Abstract
Objective
To assess the authors’ experience with intraductal papillary mucinous neoplasms of the pancreas (IPMNs).
Summary Background Data
Intraductal papillary mucinous neoplasms of the pancreas are being recognized with increasing frequency.
Methods
All patients who underwent pancreatic resection for an IPMN at the Johns Hopkins Hospital between January 1987 and December 2000 were studied. The data were compared with those of 702 concurrent patients with infiltrating ductal adenocarcinoma of the pancreas not associated with an IPMN resected by pancreaticoduodenectomy.
Results
In the 13-year time period, 60 patients underwent pancreatic resection for IPMNs, with 40 patients undergoing resection in the past 3 years. Mean age at presentation was 67.4 ± 1.4 years. The most common presenting symptom in patients with IPMNs was abdominal pain (59%). Most IPMNs were in the head of the pancreas or diffusely involved the gland, with 70% being resected via pancreaticoduodenectomy, 22% via total pancreatectomy, and 8% via distal pancreatectomy. Twenty-two patients (37%) had IPMNs with an associated infiltrating adenocarcinoma. In a subset of IPMNs immunohistochemically stained for the Dpc4 protein (n = 50), all of the intraductal or noninvasive components strongly expressed Dpc4, whereas 84% of associated infiltrating cancers expressed Dpc4. The 5-year survival rate for all patients with IPMNs (n = 60) was 57%.
Conclusion
Intraductal papillary mucinous neoplasms represent a distinct clinicopathologic entity being recognized with increasing frequency. IPMNs are clinically, histologically, and genetically disparate from pancreatic ductal adenocarcinomas. The distinct clinical features, the presumably long in situ or noninvasive phase, and the good long-term survival of patients with IPMNs offer a unique opportunity for early diagnosis, curative resection, and further studies of the molecular genetics and natural history of these unusual neoplasms.
Cystic neoplasms of the pancreas account for less than 5% of primary pancreatic malignancies. 1–4 Intraductal papillary mucinous neoplasms of the pancreas (IPMNs) are a clinicopathologic entity being recognized with increasing frequency. In 1986, Itai et al 5 described a subset of mucinous cystic tumors of the pancreas they termed “ductectatic mucinous cystadenoma and cystadenocarcinoma.” They described five such tumors with localized cystic dilatations of the main pancreatic duct and major side branches. In contrast to mucinous cystic neoplasms (MCNs), these neoplasms involved the major pancreatic ducts and lacked an associated ovarian stroma. IPMN is a relatively new nomenclature used to designate such pancreatic tumors, accepted by the World Health Organization in 1996. 6 Many tumors previously termed papillary carcinoma, ductectatic mucinous cystadenoma, villous adenoma, and mucin-producing tumors of the pancreas are now classified as IPMNs. 7 Therefore, part of the increased incidence of this clinicopathologic entity may be a result of better recognition and appropriate classification. Also, it has been suggested that the observed increase in IPMNs may be partially attributed to improved diagnostic imaging. 8
Several case series of IPMNs have been reported. 7,9–15 In the current study we present a single-institution, retrospective analysis of the clinical presentation, treatment, pathologic features, and long-term outcome of patients with IPMNs. Of note, 67% of the patients with IPMNs in this series underwent resection in the last 3 years of the study.
PATIENTS AND METHODS
Between January 1987 and December 2000, 60 patients who underwent resection for IPMNs of the pancreas were identified in our pancreatic resection database. A retrospective review of this prospectively collected database was performed. The demographics, presenting symptoms, operative management, pathology, postoperative course, and long-term survival of those patients with IPMNs were compared with those of 702 concurrent patients undergoing surgical resection (pancreaticoduodenectomy) for infiltrating pancreatic ductal adenocarcinoma not associated with an IPMN.
Patients with neoplasms (IPMN or infiltrating ductal adenocarcinoma) in the head, neck, or uncinate process of the pancreas underwent pancreaticoduodenectomy; those with neoplasms in the body and tail underwent distal pancreatectomy. Total pancreatectomy was performed for tumors diffusely involving the gland or those involving the head and extending distally into the body of the pancreas. After the initiation of an ongoing prospective, randomized trial in 1996, retroperitoneal lymphadenectomy and distal gastrectomy were used more frequently in patients with an infiltrating adenocarcinoma. 16 All distal pancreatic resections included splenectomy and most extended proximally to the superior mesenteric vessels.
All pathologic specimens were reviewed to confirm the diagnosis of noninvasive IPMN (no infiltrating cancer), IPMN with an associated infiltrating carcinoma, or infiltrating ductal adenocarcinoma of the pancreas not associated with an IPMN (Fig. 1). IPMNs were classified according to the criteria established by the World Health Organization 6 as having tall, columnar, mucin-containing epithelium with or without papillary proliferations and extensively involving the pancreatic ducts. IPMN/adenoma, IPMN/borderline, and IPMN/in situ carcinoma were lumped together as noninvasive IPMNs for purposes of data analysis. All noninvasive IPMNs were submitted in entirety for histologic examination. By definition, IPMNs lacked the “ovarian”-type stroma seen in MCNs. Size was the major criterion used to distinguish IPMNs from pancreatic intraepithelial neoplasms (PanINs). To be classified as an IPMN the lesion had to be grossly and/or radiographically visible. If it was not, it was classified as a PanIN. In addition, formalin-fixed paraffin-embedded sections of a subset of IPMNs (n = 50) were immunolabeled with a previously well-characterized antibody to the Dpc4 protein, 17–19 a tumor-suppressor protein commonly mutated in pancreatic adenocarcinoma (see Fig. 1).
