Abstract
Objective
To analyze the late complications after endovascular graft repair of elective abdominal aortic aneurysms (AAAs) at the authors’ institution since November 1992.
Summary Background Data
Recently, the use of endovascular grafts for the treatment of AAAs has increased dramatically. However, there is little midterm or long-term proof of their efficacy.
Methods
During the past 9 years, 239 endovascular graft repairs were performed for nonruptured AAAs, many (86%) in high-risk patients or in those with complex anatomy. The grafts used were Montefiore (n = 97), Ancure/EVT (n = 14), Vanguard (n = 16), Talent (n = 47), Excluder (n = 20), AneuRx (n = 29), and Zenith (n = 16). All but the AneuRx and Ancure repairs were performed as part of a U.S. phase 1 or phase 2 clinical trial under a Food and Drug Administration investigational device exemption. Procedural outcomes and follow-up results were prospectively recorded.
Results
The major complication and death rates within 30 days of endovascular graft repair were 17.6% and 8.5%, respectively. The technical success rate with complete AAA exclusion was 88.7%. During follow-up to 75 months (mean ± standard deviation, 15.7 ± 6.3 months), 53 patients (22%) died of unrelated causes. Two AAAs treated with endovascular grafts ruptured and were surgically repaired, with one death. Other late complications included type 1 endoleak (n = 7), aortoduodenal fistula (n = 2), graft thrombosis/stenosis (n = 7), limb separation or fabric tear with a subsequent type 3 endoleak (n = 1), and a persistent type 2 endoleak (n = 13). Secondary intervention or surgery was required in 23 patients (10%). These included deployment of a second graft (n = 4), open AAA repair (n = 5), coil embolization (n = 6), extraanatomic bypass (n = 4), and stent placement (n = 3).
Conclusion
With longer follow-up, complications occurred with increasing frequency. Although most could be managed with some form of endovascular reintervention, some complications resulted in a high death rate. Although endovascular graft repair is less invasive and sometimes effective in the long term, it is often not a definitive procedure. These findings mandate long-term surveillance and prospective studies to prove the effectiveness of endovascular graft repair.
Since the Food and Drug Administration (FDA)’s approval of the Guidant Ancure graft (Guidant, San Jose, CA) and the AneuRx graft (Medtronic, Inc., Minneapolis, MN) for the treatment of abdominal aortic aneurysms (AAAs), interest in and use of endovascular grafts (EVGs) have increased dramatically in the United States as well as in Europe. However, FDA approval was made based on procedural safety with a follow-up of only 12 months, and there is little midterm or long-term proof of their efficacy. 1 EVGs can fail in a number of ways, including graft migration, infection, deterioration, or component separation, all of which can contribute to the occurrence of late endoleaks, graft thrombosis, and ultimately rupture of the aneurysm. 2–18
Recently, several investigators reported their midterm results and reached differing conclusions. In one positive article published by May et al, 11 they showed that the long-term patient survival rate was improved after EVG repair compared with that of patients undergoing open surgery. Zarins et al 4 reported similar encouraging findings. However, several other investigators have raised concerns regarding the midterm durability of EVG repair. Holzenbein et al 10 recently reported their midterm results after EVG repair of AAAs. During a mean follow-up period of 18 months, 26% of their patients had to undergo a secondary procedure to treat EVG-related complications. Similar concerns have also been raised by others. 2,7–9,12,17,18 In the present study, we analyzed the durability and late complications after EVG repair of AAAs at our institution, where we first used an EVG to treat an AAA in November 1992. 19
METHODS
During the past 9 years, 239 EVG repairs were performed for nonruptured AAAs. During the same period, 20 ruptured AAAs were treated with EVGs but were excluded from this study. 20 The mean age of the patients was 76 ± 7 years, and 85% were men. The prevalence of associated comorbidities was as follows: coronary artery disease 87%, chronic obstructive pulmonary disease 59%, diabetes mellitus 30%, hypertension 89%, and chronic renal insufficiency 15%. The mean American Society for Anesthesiology (ASA) score was 3.0 ± 0.7, and 86% of the patients had an ASA score of 3 or more. Many of the patients were high risk or had complex anatomy. The mean size of the AAA was 6.3 ± 1.2 cm. The EVGs used were Montefiore EVG or MEGS (n = 97), Ancure/EVT (n = 14), Vanguard (Boston Scientific Corp., Natick, MA) (n = 16), Talent (World Medical/Medtronic) (n = 47), Excluder (W.L. Gore, Flagstaff, AZ) (n = 20), AneuRx (n = 29), and Zenith (Cook Inc., Indianapolis, IL) (n = 16) (Fig. 1). All but the AneuRx and most of the Ancure EVG repairs were performed as part of a phase 1 or phase 2 U.S. clinical trial under either an investigator- or an industry-sponsored IDE from the FDA. The procedures were conducted in accordance with the ethical standards of our institutional review board. Each patient was informed of the investigational nature of the procedure (for trial EVGs), and informed consent was then obtained. All patients were followed with computed tomographic scans taken 1, 3, 6, and 12 months after the procedure and every 12 months thereafter. Procedural outcomes and follow-up results were prospectively recorded.

Figure 1. Types of endovascular grafts currently undergoing clinical trials (from left): Montefiore endovascular graft; Ancure graft (EVT-Guidant, Menlo Park, CA); Vanguard graft (Boston Scientific Corp., Oakland, NJ); Talent graft (World Medical Corp., Miami, FL); Corvita graft (Boston Scientific Corp.); AneuRx graft (Medtronic, Minneapolis, MN); Excluder graft (W.L. Gore, Flagstaff, AZ); Zenith graft (Cook, Bloomington, IL); Power Link graft (Endologix, Phoenix, AZ); Quantum graft (Cordis, Warren, NJ).
Operative Technique
The operative techniques for EVG deployment have been described elsewhere. 14,20–23 Briefly, all procedures were performed in the operating room under either general (51%) or epidural (49%) anesthesia. Bilateral groin incisions were made and the femoral arteries were exposed. The EVG was deployed under fluoroscopic control using the OEC 9800 (GE/OEC, Salt Lake City, UT) or the BV 300 (Philips Medical Systems, the Netherlands). Completion angiography was obtained at the end of the procedure, and the patency of the EVG and renal and hypogastric arteries and the presence or absence of various types of endoleaks were assessed (Figs. 2 and 3).
