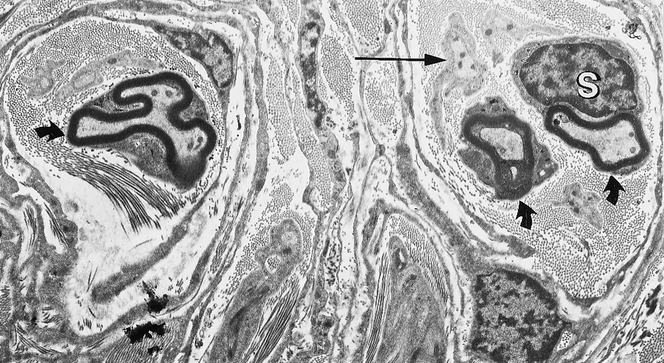
Figure 6. Transmission electron microscopy of a human adhesion showing myelinated (thick arrows) and nonmyelinated nerve fibers (thin arrows) embedded within the extracellular matrix. Schwann’s cells (S) were found surrounding the axons with associated collagen fibers (×4,800).
DISCUSSION
In this study, all the peritoneal adhesions examined contained nerve fibers. The fibers were immunoreactive for all the neuronal markers assessed, irrespective of the site of the adhesion, its size, or its estimated age. Past surgical intervention, inflammation, or reports of chronic pelvic pain did not influence the proportion or type of nerve fibers found. Substance P-immunoreactive and CGRP-immunoreactive nerve fibers were observed throughout the samples, and because these peptides are abundant in sensory nerves, it is likely that these fibers are part of a sensory pathway. Moreover, the small-diameter nonmyelinated fibers, shown by electron microscopy, could be C or A-delta fibers, both known to be involved in the conduction of pain stimuli. 22,23
The association of nerve fibers with blood vessels, as shown by dual immunolocalization and acetylcholinesterase histochemistry, suggests that angiogenesis may play an important role in regulating the growth of nerve fibers into adhesions. Hill et al 25 found that the reinnervation of arterial smooth muscle and intrinsic neurons of the gut, after disruption of their nerve supply, was predominantly by sympathetic fibers (TH- and VIP-immunoreactive) regenerating from the interrupted paravascular nerve bundles. Further, after ileal transplantation, regenerating fibers expressing a number of neuronal markers such as CGRP, substance P, and TH, extend toward the anastomotic site from paravascular fibers in the mesentery. 26 In the developing adhesion, blood vessels appear 3 days after injury. 24 They may therefore guide nerve growth through the release of trophic factors and the deposition of extracellular matrix tracts. The finding that some nerve fibers appeared to be independent of blood vessels suggests that their growth was influenced by additional stimuli, such as chemotactic inflammatory mediators, or inhibited by dense fibrotic areas within the adhesions.
The effect of innervation on adhesion function is not known, but neuropeptides released by nerves are thought to be involved in a number of processes, including the control of blood flow and the regulation of neurogenic inflammation. 27 Both neurokinin A and substance P have been shown to stimulate fibroblast and arterial smooth muscle cell proliferation, and neurokinin A also induces fibroblast chemotaxis. 28,29 Neuropeptides such as CGRP and substance P are thought to be essential for normal wound healing: denervated wounds heal more slowly and have a reduced inflammatory infiltrate compared with controls. 30 In terms of the peritoneum, it has been postulated that increasing the intensity of neural stimuli, through stretch or distension of viscera, may lead to antidromic release of neuropeptides, resulting in the recruitment of peripheral neural sensory branches and the release of inflammatory mediators. 31 Hence, repeated distortion and stretching of adhesions may lead to the release of neuropeptides and so influence fibroblast activity and modulate the deposition of connective tissue during adhesion maturation.