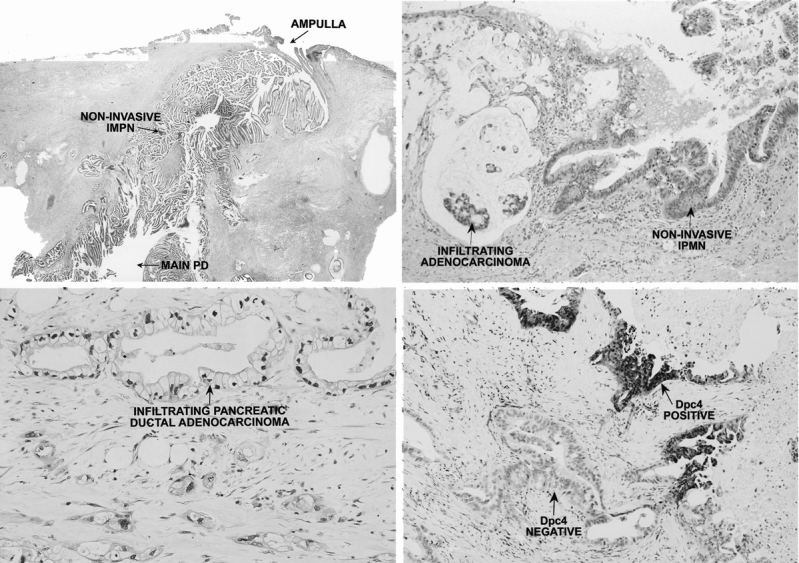
Figure 1. (A) Noninvasive intraductal papillary mucinous neoplasm of the pancreas (IPMN). Note the tall, columnar, mucin-filled epithelium and the papillary proliferations extensively involving the main pancreatic duct (main PD). The basement membrane is intact. The ampulla of Vater is shown. (B) IPMN with associated infiltrating adenocarcinoma. Note the noninvasive component at the right and the infiltrating component at the left, characterized by dissecting pools of mucin in which neoplastic glandular cells are embedded. (C) Infiltrating ductal adenocarcinoma of the pancreas. The tumor consists of cuboidal epithelial cells with irregular nuclei. Note the striking desmoplastic response typical of pancreatic ductal adenocarcinoma. (D) Dpc4 protein staining of an IPMN with associated infiltrating adenocarcinoma. The noninvasive component is strongly positive for Dpc4, whereas the infiltrating component no longer expresses the Dpc4 protein (Dpc4 negative).
The perioperative death rate was defined as in-hospital death or death within 30 days of surgery. The overall incidence of postoperative complications was evaluated using previously defined criteria. 16
Survival information was available on all 60 IPMN patients and 698 of 702 pancreatic adenocarcinoma patients. Follow-up information was obtained by contacting the U.S. Social Security Administration and through direct patient contact, hospital charts, and surgeons’ records. Many patients in the current report have been included in previous studies from this institution. 2,16
All continuous data are presented at mean ± standard error of the mean. Differences between categorical variables were evaluated by chi-square analysis; the Student t test was used for all comparisons among continuous variables. Survival analysis was performed using the Kaplan-Meier method. 20 Differences in survival were compared using the log-rank test. Significance was accepted at the 5% level.
RESULTS
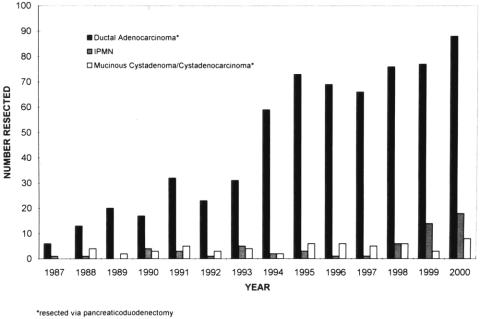
In the 13 years of this study, 60 patients underwent surgical resection for an IPMN and 702 concurrent patients underwent pancreaticoduodenectomy for an infiltrating ductal adenocarcinoma of the pancreas not associated with an IPMN. Of the 60 IPMNs resected, 22 (37%) had an associated infiltrating component in the resection specimen, whereas 38 (63%) had only a noninvasive intraepithelial component. The numbers of pancreaticoduodenectomies for infiltrating pancreatic ductal adenocarcinoma, resections for IPMNs, and pancreaticoduodenectomies for mucinous cystadenoma/cystadenocarcinoma over time are depicted in Figure 2. The increase in pancreatic ductal adenocarcinomas resected in the late 1980s and early 1990s largely reflects referral patterns during this time period, secondary to trends toward regionalization of care at centers of excellence. 21,22 Although the number of IPMNs resected increased during the last 3 years of the study, the number of mucinous cystadenomas/cystadenocarcinomas resected remained relatively constant.
Figure 2. Histogram representing the number per year of pancreaticoduodenal resections for pancreatic ductal adenocarcinoma, resections for intraductal papillary mucinous neoplasms (IPMNs), and pancreaticoduodenal resections for mucinous cystadenomas/cystadenocarcinomas.
Presentation
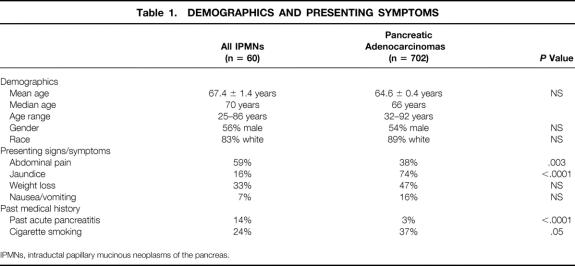
The mean age of patients with IPMNs was 67.4 ± 1.4 years (median 70, range 25–86); 56% of patients were men and 83% were white (Table 1). These demographics were similar to those seen in patients undergoing pancreaticoduodenectomy for pancreatic ductal adenocarcinoma, where the mean age was 64.6 ± 0.4 years (median 66, range 32–92); 54% of patients were men and 89% were white. The demographics were statistically similar in patients having IPMNs with and without associated infiltrating components.
Table 1. DEMOGRAPHICS AND PRESENTING SYMPTOMS
IPMNs, intraductal papillary mucinous neoplasms of the pancreas.
Unlike the demographic factors, the presenting signs and symptoms in patients with IPMNs differed markedly from those in patients with pancreatic ductal adenocarcinoma. Patients with IPMNs were more likely to have abdominal pain (59% vs. 38%, P = .003) but less likely to have obstructive jaundice (16% vs. 74%, P < .0001). There were no differences in presenting signs and symptoms between patients having IPMNs with and without associated infiltrating cancers.
Patients with IPMNs were more likely to have had an episode of acute pancreatitis (14% vs. 3%, P < .0001) and were less likely to smoke (24% vs. 37%, P = .05) than those with pancreatic ductal adenocarcinoma.