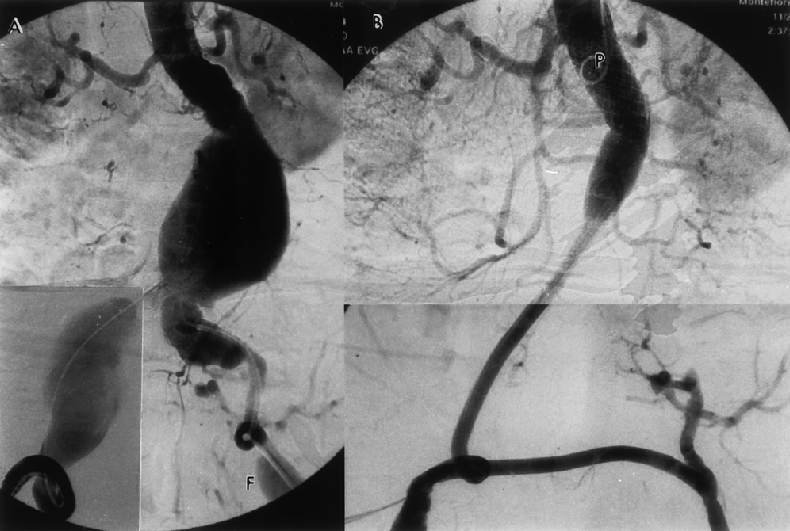
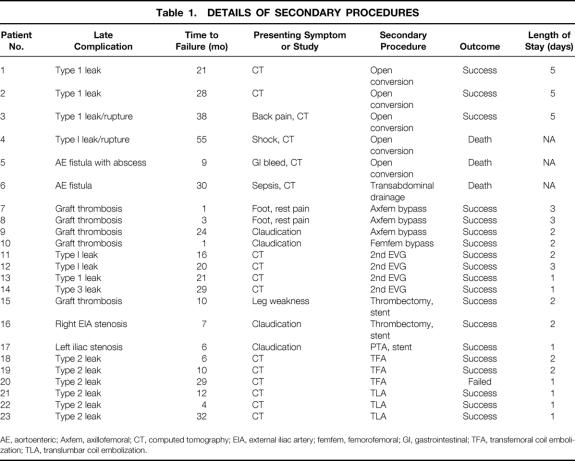
Figure 2. Large complex abdominal aortic aneurysm (AAA) in an elderly patient with severe chronic obstructive pulmonary disease. (A) Intraoperative angiogram reveals complex aneurysm with 1) angulated proximal neck; 2) 7.5-cm AAA; 3) 9-cm right common iliac artery aneurysm; 4) 3-cm left common iliac artery aneurysm; 5) severely tortuous access vessels; and 6) left femoral aneurysm (F). (B) Successful endovascular graft repair was performed with the Montefiore endovascular graft. Note the absence of endoleaks as well as lack of visualization of all the aneurysms.
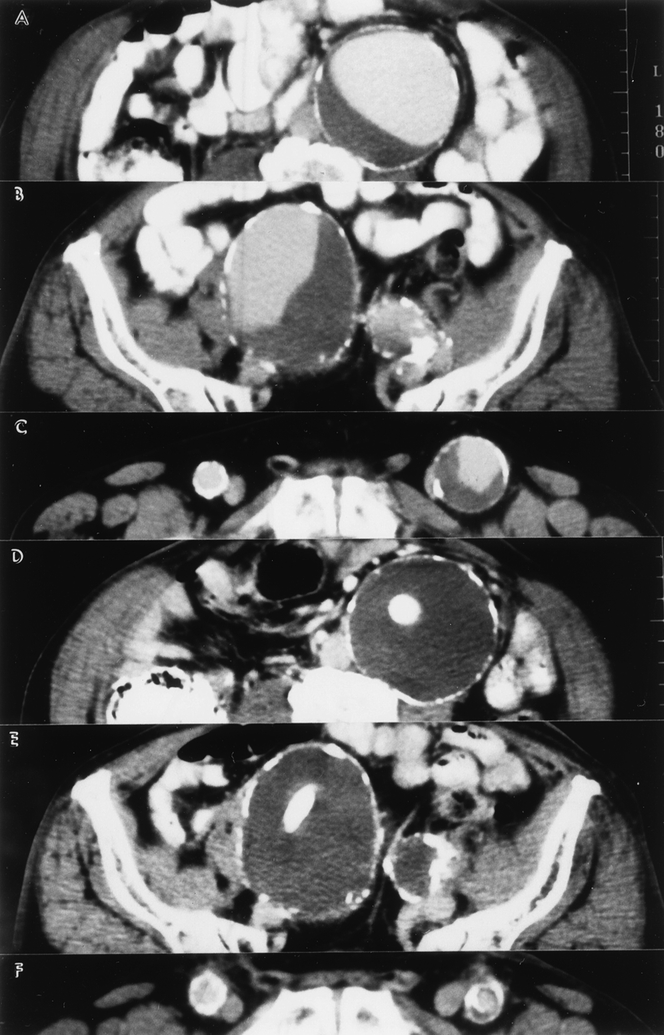
Figure 3. Same patient as in Figure 2. (A-C) Preoperative computed tomography scan shows a 7.5-cm abdominal aortic aneurysm, 9-cm right and 3-cm left common iliac artery aneurysms, and a 3.5-cm left femoral aneurysm. Note severe calcification in the entire arterial system. (D-F) Postoperative computed tomography scan shows complete exclusion of all aneurysms.
Definitions
Technical success was defined according to the Society for Vascular Surgery/International Society for Cardiovascular Surgery reporting standards as successful EVG deployment without the need for surgical conversion or death; lack of a persistent (>48 hours) type 1 or type 3 endoleak; and a patent graft. 6
Primary clinical success was defined as the lack of enlargement of the aneurysm sac (>0.5 cm); the lack of any endoleak (spontaneously sealed endoleaks within 6 months were considered a success); and the lack of the need for any secondary intervention or additional open surgical procedure. Because we were evaluating the midterm and long-term outcomes, for the purpose of this study only patients who survived the operation with a technical success were analyzed by the life table method.