Nerves in the adhesions may also be involved in the occurrence of chronic pelvic pain. This study is the first demonstration of sensory nerve fibers, including those that may generate pain stimuli, in human peritoneal adhesions. However, five patients reported no abdominopelvic pain 3 to 6 months before surgery, even though their adhesions also contained nonmyelinated axons and fibers immunoreactive for the sensory neuronal markers present in pain-conducting fibers. This suggests that the presence of sensory nerves in adhesions does not necessarily involve chronic abdominopelvic pain. These nerves may be nonfunctional or require a series of inducing stimuli to transmit pain sensations, as in the visceral peritoneum. 32 The number of A-delta and C primary afferents supplying the viscera is small in comparison with the number of fine afferents in somatic nerves to the abdominal wall. 33 These visceral afferents, unlike somatic fibers, probably have neither nociceptive nor high-threshold, specialized nerve endings that, when stimulated, specifically result in the sensation of pain. Instead, they terminate in mechanoreceptors that have the capacity of a graded response that depends on the intensity of the stimulus. Visceral pain is thought to reflect an abnormal involvement of the neural mechanisms usually concerned with the mediation of reflexes and amorphous sensations, which with added mechanical or chemical stimuli respond with increased intensity and are therefore perceived as painful stimuli. A similar mechanism may exist in human adhesions, where a combination of distention, hypoxia, and inflammation may result in the stimulation of sensory fibers in the adhesions and the perception of chronic abdominopelvic pain. In addition to this direct role, adhesions may cause abdominopelvic pain by restricting the mobility of internal organs and thus stimulating preexisting stretch-sensitive fibers in the muscle of viscera. 5,16,17
In conclusion, this study has shown the presence of sensory nerves within all the human peritoneal adhesions examined. Although pain physiologic studies were not conducted, it is possible that the thin nonmyelinated fibers observed conduct pain stimuli. However, not all patients in this study experienced chronic pelvic pain; therefore, although all adhesions may be able directly to give rise to pain sensations, there are likely to be other factors to consider, in addition to the innervation, such as peritoneal pathology, organ mobility, and psychosomatic manifestations.
Footnotes
Supported by Johnson & Johnson Medical, Gargrave, Skipton, UK. The authors do not have a fiduciary interest in the sponsor.
Correspondence: Sarah E. Herrick, PhD, Department of Medicine, University College London, Rayne Institute, 5 University St., London, UK WC1E 6JJ.
E-mail: s.herrick@ucl.ac.uk
Accepted for publication October 26, 2000.
References
- 1.Menzies D, Ellis H. Intestinal obstruction from adhesions: how big is the problem? Ann R Coll Surg Engl 1990; 72: 60–63. [PMC free article] [PubMed] [Google Scholar]
- 2.DeCherney AH, Mezer HC. The nature of posttuboplasty pelvic adhesions as determined by early and late laparoscopy. Fertil Steril 1984; 41: 643–666. [DOI] [PubMed] [Google Scholar]
- 3.Tulandi T, Collins JA, Burrows E, et al. Treatment-dependent and treatment-independent pregnancy among women with periadnexal adhesions. Am J Obstet Gynecol 1990; 162: 354–357. [DOI] [PubMed] [Google Scholar]
- 4.Stricker B, Blanco J, Fox HE. The gynecologic contribution to intestinal obstruction in females. J Am Coll Surg 1994; 178: 617–620. [PubMed] [Google Scholar]
- 5.Kresch AJ, Seifer DB, Sachs LB, et al. Laparoscopy in 100 women with chronic pelvic pain. Obstet Gynaecol 1984; 64: 672–674. [PubMed] [Google Scholar]
- 6.Alexander-Williams J. Do adhesions cause pain? Br Med J 1987; 294: 659–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Punch MR, Roth RS. Adhesions and chronic pain: an overview of pain and a discussion of adhesions and pelvic pain. Clin Biol Res 1993; 381: 101–120. [PubMed] [Google Scholar]
- 8.Duffy DM, diZerega GS. Pelvic pain as a cause of adhesions, crystalloids in preventing them. J Reprod Med 1996; 41: 19–26. [PubMed] [Google Scholar]
- 9.Sutton C, MacDonald R. Laser laparoscopic adhesiolysis. J Gynaecol Surg 1990; 6: 155–159. [DOI] [PubMed] [Google Scholar]