Intraoperative Course
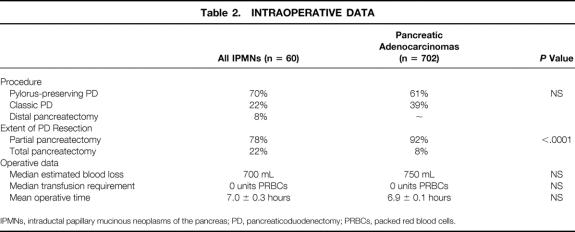
Ninety-two percent of patients (n = 55) underwent pancreaticoduodenal resection for IPMNs (Table 2). Eight percent of patients (n = 5) underwent distal pancreatectomy for IPMNs in the body and tail of the gland. The distribution of pylorus-preserving versus classic procedures was similar in the pancreatic ductal adenocarcinoma group, with 61% of patients (n = 428) undergoing pylorus preservation and 39% having a distal gastrectomy (n = 274, P = NS). However, those with ductal adenocarcinoma were less likely to be managed via total pancreatectomy (8% ductal adenocarcinoma vs. 22% IPMN, P < .0001).
Table 2. INTRAOPERATIVE DATA
IPMNs, intraductal papillary mucinous neoplasms of the pancreas; PD, pancreaticoduodenectomy; PRBCs, packed red blood cells.
Pathologic and Immunohistochemical Features
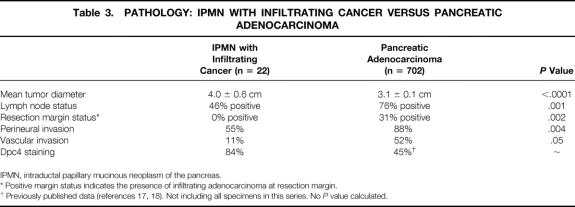
Patients with IPMNs associated with an infiltrating adenocarcinoma (n = 22) had larger tumors (4.0 ± 0.6 cm, median 3.0 cm) than those with ductal adenocarcinomas (n = 702, 3.1 ± 0.1 cm, median 3.0 cm, P < .0001) (Table 3). Despite having larger tumors, patients with IPMNs with an associated infiltrating adenocarcinoma had a lower incidence of lymph node metastases (46% vs. 76%, P = .001) and a lower incidence of invasive cancer at the surgical margins (0% vs. 31%, P = .002) than those with infiltrating ductal adenocarcinoma not arising in an IPMN.
Table 3. PATHOLOGY: IPMN WITH INFILTRATING CANCER VERSUS PANCREATIC ADENOCARCINOMA
IPMN, intraductal papillary mucinous neoplasm of the pancreas.
* Positive margin status indicates the presence of infiltrating adenocarcinoma at resection margin.
† Previously published data (references 17, 18). Not including all specimens in this series. No P value calculated.
The mean tumor diameter for noninvasive IPMNs (n = 38) was 4.2 ± 0.4 cm (median 3.6 cm). These tumors were submitted in entirety for histologic examination and, by definition, lacked an invasive component, were node-negative, and lacked perineural or vascular invasion. Six patients with noninvasive IPMNs had residual neoplasm (noninvasive) at the surgical margin of resection.
Fifty of the 60 IPMN specimens were immunohistochemically labeled for the Dpc4 protein. All of the noninvasive IPMNs strongly expressed Dpc4, whereas 84% of the infiltrating adenocarcinomas associated with an IPMN expressed Dpc4. This is in contrast to previously published reports concerning pancreatic ductal adenocarcinomas not associated with an IPMN, where only 70% of in situ carcinomas (PanIN-3) and only 45% of infiltrating adenocarcinomas stained positive for the Dpc4 protein. 18,19
Postoperative Course
There were four perioperative deaths among the IPMN patients and 22 among the pancreatic adenocarcinoma patients, for death rates of 6.6% and 3.1%, respectively (P = NS) (Table 4). The most common cause of perioperative death was intraabdominal sepsis and associated multisystem organ failure, which was seen in 3 IPMN patients and 15 ductal adenocarcinoma patients. Three patients died of bleeding problems (intraabdominal or gastrointestinal), and single patients died of ischemic bowel, pulmonary embolism, portal vein/superior mesenteric vein (PV/SMV) injury, unexplained bradycardic arrest, and brain stem infarct. The overall complication rates were 39% in patients with IPMNs and 31% in those with ductal adenocarcinoma (P = NS).
Table 4. POSTOPERATIVE COURSE
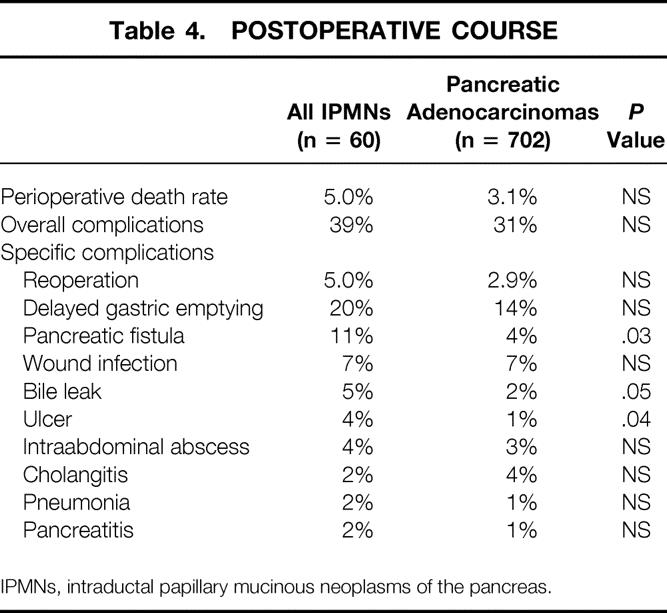
IPMNs, intraductal papillary mucinous neoplasms of the pancreas.
Long-Term Survival
The mean live patient follow-up was 28 months in the IPMN group and 31 months in the pancreatic ductal adenocarcinoma group. Forty-one of 60 IPMN patients (68%) and 220 of 698 (31%) pancreatic ductal adenocarcinoma patients remained alive at the time of survival analysis. The survival rates for all patients with IPMNs were 82% at 1 year, 67% at 3 years, and 57% at 5 years, with a median survival of 74 months (Fig. 3). Patients with noninvasive IPMNs had 1-, 2-, and 4-year survival rates of 87%, 71%, and 64%; those with IPMNs with an associated infiltrating adenocarcinoma had 1-, 2-, and 4-year survival rates of 73%, 73%, and 62%, (P = NS, Fig. 4).