Secondary success was defined as continued clinical success after a salvage interventional procedure without the need for an open conversion to replace the previously deployed EVG.
Survival Analysis
Life table survival analysis was determined for all causes of death, including both aneurysm-related and -unrelated deaths, and for aneurysm-related deaths alone. Aneurysm-related deaths included all perioperative (30-day) deaths, death related to secondary procedures, open conversions, and other graft-related deaths from aortoenteric fistulas and aneurysm ruptures.
Treatment methods of late failures
Endoleaks
When there was evidence of an endoleak or aneurysm enlargement, computed tomography scans, duplex ultrasonography, and arteriography were obtained as needed. The basic treatment strategies for various endoleaks are shown in Figure 4. 7,15,24 In summary, when the AAA was enlarging, short and large-diameter endoleak channels (mostly types 1 and 3) were treated with either insertion of a second EVG or a proximal or distal cuff (Figs. 5–9) or surgical conversion (Fig. 10). Long and small-diameter endoleak channels were treated by inducing thrombosis. This included transarterial and translumbar coil embolization as well as the temporary termination of chronic anticoagulation therapy (Figs. 11–13). Conservative (nonoperative) or endovascular treatments were preferentially performed when possible. Some patients were managed conservatively if the AAA was smaller than 5.5 cm and not enlarging, or the patient was a prohibitive risk for any intervention.
Figure 4. Basic treatment strategy for various endoleaks (ELS).
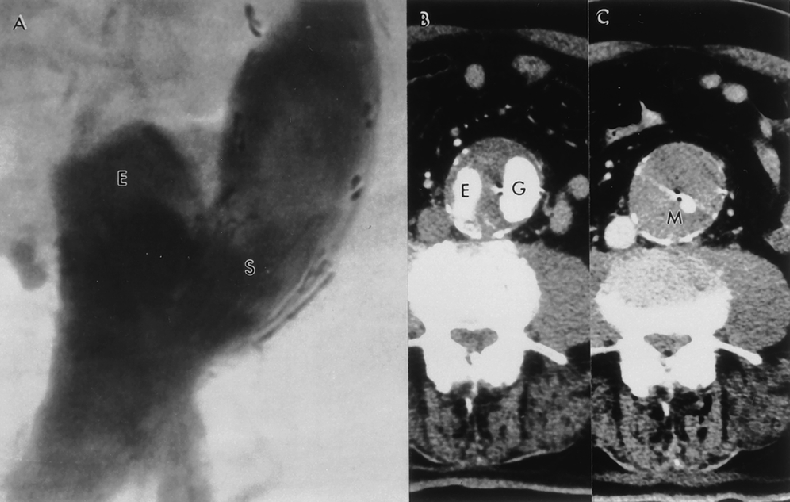
Figure 5. This 80-year-old patient received a tube endovascular graft 75 months ago. (A,B) Eighteen months after the original endovascular graft (G) repair, the patient developed a distal type 1 endoleak (E). Because the endoleak channel was short and had a large diameter, inducing thrombosis would not be effective in reducing intrasac pressure; therefore, a second EVG was needed to exclude the aneurysm. (C) The Montefiore endovascular graft (M) was inserted via a femoral approach within the previous endovascular graft and the endoleak was treated. Seventy-five months after the initial procedure, the patient continues to do well with continued secondary clinical success.
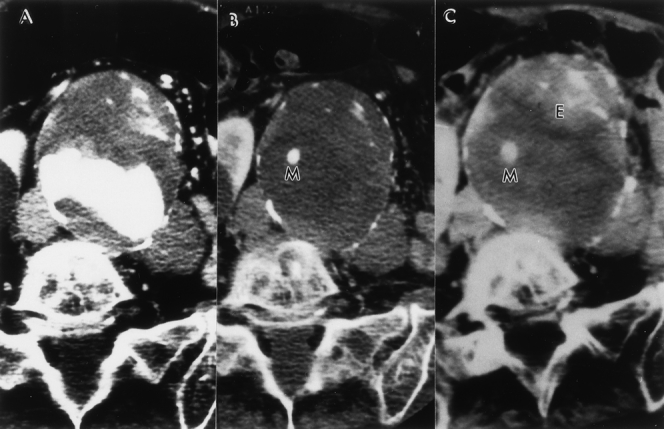
Figure 6. (A) Preoperative computed tomography scan of an 81-year-old woman who had oxygen-dependent chronic obstructive pulmonary disease and an 8.5-cm abdominal aortic aneurysm (AAA). (B) This AAA was successfully treated with a Montefiore endovascular graft with no evidence of an endoleak. (C) Eighteen months after endovascular graft repair, the patient developed a proximal type 1 endoleak (E) and subsequently developed abdominal pain. The AAA measured 9 cm. Insertion of a second Montefiore endovascular graft (M) resulted in resolution of both the endoleak and abdominal pain. Thirty-six months after surgery, the patient is doing well with continued secondary success.
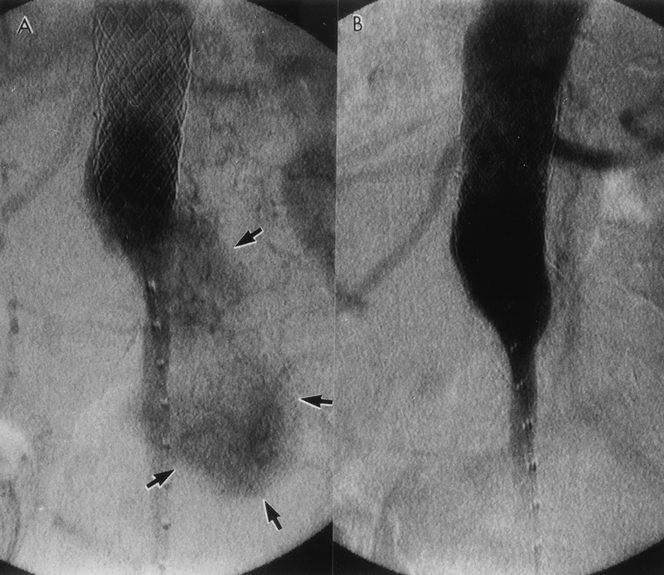
Figure 7. (A) Same patient as in Figure 6. An intraoperative angiogram obtained at the time of the secondary intervention reveals the presence of a proximal type 1 endoleak with a short and large-diameter channel. (B) The insertion of a second Montefiore endovascular graft resulted in resolution of the endoleak.