- 10.Steege JF, Stout AL. Resolution of chronic pelvic pain after laparoscopic lysis of adhesions. Am J Obstet Gynaecol 1991; 165: 278–283. [DOI] [PubMed] [Google Scholar]
- 11.Fayez JA, Clark RR. Operative laparoscopy for the treatment of localized chronic pelvic-abdominal pain caused by postoperative adhesions. J Gynaecol Surg 1994; 10: 79–83. [DOI] [PubMed] [Google Scholar]
- 12.Saravelos HG, Li TC, Cooke ID. An analysis of the outcome of microsurgical and laparoscopic adhesiolysis for chronic pelvic pain. Hum Reprod 1995; 10: 2895–2901. [DOI] [PubMed] [Google Scholar]
- 13.Reiter RC, Gambone JC. Nongynecologic somatic pathology in women with chronic pelvic pain and negative laparoscopy. Reprod Med 1991; 36: 253–259. [PubMed] [Google Scholar]
- 14.Howard FM. Laparoscopic evaluation and treatment of women with chronic pelvic pain. J Am Assoc Gynecol Laparosc 1994; 1: 325–331. [DOI] [PubMed] [Google Scholar]
- 15.Davies HT. First European Conference on Pain Research, Brussels, December 11–13, 1991. Qual Life Res 1992; 1: 225–227. [DOI] [PubMed] [Google Scholar]
- 16.Lundberg WI, Wall JE, Mathers JE. Laparoscopy in evaluation of pelvic pain. Obstet Gynecol 1973; 42: 872–876. [PubMed] [Google Scholar]
- 17.Keltz MD, Peck L, Liu S, et al. Large bowel-to-pelvic sidewall adhesions associated with chronic pelvic pain. J Am Assoc Gynecol Laparosc 1995; 3: 55–59. [DOI] [PubMed] [Google Scholar]
- 18.Almeida OD, Val-Gallas JM. Conscious pain mapping. J Am Assoc Gynecol Laparosc 1997; 4: 587–590. [DOI] [PubMed] [Google Scholar]
- 19.Kligman I, Drachenberg C, Papadimitriou J, et al. Immunohistochemical demonstration of nerve fibres in pelvic adhesions. Obstet Gynaecol 1993; 82: 566–568. [PubMed] [Google Scholar]
- 20.Tulandi T, Chen MF, Al-Took S, et al. A study of nerve fibers and histopathology of postsurgical, postinfectious, and endometriosis-related adhesions. Obstet Gynecol 1998; 92: 766–768. [DOI] [PubMed] [Google Scholar]
- 21.Herrick SE, Mutsaers SE, Ozua P, et al. Human peritoneal adhesions are highly cellular, innervated and vascularised. J Pathol 2000; 192: 67–72. [DOI] [PubMed] [Google Scholar]
- 22.Henry JL. Relation of substance P to pain transmission: neurophysiological evidence. In: Porter R, O’Connor M, eds. Substance P in the nervous system: Ciba Foundation Symposium Vol 19. London: Pitman; 1982: 206–224. [DOI] [PubMed]
- 23.Woolf C, Wiesenfeld-Hallin Z. Substance P and calcitonin gene-related peptide synergistically modulate the pain of the nociceptive flexor withdrawal reflex in the rat. Neurosci Lett 1986; 66: 226–230. [DOI] [PubMed] [Google Scholar]
- 24.Milligan DW, Raftery AT. Observations on the pathogenesis of peritoneal adhesions: a light and electron microscopical study. Br J Surg 1974; 61: 274–280. [DOI] [PubMed] [Google Scholar]
- 25.Hill CE, Hirst GD, Ngu MC, et al. Sympathetic postganglionic reinnervation of mesenteric arteries and enteric neurones of the ileum of the rat. J Auton Nerv Syst 1985; 14: 317–334. [DOI] [PubMed] [Google Scholar]
- 26.Sugitani A, Reynolds JC, Tsuboi M, et al. Extrinsic intestinal reinnervation after canine small bowel autotransplantation. Surgery 1998; 123: 25–35. [PubMed] [Google Scholar]
- 27.Sternini C. Organisation of the peripheral nervous system: Autonomic and sensory ganglia. J Invest Dermatol Symp Proceed 1997; 2: 1–7. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson J, von Euler AM, Dalsgaard CJ. Stimulation of connective tissue cell growth by substance P and substance K. Nature 1985; 315: 61–63. [DOI] [PubMed] [Google Scholar]
- 29.Harrison NK, Dawes KE, Kwon OJ, et al. Effects of neuropeptides on human lung fibroblast proliferation and chemotaxis. Am J Physiol 1995; 268: L278–283. [DOI] [PubMed] [Google Scholar]
- 30.Richards AM, Mitsou J, Floyd DC, et al. Neural innervation and healing. Lancet 1997; 350: 339–340. [DOI] [PubMed] [Google Scholar]
- 31.Rapkin AJ. Neuroanatomy, neurophysiology, and neuropharmacology of pelvic pain. Clin Obstet Gynecol 1990; 33: 119–129. [DOI] [PubMed] [Google Scholar]
- 32.Cervero F, Laird JM. Visceral pain. Lancet 1999; 353: 2145–2148. [DOI] [PubMed] [Google Scholar]
- 33.Janig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol Psychol 1996; 42: 29–51. [DOI] [PubMed] [Google Scholar]