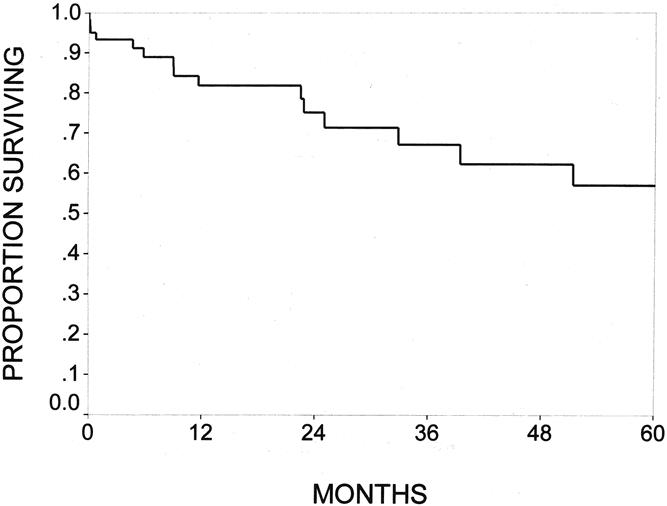
Figure 3. The Kaplan-Meier actuarial survival for the entire cohort of 60 patients with resected intraductal papillary mucinous neoplasms (IPMNs) of the pancreas. The survival rates were 82% at 1 year, 67% at 3 years, and 57% at 5 years.
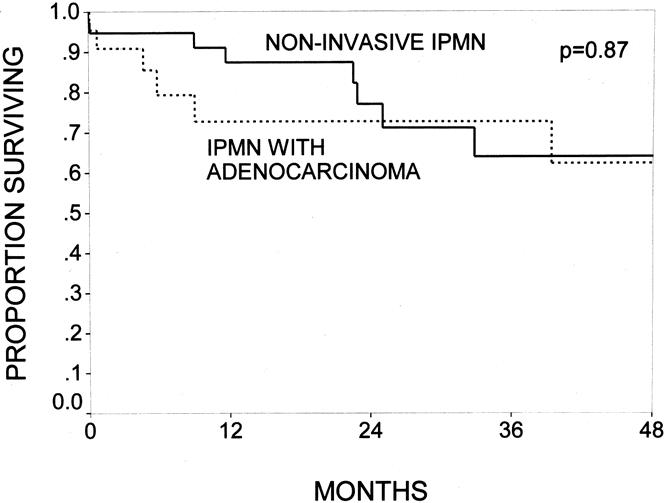
Figure 4. The Kaplan-Meier actuarial survival curves comparing patients with noninvasive intraductal papillary mucinous neoplasms (IPMNs) (n = 38) and patients with IPMNs with an infiltrating adenocarcinoma component (n = 22, P = .87). Patients with noninvasive IPMNs had 1-, 2-, and 4-year survival rates of 87%, 71%, and 64%; patients with IPMNs with an associated infiltrating adenocarcinoma had survival rates of 73%, 73%, and 62%.
There have been 19 deaths in IPMN patients during the follow-up period. There were two postoperative deaths in the noninvasive IPMN group and two postoperative deaths in the patients with IPMN with associated infiltrating adenocarcinoma. The cause of death was known in 11 of the remaining 15 patients.
All six patients with IPMNs with an infiltrating adenocarcinoma who died, died of recurrent adenocarcinoma. The one patient with an IPMN with an associated infiltrating adenocarcinoma, who had a positive surgical margin for noninvasive IPMN, remains alive. Two patients with IPMNs with an associated infiltrating adenocarcinoma were discovered to have infiltrating adenocarcinoma in the remnant pancreas approximately 10 years after margin-negative pancreaticoduodenectomy. Both underwent distal pancreatectomy (completion pancreatectomy). One subsequently died of disseminated disease; the other remains alive without evidence of disease.
Information on the cause of death was available in five of the nine patients with noninvasive IPMNs who died during follow-up. Of these five patients with known causes of death, four died of disseminated adenocarcinoma presumably of pancreatic origin, and one died of complications of diabetes. Three of the four who died of disseminated adenocarcinoma had negative resection margins. Further, in this group of 38 patients with noninvasive IPMN, 6 had a positive surgical margin for noninvasive IPMN. One of these six patients died during follow-up of disseminated adenocarcinoma presumably of pancreatic origin. Five of these six patients remain alive without evidence of disease. There is one additional patient with a noninvasive IPMN, who developed adenocarcinoma in the tail of the pancreas 5 years after margin-negative pancreaticoduodenectomy. The patient underwent distal pancreatectomy (completion pancreatectomy) in November 2000 and remains alive without evidence of disease.
The outcomes of patients with IPMNs with an associated infiltrating adenocarcinoma (n = 22) were compared with the outcomes of patients with pancreatic ductal adenocarcinoma not associated with an IPMN (n = 698). Patients having IPMNs with an associated infiltrating adenocarcinoma had 1-, 3-, and 5-year survival rates of 73%, 73%, and 62% (median survival not reached at 5 years), compared with 63%, 27%, and 19% (median survival 19 months) in patients with ductal adenocarcinomas not arising in the setting of an IPMN (P = .01, Fig. 5).
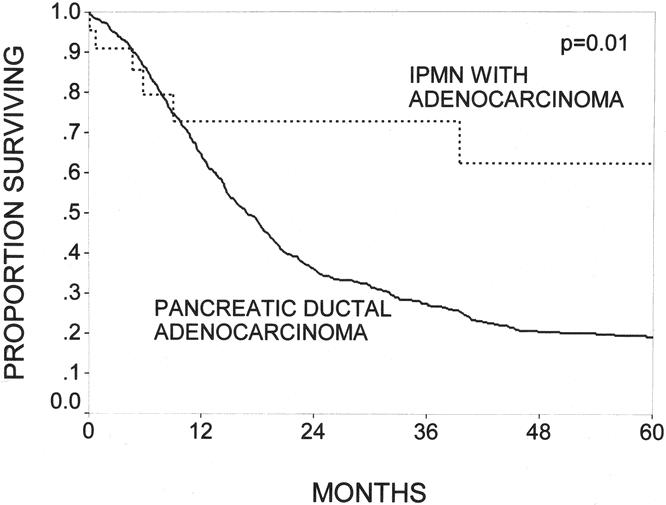
Figure 5. The Kaplan-Meier actuarial survival curves comparing patients with intraductal papillary mucinous neoplasms (IPMNs) with an infiltrating adenocarcinoma component (n = 22) and patients with infiltrating ductal adenocarcinoma of the pancreas without an associated IPMN (n = 698, P = .01). IPMN patients had 1-, 3-, and 5-year survival rates of 73%, 73%, and 62%; patients with pancreatic ductal adenocarcinoma had corresponding rates of 63%, 27%, and 19%.