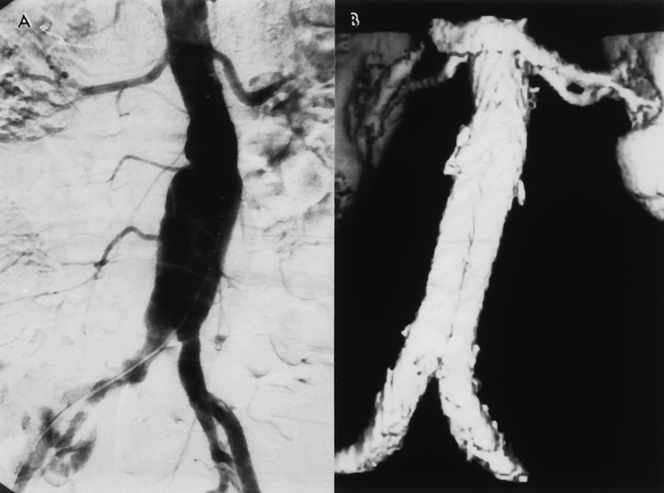
Figure 8. (A) Preoperative angiogram of a patient with a 6-cm abdominal aortic aneurysm. (B) Postoperative computed tomography scan shows complete exclusion of the aneurysm.
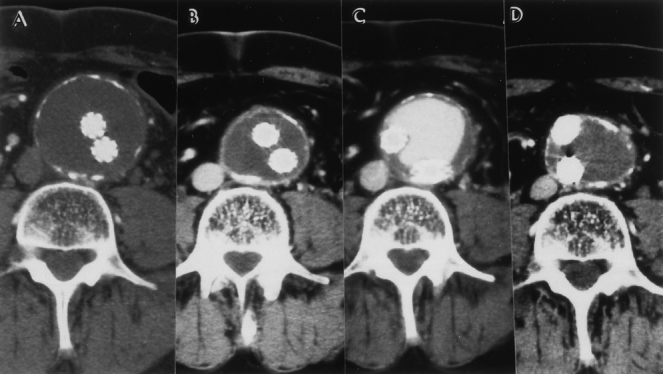
Figure 9. (A) Immediate postoperative contrast computed tomography (CT) scan of the patient in Figure 8. The CT scan shows complete exclusion of the 6-cm abdominal aortic aneurysm (AAA) with no evidence of an endoleak. (B) Postoperative CT scan (12 months) shows continued exclusion of the AAA with shrinkage of the AAA sac (4 cm). (C) Postoperative CT scan (21 months) shows an endoleak with an acutely enlarging AAA sac. This endoleak was a type 1 endoleak (distal attachment). It was treated by deploying a second endovascular graft to bridge the defect between the separated limb and the left common iliac artery. (D) CT scan obtained 52 months after the initial repair and 34 months after the secondary intervention. The AAA has shrunk without evidence of further endoleak.
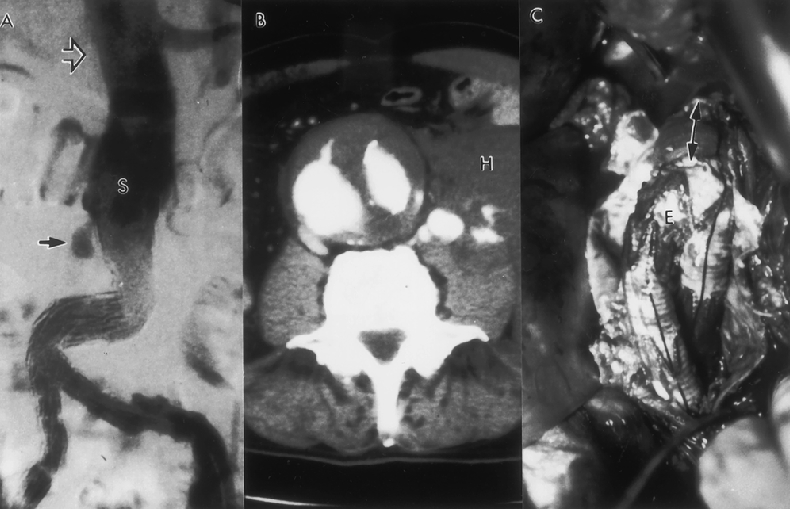
Figure 10. (A) Type 1 endoleak (arrow) developed 24 months after endovascular graft repair. This endoleak was due to migration of the proximal portion of the stent-graft (S). Note the excessive kinking of the limb of the graft as a result of distal migration. The open arrow denotes the location where the proximal stent was deployed initially. (B) Computed tomography scan reveals an endoleak. In addition, the aneurysm had ruptured and a retroperitoneal hematoma (H) can be seen. (C) Open conversion was performed on an urgent basis. The proximal part of the graft was excised and the remainder of the endovascular graft (E) was bridged to the proximal aneurysm neck with a short segment of standard graft (arrows).