Ten of the 22 patients with IPMNs with an associated infiltrating component had lymph nodes with metastatic tumor in the resection specimen. When compared with 529 node-positive patients with pancreatic ductal adenocarcinoma, the differences in survival were not significant. These 10 patients with node-positive IPMNs had a 1-year survival rate of 50% and a median survival of 12 months, whereas patients with node-positive ductal adenocarcinoma had a 1-year survival rate of 60% and a median survival of 15 months (P = NS). All patients with node-positive infiltrating IPMNs were deceased or censored at 39 months, so 5-year actuarial survival data are not presented.
Twelve of the 22 patients with IPMNs with an infiltrating component were node-negative. These node-negative IPMN patients had 1-, 5-, and 10-year actuarial survival rates of 92%, 92%, and 69%, compared with 71%, 26%, and 13% for patients with node-negative ductal adenocarcinoma not arising in association with an IPMN (n = 169, P = .008).
Log-rank tests were used to analyze univariate factors predictive of survival. Considering all 60 IPMN patients, neither tumor diameter nor the presence of noninvasive IPMN at the surgical margin significantly influenced survival. Patients treated via partial pancreatectomy (n = 48) were observed to have significantly improved survival compared with those undergoing total pancreatectomy (n = 12, P = .003). However, total pancreatectomy was performed in patients with advanced disease, because 7 of the 12 patients undergoing total pancreatectomy were lymph node-positive, and the mean tumor diameter in this group was 5.2 cm. Considering only the subset of patients with an IPMN with an associated infiltrating adenocarcinoma (n = 22), positive lymph node status was a significant predictor of an adverse outcome compared with negative lymph node status (P = .02).
DISCUSSION
Intraductal papillary mucinous neoplasms of the pancreas represent a distinct clinicopathologic entity being recognized and managed with increasing frequency (see Fig. 2). During the period of this study, the overall number of pancreatic resections per year at our institution has generally increased. Despite reports attributing the IPMN increase to the increased use and improved accuracy of diagnostic imaging, 8,15 this is likely not solely the case, as suggested by the disproportionate increase in IPMNs relative to mucinous cystadenomas, cystadenocarcinomas, and pancreatic ductal adenocarcinomas seen at our institution between 1995 and 2000. Increased recognition of the pathologic entity of IPMN may play some role. However, because the number of mucinous cystadenomas and cystadenocarcinomas has not decreased as the number of IPMNs has increased, it is unlikely that the increase in IPMNs is solely due to prior misclassification. Recently, the pathology on all mucinous cystadenomas, cystadenocarcinomas, and IPMNs resected at our institution has been retrospectively reviewed and reclassified using current diagnostic criteria. 18,23 Overall, we believe that the observed increase in IPMNs is real, multifactorial, and not fully explained.
Clinically, IPMNs present in a manner somewhat distinct from infiltrating ductal adenocarcinomas of the pancreas, although their demographics are similar. Whereas patients with ductal adenocarcinoma typically present with painless jaundice, abdominal pain is the most common feature associated with IPMNs (59% of patients) and obstructive jaundice is seen only infrequently (16%). Interestingly, 14% of patients with IPMNs in our series have a history of acute pancreatitis, a finding uncommon in patients with ductal adenocarcinoma (3%).
IPMNs are pathologically distinct from pancreatic ductal adenocarcinomas and from MCNs of the pancreas. IPMNs typically occur in the main pancreatic duct and major branches, whereas ductal adenocarcinomas are believed to arise from smaller precursors (PanINs) in the intralobular and interlobular ducts of the pancreas. Most IPMNs and ductal adenocarcinomas arise on the right side of the pancreas (head and uncinate process), whereas most MCNs arise on the left side of the pancreas (body and tail). IPMNs communicate with the pancreatic ductal system; MCNs typically do not. Histologically, in women with MCNs a dense “ovarian”-like stroma surrounds the epithelial cells. Such a stroma is never seen in IPMNs. Despite being larger than ductal adenocarcinomas, IPMNs with an infiltrating adenocarcinoma appear to be biologically less aggressive than ductal adenocarcinomas, with a lower incidence of nodal positivity, perineural invasion, and vascular invasion and a significantly better 5-year survival rate (62% vs. 19%;P = .01).
With the help of immunohistochemical techniques and a unified nomenclature system to classify the intraductal precursor lesions of infiltrating pancreatic ductal adenocarcinoma (see http://pathology.jhu.edu/pancreas/panin, proposed by the 1999 NIH-sponsored Pancreatic Cancer Think Tank held in Park City, UT), a histologic and genetic progression model of pancreatic ductal cancer has been developed. 19 In this model, noninvasive precursor lesions (PanINs) can progress to infiltrating ductal adenocarcinoma. The association of some IPMNs with an infiltrating adenocarcinoma suggests a similar progression model, but one that is likely genetically disparate from that of PanINs and pancreatic ductal adenocarcinomas.
Labeling of IPMNs for Dpc4 protein expression supports the contention that they are genetically different from PanINs and infiltrating ductal adenocarcinomas. All noninvasive IPMNs and 84% of IPMNs associated with an infiltrating adenocarcinoma expressed the Dpc4 protein, whereas only 70% of intraductal (PanIN-3) and only 45% of infiltrating ductal pancreatic adenocarcinomas did so. 19 Further studies of the molecular genetics of IPMNs need to be undertaken in an attempt to determine the genetic alterations that are critical for an aggressive phenotype and to characterize differences between IPMNs and ductal adenocarcinomas.