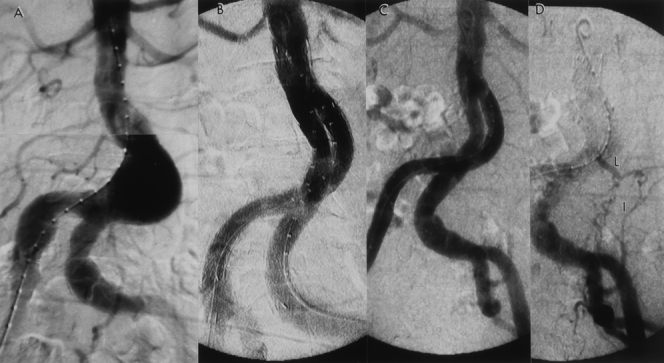
Figure 11. (A) Preoperative angiogram reveals a 5-cm abdominal aortic aneurysm (AAA) with tortuous iliac arteries. (B) Completion angiogram following the Gore-Excluder graft implantation, shows successful exclusion of the AAA with no signs of an endoleak. (C) The patient developed a late endoleak at 18 months, and a transfemoral angiogram was obtained. Note the lack of graft migration. (D) Delayed image of the angiogram reveals a type 2 endoleak arising from the left iliolumbar artery (I), which was feeding the aneurysm via a patent lumbar artery (L). Chronic anticoagulation therapy was terminated for 3 months; however, this endoleak persisted with further enlargement of the AAA.
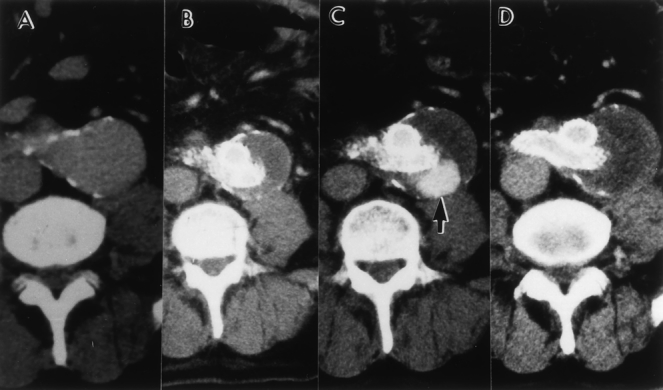
Figure 12. Serial computed tomography (CT) scans of the patient in Figure 11. (A) Preoperative CT shows a 5-cm abdominal aortic aneurysm (AAA). (B) Six months after endovascular graft repair. An endoleak is not visualized and the AAA has shrunk. (C) CT scan after 12 months shows the presence of an endoleak and an enlarging AAA. (D) CT scan obtained 20 months after translumbar coil embolization. The endoleak has resolved.
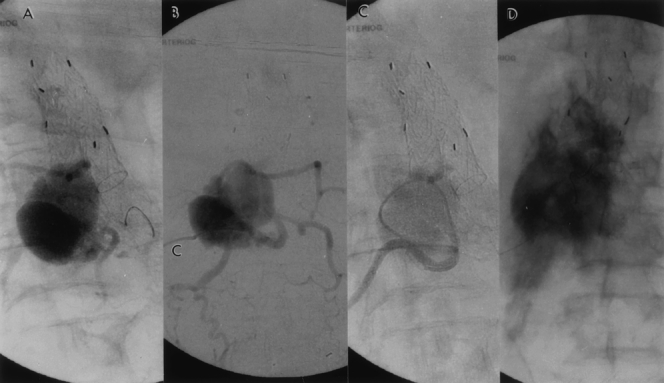
Figure 13. (A,B) Because conservative therapy failed, translumbar puncture of the sac was performed. The sac-gram reveals the presence of multiple feeding arteries in addition to the iliolumbar artery that was depicted by the standard angiogram in Figure 11D. Sac pressure was equivalent to systemic blood pressure (c, translumbar catheter). (C) Selective coil embolization of all four lumbar arteries was performed. (D) Completion sac-gram shows lack of communication between the lumbar arteries and the abdominal aortic aneurysm (AAA) sac, and the contrast is stagnant in the isolated AAA. The AAA sac pressure measured 40 mm Hg on completion of the selective coil embolization.
Failing or Failed Grafts
Failing EVGs detected during physical examination or during routine duplex scans were confirmed arteriographically. Percutaneous balloon angioplasty and stenting were preferentially performed for graft narrowing or kinking. Thrombolysis or thrombectomy via an open femoral arteriotomy was performed when the EVG or one limb of it had completely thrombosed. After removal of the clot, an effort was made to correct the underlying defect by stenting. If this proved impossible, an extraanatomic bypass was performed.
Graft Infection
The diagnosis of graft infection, including aortoenteric fistula, was made by information derived from multiple tests including physical examination, endoscopy, blood cultures, white blood cell counts, computed tomography and duplex scans. Treatment was by open conversion or operative drainage (Table 1).
Table 1. DETAILS OF SECONDARY PROCEDURES
AE, aortoenteric; Axfem, axillofemoral; CT, computed tomography; EIA, external iliac artery; femfem, femorofemoral; GI, gastrointestinal; TFA, transfemoral coil embolization; TLA, translumbar coil embolization.
Secondary Procedures
The details of the secondary procedures, including the reasons for performing the salvage interventions, the clinical presentation of the problem or study leading to the diagnosis, the time from the initial procedure to its occurrence, and the outcome, are shown in Table 1.
Statistical Analysis
Descriptive data are expressed as mean ± standard deviation. The Kaplan-Meier method was used to determine the success and survival rates (SAS Statistical Software, Cary, NC).
RESULTS
The mean operating room time for the initial EVG placement procedure was 5.9 ± 0.35 hours (range 1.7–14.1) and the mean blood loss was 468 ± 790 mL. Twenty-five percent of the patients required a homologous blood transfusion. The mean length of stay was 4.1 ± 3.6 days.
The major complication and death rates within 30 days of EVG repair were 17.6% and 8.5%, respectively. The death rate for the first 103 patients treated between 1992 and 1999 was 13.6%, whereas it was 4.4% for the last 136 patients treated between 1999 and 2001. The technical success rate (complete AAA exclusion without perioperative death) was 88.7%. During follow-up to 75 months (mean 15.7), 53 patients (22%) died of unrelated causes. The overall life table patient survival rate at 5 years was 37% (Fig. 14). Fifteen patients (6.3%) were lost to follow-up despite multiple attempts to contact them, the family, or the primary physician. During this period, only two patients suffered an aneurysm rupture. Of the patients with AAA rupture, one had been lost to follow-up and the other had known graft migration, a proximal type 1 endoleak, and an enlarging aneurysm. The patient suffered the rupture while awaiting a secondary open repair but survived an emergent open repair (see Fig. 10).
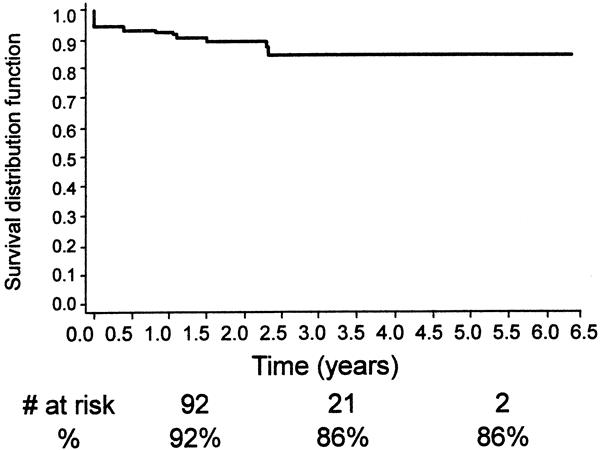
Figure 14. Kaplan-Meier analysis of continued primary clinical success (the number at risk at 1, 3, and 5 years is also shown).
Other late complications included type I endoleak (n = 7), aortoduodenal fistula (n = 2), one with an abdominal abscess, graft thrombosis/stenosis (n = 7), and limb separation or fabric tear with a subsequent type 3 endoleak (n = 1), and a persistent type 2 endoleak (n = 13). A secondary intervention or open surgical procedure was required in 23 patients (10%) (see Table 1). These included deployment of a second EVG (n = 4), open AAA repair (n = 5), transarterial coil embolization (n = 3), translumbar coil embolization (n = 3), extraanatomic bypass (n = 4), and stent placement (n = 3). The technical success of the nonoperative secondary interventions was 92% and the mean length of hospital stay for these secondary procedures was 1.5 days. Continued clinical success, assisted clinical success, freedom from aneurysm-related death, and freedom from death from any cause are shown in Figures 14 through 17 using life table analyses.
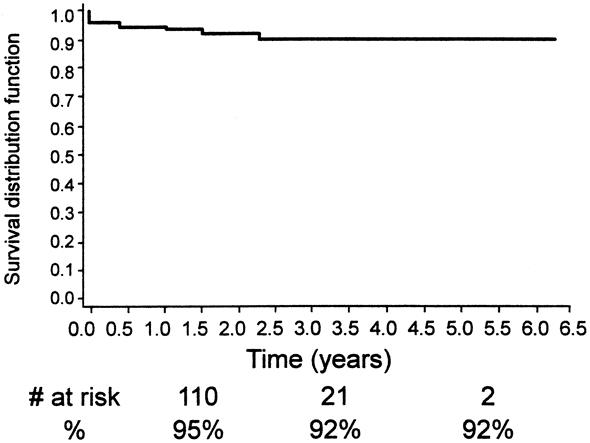
Figure 15. Kaplan-Meier analysis of continued secondary clinical success.
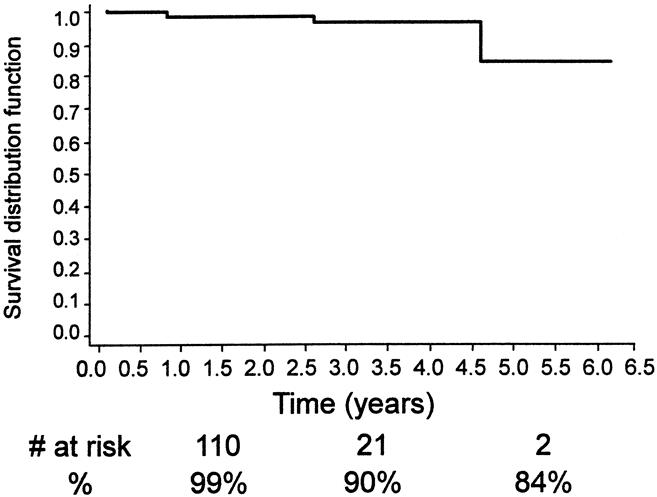
Figure 16. Kaplan-Meier analysis of freedom from aneurysm-related death.
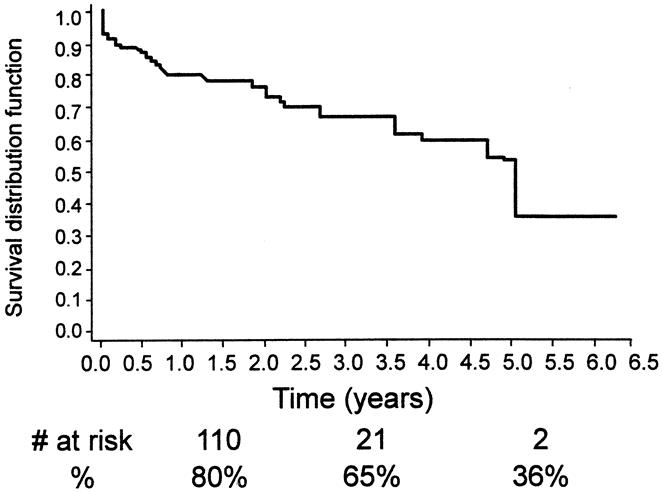
Figure 17. Kaplan-Meier analysis of freedom from any death.
DISCUSSION
A decade has passed since the first EVG repair of an AAA was performed and reported by Parodi et al. 25 During this period, significant advances have been made in the field. These include the use of EVGs to treat other vascular lesions such as thoracic aneurysms, aortoiliac occlusive disease, iliac aneurysms, vascular trauma, and finally ruptured AAAs. 20,22,23,26,27 In addition, patient selection and the technology itself have improved markedly. In the early days, the EVGs used were largely surgeon-made devices that required large-caliber delivery systems that made the procedures difficult and risky. In the early 1990s, EVGs were mostly reserved for patients who were deemed high risk for standard surgical repair. When the more sophisticated industry-made EVGs became available, the safety of the procedures improved, 1,28,29 and EVGs were also used to treat patients who were good surgical candidates. Since the FDA’s approval of the Guidant Ancure graft and the Medtronic AneuRx graft in 1999, this trend has accelerated. Currently, most AAAs are treated with EVGs at many hospitals around the world, including our own center. 21 However, the FDA’s approval was largely based on procedural safety issues, and little long-term proof of efficacy exists to support the widespread use of EVGs, particularly in patients who might be good candidates for a standard open repair.