The appropriate extent of pancreatic resection indicated for patients with IPMNs remains uncertain. In the current series, 22% of patients with an IPMN underwent total pancreatectomy, typically performed for extensive tumors involving much of the pancreas. The need for such extensive pancreatic resection is supported by the past experiences of others, where 10% to 29% of IPMN patients have undergone total pancreatectomy. 13,14,24 The survival data in the current series for all patients with IPMNs indicate 1-, 3-, and 5-year actuarial survival rates of 82%, 67%, and 57%, respectively. Interestingly, no difference in survival was observed between patients with or without infiltrating components to their tumors. However, the cause of death was unknown for four of the noninvasive IPMN patients, and one patient died of complications of diabetes and not from tumor.
The subsequent recognition of cancer in patients with noninvasive IPMNs, and the observation of a high risk of recurrence (or second metachronous primary) in patients with apparently completely resected IPMNs with an infiltrating adenocarcinoma component appear to support the concept that IPMNs may represent a widespread neoplastic field defect in the pancreatic ductal epithelium. If the entire pancreas is at risk for the development of invasive cancer, then issues related to the extent of pancreatic resection and postoperative surveillance are critical. Based on our limited data concerning the cause of death in IPMN patients, it appears that patients who undergo partial pancreatic resection for IPMNs remain at risk for the development of invasive cancer in their pancreatic remnant. Certainly, patients who undergo partial pancreatectomy for IPMNs are candidates for surveillance, early detection strategies, and future chemopreventive approaches. Although the most effective means of such surveillance is not known, the use of pancreatic ductal visualization and sampling techniques (endoscopic ultrasound, fine-needle aspiration, or pancreatoscopy) plus the application of imaging techniques (computed tomography, magnetic resonance cholangiopancrea-tography, endoscopic ultrasound) and tumor marker strategies recently proposed to screen high-risk families for pancreatic cancer 25 may be appropriate for IPMN patients who have undergone partial pancreatic resection. The role of total pancreatectomy remains uncertain. Although patients with large tumors involving the entire pancreas typically undergo resection via total pancreatectomy, this extensive resection does not prevent tumor-related death. In this series, although the number of patients managed with total pancreatectomy was only 12, 4 of these patients (33%) died of adenocarcinoma. Thus, in this small series, total pancreatectomy did not prevent tumor-related death.
In this and previous reports, 11,13 patients with IPMNs have been observed to have improved long-term survival compared with patients with pancreatic ductal adenocarcinoma not arising in the setting of an IPMN. The higher incidences of nodal and margin positivity, as well as perineural and vascular invasion, seen with ductal adenocarcinomas may account for some of this survival difference. There was no difference in survival between the two groups when only node-positive patients were examined. However, in node-negative patients, there was an improvement in long-term survival in patients who had IPMNs with an invasive component. This observation suggests a benefit to resection of IPMNs before the development of node-positive invasive cancer, supporting an aggressive approach to the management of these tumors. The role of chemotherapy or radiation therapy after resection in patients with IPMNs remains unknown.
The treatment approach to patients with IPMNs clearly contrasts with the management strategy for patients with MCNs of the pancreas. When MCNs are completely resected and fully examined histologically, they can be categorized into one of four groups: invasive mucinous cystadenocarcinoma, MCN with in situ carcinoma, borderline MCN, and mucinous cystadenoma. Recent data have indicated that neoplasms in the latter three categories never recur or metastasize. 26,27 Obviously, the pathologic distinction between IPMN and MCN is crucial to allow for appropriate management.
Additional information is needed about the incidence, natural history, etiologic factors, and molecular genetics of IPMNs. We hope that as additional data are accumulated, advances can be made in the detection, surveillance, and management of what appears to be an increasing volume of patients with IPMNs.
Addendum
From January 1, 2001, through April 30, 2001, 10 additional patients with IPMNs of the pancreas have undergone surgical resection at the Johns Hopkins Hospital.
DISCUSSION
Dr. Andrew L. Warshaw (Boston, Massachusetts): I congratulate Dr. Sohn on her continuing productivity at Johns Hopkins. Like all outstanding papers, it raises many questions.
Clearly, this is a spectrum of disease from small papillations and benign adenomas through aggressive and lethal malignancy. We are all seeing more of these. Our own series is just over 80 in this same time period.
I am struck by the not-so-good survival of what appears to be benign tumors. At four years, the survival in the benign non-invasive tumors was 64% compared to 62% for the invasive cancers. Does this mean that we can’t distinguish benign from malignant neoplasm during the long ramp-up of this disease? Or that the disease was not fully clear at operation? Or that there is really a field defect manifesting as new neoplasm arising in the remnant?
I notice that 6 out of 38 of your non-invasive tumors had positive margins while none of 22 of the invasive cancers had positive margins. Does this mean that we can’t rely on frozen sections? Do you use pancreatoscopy intraoperatively to try to evaluate the remnant that you plan to leave?
How close are you to recommending total pancreatectomy for what appears to be a field defect? If you are not going to do a total pancreatectomy, how do you decide the extent of resection? Some of these tumors are really quite small, less than a centimeter in size, and it seems a little excessive in such a case to excise the whole pancreas.
What is the appropriate post-resection surveillance? Do you use endoscopic ultrasound for looking at the pancreatic remnant? Is there any reliable strategy?
Differing from your experience, we have encountered only one recurrence in the pancreatic remnant after resection for non-invasive neoplasm. In our experience these tumors are indolent and highly curable early, even though they progress to high-grade cancers if not removed. You have looked at the loss of the dpc4 gene as one of the markers for evolving malignancy. We have looked at K-ras and P-53. We have found K-ras codon 12 mutations in most malignant IPMN. P-53, however, changes when the tumor becomes aggressive, invasive, and metastatic. Like colon cancer, there appears to be an accumulation of genetic hits which define the malignant phase.
Have you any data regarding the BRCA-2 mutation in IPMN, as in some pancreatic cancers? And that leads to my final question, which is whether you have detected any familial links or predispositions?
Presenter Dr. Taylor A. Sohn (Baltimore, Maryland): Dr. Warshaw, I thank you for your questions. You talked initially about the survival rate being similar between our patients with non-invasive tumors and those with invasive adenocarcinomas. You need to remember that this is based on limited survival data. In four patients in our non-invasive group we do not know the cause of death. It remains striking, however, that four of the patients in the non-invasive group died of presumed recurrent pancreatic adenocarcinoma.