Recently, several investigators have reported their midterm results with mixed conclusions. In one favorable article published by May et al, 11 they showed that the long-term patient survival rate was better during a follow-up period up to 5 years after EVG repair than it was after open surgical repair in a control group of patients. Zarins et al 4 reported an encouraging rupture-free rate of 99.5% at 3 years using the AneuRx graft. There have been similar encouraging reports on the outcome of the Ancure graft as well. 28,29 However, several others have raised concerns regarding the midterm durability of EVG repair. Zarins et al 18 reported seven unexpected AAA ruptures after EVG repair, with five deaths. Further, the European collaborators 2 reported their experience with 2,464 EVGs during a 4-year period. Of these, 14 patients presented with aneurysm rupture 0 to 24 months after EVG repair, with nine deaths. Holzenbein et al 10 reported that 26% of their patients needed to undergo a secondary procedure to treat EVG-related complications during a follow-up period of 18 months. Others reported similar results and raised similar concerns. 2,7–9,12,17
Our results expand on these findings and concerns. In our series, the procedural time and blood loss as well as the perioperative death rate (8.5%) were higher than in most other reports. This is probably because most (86%) of our patients were elderly high-risk patients, many of whom had large, complex AAAs (see Figs. 2, 3, 6, 7). 14 In addition, many of our patients had previously been denied both open and EVG repair by other surgeons as a result of medical comorbidities, aortoiliac anatomic complexity, or both. 21 This made our patient cohort unusually challenging. The death rate for patients treated between 1992 and 1999 was 13.6%, whereas it was 4.4% for those treated between 1999 and 2001. During the initial period, devices were limited to first-generation custom-made devices, and also EVG repair was reserved for patients considered to be at prohibitive risk for standard surgical repair. During the latter period, more sophisticated devices became available, and patients with less severe comorbid conditions were treated with EVGs. These factors, as well as the learning curve, probably contributed to the decline in the death rate. Even in this difficult group of patients, our technical success rate was high and perioperative complications, length of hospital stay, and estimated blood loss were reasonably and acceptably low. Nevertheless, the increasing occurrence of late failure with time in our patients whose treatment was originally successful for more than 1 year is alarming. In this regard, it is worth noting that the FDA’s approval of these devices was made based on a follow-up period of only 12 months. Our observation of frequent problems developing after 1 year is consistent with the findings of others. 2–18 From our experience and that of others, it is clear that EVG repair is not as durable as open surgical repair. EVGs can fail in a greater number of ways and with greater frequency than standard AAA grafts placed during an open operation. These modes of endovascular graft failure included late endoleaks, graft thrombosis, aortoenteric fistula, and ultimately rupture of the aneurysm with and sometimes without a known endoleak.
Such disadvantages of EVG repair must be weighed against several positive attributes. These include the low death rate, even in patients at high risk for open surgery, and the short hospital stay. In addition, the fact that only two of our patients suffered an aneurysm rupture during the entire study period is encouraging. Of our patients who were not lost to follow-up, none had an aneurysm rupture without a preceding known endoleak. The vast majority of the late deaths in our series were from causes not related to the aneurysm, and our aneurysm-related death rate was low (15% at 5 years; see Fig. 16), despite the preponderance of high-risk patients and the inclusion of all perioperative deaths in our analysis. Also, the fact that most late failures can be detected before causing a catastrophic event or death is a positive finding. This allows one to perform secondary salvage procedures in a timely fashion to prevent aneurysm rupture or limb loss. Moreover, the secondary procedures, when required, were mostly minimally invasive, and the technical success rate was high (see Table 1). Most of the late problems that we encountered could be treated with percutaneous procedures, and many were done transfemorally. Therefore, late failure in itself does not necessarily produce a bad overall outcome. Others have also reported successful outcomes after secondary interventions for late EVG failures. 7,10,13,16,30 However, failure to detect late failure can lead to aneurysm rupture and death. 2,18 Therefore, diligent postoperative surveillance is critical after an EVG repair.
Various methods of endoleak treatment have been attempted and reported. Options include coil embolization by transarterial or translumbar access routes, addition of stent-graft cuffs and extensions, endoscopic ligation of inferior mesenteric and lumbar arteries, redo endovascular stent-graft repair, and open surgical repair (see Fig. 4). 7,10,13,15–17,30,31 The method of treatment for a given endoleak is the subject of considerable controversy. Based on the findings of various experimental models, we believe that when an endoleak has a long and narrow channel, pressure transmission across thrombus induced in this channel may be significantly reduced. 24 Thus, inducing thrombosis in this circumstance may lead to a successful outcome. 7,15,24,30,32,33 In contrast, short endoleak channels with a wide diameter will require covering of the feeding orifice with graft material to obliterate pressure transmission. Thus, although the type of endoleak influences the method of treatment, we believe that the treatment method should also be determined by the length and diameter of the endoleak channel, and we applied this concept in the treatment of endoleaks in the latter part of the current study (see Fig. 4). For endoleaks with short, large-diameter channels (most type 1 and 3 endoleaks), deployment of a second EVG or an extension cuff is needed as opposed to inducing thrombosis. Placement of such a second graft was often possible. However, when it was not, surgical conversion was performed if the patient was deemed an acceptable risk. For patients with long, narrow endoleak channels (most type 2 endoleaks and some type 1), inducing thrombosis was performed by either temporarily terminating chronic anticoagulation therapy or by percutaneous coil embolization or injection of a biologic glue. For methods of access, we have used both transarterial and translumbar approaches. Because most type 2 endoleaks have more than one inflow or outflow, it may be difficult to coil-embolize all the endoleak channels using a transarterial approach. Thus, we recommend the translumbar approach for all type 2 endoleaks (see Figs. 11–13). 7
Based on the results in our series of patients with follow-up to 5 years, we believe that the value of EVGs for treating large AAAs in high-risk patients, including those with ruptured AAAs, seems reasonable. 20 For good-risk patients, however, the lack of durability is a concern. Patients should be informed beforehand about the requirement for life-long surveillance and the possibility of the need for a secondary intervention. Good-risk patients should be given a choice between an open operation and an EVG repair. Indeed, patients may be encouraged to view the two treatment options—standard surgical repair and EVG repair—as the choice between one big operation or the possibility of several smaller operations. However, open surgical repair is also not a perfectly durable operation. In addition to the higher perioperative complication rate, graft infection, paraanastomotic aneurysm formation, and graft thrombosis occur after open repair, although the incidence appears to be lower than after EVG repair. 34–36 Moreover, durability is but one important aspect of the procedure and will not alone determine the superiority of one approach over another. Whether one chooses an EVG repair that provides early benefit because of its minimally invasive nature at the cost of possibly undergoing a secondary procedure, or whether one undergoes a single more definitive but more invasive procedure will depend not only on the results of prospective comparative studies but also on patient preference.