In order to be defined as having a non-invasive IPMN, the specimen had to be submitted in its entirety. The diagnosis was not made on frozen sections. So I think that our diagnosis of IPMN-adenoma is correct and that, in fact, this is a field defect that we are seeing and the patients who develop adenocarcinoma down the road are developing new primaries in mucosa at risk.
There were no patients who had invasive cancer at the surgical margin; however, it should be noted that six patients had non-invasive IPMN at the surgical margin. Only one of these six patients has died in follow-up.
We do not routinely use intraoperative pancreatoscopy, although perhaps in the future intraoperative pancreatoscopy would be useful in identifying small lesions that we miss on our routine staging and imaging.
You bring up the important question regarding the extent of pancreatectomy. If we are suggesting this is a field defect, do we do a total pancreatectomy? If you look at the 12 patients who had a total pancreatectomy in our series, their survival was worse than those who had a partial pancreatectomy. But this is largely because the majority of the total pancreatectomy patients were node positive and they had much larger tumors with a mean diameter of 5.2 centimeters. At this time we do not recommend total pancreatectomy for all patients, but we would recommend resecting all gross disease and using careful surveillance.
At Hopkins currently we have identified high risk patients in our National Familial Pancreas Tumor Registry (NFPTR): We have initiated a screening program for those individuals with two or more first degree relatives with pancreatic cancer. This screening involves physical exam, tumor markers, ERCP with sampling of pancreatic juice, spiral CT and endoscopic ultrasound imaging. We would propose to put these IPMN patients treated via partial pancreatectomy in a similar high risk category and suggest a surveillance program similar to that.
You asked for our experience with regard to BRCA-2 mutations. I don’t have any information on that. In addition, I don’t know of any familial syndrome specifically associated with IPMNs.
Dr. L. William Traverso (Seattle, Washington): Dr. Sohn has effectively presented a review of your experience with this process. I want to commend you on a great manuscript, which I was able to review well in advance. Thank you.
The issue today for us as surgeons taking care of these problems is when to resect, how much to resect, and then how to follow them. And these are very critical issues, particularly when these people are older and some of them have no symptoms. In addition, some are not good candidates in surgery. So balancing candidate fitness for surgery, symptoms and imaging, surgeons should use judgment to come up with some plan to make resection decisions.
So what items are provided to date to help surgeons make these decisions? We have 63 patients that we have resected in the same time period. I would like to compare these small numbers to your small numbers to give some idea of what to do and ask a few questions.
First of all, in 1995 there were only 105 cases in the literature. You have now added 60 — in fact, the abstract was written with 55 cases, so in the meantime you have added five more. I see one of these cases once a week where beginning in the 1990s it might have been once every couple of months. So indeed they are increasing in frequency.
37% of your cases were invasive and 44% of ours were. About half of your patients presented with pain and almost every one of our patients presented with pain, 85%. 14% of your patients presented with pancreatitis but the majority of our patients presented with pancreatitis. That may be due to our referral pattern. But if one sees a patient with recurrent idiopathic pancreatitis, IPMN may be one of the causes.
We have found in multivariate analysis that a predictor of the benign disease is the presence of mucus. In your manuscript you did not address this? Did you see mucus production as a predictor of malignancy or benign disease as we did?
Their is another important classification, that of main pancreatic duct involvement or a side branch involvement only — the Japanese have found and we have found that the side branch only involvement has less of a chance of developing malignancy. Did you see that?
Much to resect? 92% of your patients were resected with a pancreaticoduodenectomy, which about one out of five were total pancreatectomies. In our group, about three out of four were resected with a pancreaticoduodenectomy and about 16% were total pancreatectomies. The remainder of ours were distal pancreatectomies.
Your follow-up averaged 28 months and ours was 34 months. However, our five-year survival rates with IPMN in patients with invasive cancer was 43%, not the higher rate that you see of 64%. If we combined these patients and followed them longer, invasive cancer of IPMN would have a better prognosis than standard adenocarcinoma.
In addition to recurrence of tumor, we have had six patients recur either with symptoms or with tumor. This may be not a recurrence of a tumor but the presentation of a new one. And we have seen this three times, after a Whipple procedure the tumor will occur remotely in the pancreatic remnant.
The question is, how should we — as Dr. Warshaw indicated — how should we best follow these patients? Because that pancreatic remnant is the important thing to evaluate over time. How do we do this, with MRCP, intraoperative ultrasound, EUS, CT scan, ERCP, FISH, or all of those things?
Dr. Taylor A. Sohn: Thank you, Dr. Traverso. Similar to you, we are seeing many patients with IPMNs. We have seen nine new cases since January of this year. We feel that these tumors clearly have a malignant potential and should be identified and resected in their entirety. Again, we don’t have data to support doing a total pancreatectomy, so we feel you should resect the gross lesion and then carefully survey patients with retained at-risk glands.
That brings me to your last question, which we already talked about. We would screen these patients much as we screen our high risk families in the NFPTR and our patients with known genetic syndromes associated with pancreatic adenocarcinoma.
We did not look at mucous production in the tumor as a factor predictive of survival. However, our pathologists, with those from Wayne State, Dartmouth and others have investigated the expressions of MUC1 and MUC2-3 (glycoproteins reportedly reflecting “aggressive” and “indolent” phenotypes in pancreatic cancer) in 74 IPMNs. Fifty-four percent of the IPMNs were positive for MUC2, while only 20% of IPMNs expressed MUC1.
Dr. E. Christopher Ellison (Columbus, Ohio): Thank you, Dr. Sohn, for an excellent paper presentation. Is there any relation to CA 19-9 in these lesions? And perhaps you could comment on use of frozen section at the time of operation.
Dr. Taylor A. Sohn: We looked at CA 19-9 levels. Some IPMN patients have normal CA 19-9s, while others are markedly elevated. There was no correlation between survival or the presence of adenocarcinoma when we looked at the CA 19-9 levels.
Dr. Lawrence W. Way: In your presentation you didn’t include patients who were unresectable, for example, because of local extention of the disease. Did you encounter such patients? How frequent were they? And what were the causes of unresectability?
Dr. Taylor A. Sohn: This series does not include any of our unresectable patients. Unfortunately, when you are looking at resectability rates it is often difficult to get a denominator, as many patients are referred to our center and have clearly unresectable lesions and are never seen by a surgeon. A tumor that would be unresectable would be the same as that for a pancreatic adenocarcinoma, anything obstructing the superior mesenteric vessels or portal vein, encasing the celiac or mesenteric arteries, or a tumor with distant metastases.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association, April 26–28, 2001, the Broadmoor Hotel, Colorado Springs, Colorado.
Supported in part by a grant from the National Institute of Health (P50-CA62924).
Correspondence: Charles J. Yeo, MD, The Johns Hopkins Medical Institutions, Blalock 606, 600 N. Wolfe St., Baltimore, MD 21287-4606.
E-mail: cyeo@jhmi.edu
Accepted for publication April 26, 2001.
References
- 1.ReMine SG, Frey D, Rossi RL. Cystic neoplasms of the pancreas. Arch Surg 1987; 122: 443–446. [DOI] [PubMed] [Google Scholar]
- 2.Moesinger RC, Talamini MA, Hruban RH, et al. Large cystic pancreatic neoplasms: pathology, resectability, and outcome. Ann Surg Oncol 1999; 6: 682–690. [DOI] [PubMed] [Google Scholar]
- 3.Compagno J, Oertal JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas). Am J Clin Pathol 1978; 69: 289–298. [DOI] [PubMed] [Google Scholar]
- 4.Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with over and latent malignancy (cystadenocarcinoma and cystadenoma). Am J Clin Pathol 1978; 69: 573–580. [DOI] [PubMed] [Google Scholar]
- 5.Itai Y, Ohhashi K, Nagai H, et al. “Ductectatic” mucinous cystadenoma and cystadenocarcinoma of the pancreas. Radiology 1986; 161: 697–700. [DOI] [PubMed] [Google Scholar]
- 6.Kloppel G, Solcia E, Longnecker DS, et al. Histological typing of tumours of the exocrine pancreas. In: World Health Organization international classification of tumors, 2d ed. Berlin: Springer; 1996: 11–20.
- 7.Adsay NV, Longnecker DS, Klimstra DS. Pancreatic tumors with cystic dilatation of the ducts: intraductal papillary mucinous neoplasms and intraductal oncocytic papillary neoplasms. Sem Diag Pathol 2000; 17: 16–30. [PubMed] [Google Scholar]
- 8.Agostini S, Choux R, Payan MJ, et al. Mucinous pancreatic duct ectasia in the body of the pancreas. Radiology 1989; 170: 815–816. [DOI] [PubMed] [Google Scholar]
- 9.Bastid C, Bernard JP, Sarles H, et al. Mucinous ductal ectasia of the pancreas: a premalignant disease and a cause of obstructive pancreatitis. Pancreas 1991; 6: 15–22. [DOI] [PubMed] [Google Scholar]
- 10.Ohta T, Nagakawa T, Akiyama T, et al. The “duct-ectatic” variant of mucinous cystic neoplasm of the pancreas: clinical and radiological studies of seven cases. Am J Gastroenterol 1992; 87: 300–304. [PubMed] [Google Scholar]
- 11.Kench JG, Eckstein RP, Smith RC. Intraductal papillary-mucinous neoplasms of the pancreas: a report of five cases with immunohistochemical findings. Pathology 1997; 29: 7–11. [DOI] [PubMed] [Google Scholar]
- 12.Rivera JA, Fernandez-del Castillo C, Pins M, et al. Pancreatic mucinous ductal ectasia and intraductal papillary neoplasms: a single malignant clinicopathologic entity. Ann Surg 1997; 225: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paal E, Thompson LD, Pryzygodzki RM, et al. A clinicopathologic and immunohistochemical study of 22 intraductal papillary mucinous neoplasms of the pancreas, with a review of the literature. Mod Pathol 1999; 12: 518–528. [PubMed] [Google Scholar]
- 14.Siech M, Tripp K, Schmidt-Rohlfing B, et al. Intraductal papillary mucinous tumor of the pancreas. Am J Surg 1999; 177: 117–120. [DOI] [PubMed] [Google Scholar]
- 15.Longnecker DS. Observations on the etiology and pathogenesis of intraductal papillary-mucinous neoplasms of the pancreas. Hepato-Gastroenterology 1998; 45: 1973–1980. [PubMed] [Google Scholar]
- 16.Yeo CJ, Cameron JL, Sohn TA, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma. Comparison of morbidity, mortality and short-term outcome. Ann Surg 1999; 229: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilentz RE, Su GH, Dai JL, et al. Immunohistochemical labeling for dpc4 mirrors genetic status in pancreatic adenocarcinomas: a new marker for dpc4 inactivation. Am J Pathol 2000; 156: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, et al. Dpc4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol 2000; 157: 755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that dpc4 inactivation occurs late in neoplastic progression. Cancer Res 2000; 60: 2002–2006. [PubMed] [Google Scholar]
- 20.Kaplan E, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 21.Sosa JA, Bowman HM, Bass EB, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg 1998; 228: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieberman MD, Kilburn H, Lindsey, et al. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg 1995; 222: 638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iacobuzio-Donahue CA, Wilentz RE, et al. Dpc4 protein in mucinous cystic neoplasms of the pancreas: frequent loss of expression in invasive carcinomas suggests a role in genetic progression. Am J Surg Pathol 2000; 24: 1544–1548. [DOI] [PubMed] [Google Scholar]
- 24.Sugiyama M, Atomi Y. Intraductal papillary mucinous tumors of the pancreas. Imaging studies and treatment strategies. Ann Surg 1998; 228: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hruban RH, Canto MI, Yeo CJ. Prevention of pancreatic cancer and strategies for management of familial pancreatic cancer. Digestive Dis 2000, 19: 76–84. [DOI] [PubMed] [Google Scholar]
- 26.Wilentz RE, Albores-Saavedra J, Zahurak M, et al. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol 1999; 23: 1320–1327. [DOI] [PubMed] [Google Scholar]
- 27.Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: Clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 1999; 23: 410–422. [DOI] [PubMed] [Google Scholar]