This state of affairs may change as better devices become available. Nevertheless, the long-term effectiveness of such devices must be proven by appropriate long-term studies. In addition, there exists an ultimate need for prospective, randomized comparisons between open and endovascular repair in good-risk patients and endovascular repair and best medical treatment in high-risk patients. Moreover, such comparisons must be made in similar groups of patients with roughly comparable anatomy.
CONCLUSION
Endovascular graft repair is not as durable as open repair. However, the secondary interventions required were relatively minimally invasive procedures with high success rates. Therefore, the need for a secondary intervention does not necessarily represent a failure. Patients need to be informed of the need for life-long surveillance and the possible need for secondary procedures. For patients at good surgical risk, EVG repair should currently be performed with caution and restraint.
DISCUSSION
Dr. Gregorio A. Sicard (St. Louis, Missouri): I congratulate Dr. Ohki for an insightful and thorough evaluation of this evolving technology of endoluminal aortic graft repair. Dr. Veith continues pioneering work in this exciting new field. With the extensive experience at your institution in aortic endografting, could you summarize three brief points?
First, what are your endograft selection criteria, especially since the devices can currently be used in complex iliac anatomy? In 2001, which device would you use if you could use a commercial device or do you still prefer to use the Montefiore graft?
Secondly, what is your current recommendation for the management of Type II endoleaks at 12 months when the size and volume of the aneurysm remains unchanged? As we get a larger number of patients that have undergone this endoluminal treatment, we are finding more of those patients where the aneurysm does not shrink. How should we manage them?
Finally, I noticed in your manuscript that the intraoperative time was greater than five hours. That is significantly higher than the operative time for the commercially available devices. Could you explain if this is endograft specific and what is the difference in time, specifically between your Montefiore endograft and the commercially available graft in your series?
Again, I commend you for bringing to our attention the fact that there should be a word of caution about the use of these devices, while at the same time, if you are diligent in following these patients closely, most complications can be managed endoluminally and still make this technology safe.
presenter Dr. Frank J. Veith (Bronx, New York): Dr. Sicard asked some good questions. I will try to be very brief in the answers.
Selection criteria are constantly changing. The bottom line is, the more favorable the patient anatomy, the easier it is to do an endograft of any sort. Sometimes one of us attempts cases that others would do open. But on the average, we are doing endovascular repairs on about 60 to 70% of all comers and the remainder either get no operation or get an open operation.
Our favorite graft at present is in part regulated by graft availability in FDA studies. Our own Montefiore endovascular graft — which we are trying to commercialize — we use only when no other graft is possible. It requires a complicated operation and a lot of surgical and endovascular manipulation. We use it only when we cannot use an industry-made device. Our two favorites at present are the Cook-Zenith device and the Gore-Excluder device. The Gore device is a little easier to use. The Cook is a little more versatile but a little more complex. The AneuRx and Ancure devices are other choices because they are available and FDA approved.
Type 2 endoleaks with no change in AAA size are a major problem, and we do not know the right answer. Along with Richard Baum of Philadelphia, who devised the technique, we are using translumbar puncture to measure intrasac pressure and try to coil embolize the patent branches. We obviously do that for an enlarging aneurysm with a Type 2 endoleak. If the patient has a Type 2 leak and a large aneurysm but one that is not enlarging, at the present time, we are doing translumbar studies to evaluate what is going on. However, we do not know what the right answer is in this setting. Others are doing laparoscopic clipping of branches, both lumbar and IMA.
Our long operating times are based on the fact that in some of these cases, both industry-made and our own surgeon-made grafts, are very complicated. They just take us a long time to perform, because we keep at it until we get it right. These difficult, complicated cases are more patient specific than device specific. An easy case treated with a commercially made graft, we actually can do in one and a half to two hours. The complicated cases with any graft can sometimes take a long time.
Dr. Larry H. Hollier (New York, New York): Dr. Veith asked me last night if I would comment on this paper. I think it is because he recognizes that I do agree with him that the late complication rates and late failure rates have been underappreciated, and I think it is important that this be emphasized. I agree entirely with your findings and the late results.
I have concern with your comment that, based upon the data presented you conclude that endovascular repair is a safe procedure. You wouldn’t know that from this paper. With an 8.4% mortality rate reported in this paper, one might conclude that endovascular repair is not safe. In previous publications regarding high risk patients undering open aneurysm repair, we noted operative mortality of only 5.7%. There has got to be something else going on, either incredibly sick patients that you are doing or other technical factors. I would appreciate if you could expand on that a little bit.
The other concern is the late mortality. I think you reported 40%. That again is a much higher mortality rate over time than reported in the first paper today, which was noted to be 4%. Again, that deserves comment. Perhaps it represents a very biased referral pattern where you are being sent some incredibly sick, elderly patients. I think these comments do need to be clarified so that one does not misconstrue the article.
Dr. Frank J. Veith: Dr. Hollier, those are excellent points, and some explanation is in order.
Our high early mortality is real. I think it reflects several things. One is our early learning curve. Another is the very sick systemic nature of many of our patients, some of whom had many co-morbidities. A third is possibly our overenthusiasm for the endovascular approach. I think we have sometimes subjected patients to these procedures when conservative therapy might have been a better choice. However, they were generally patients with very large and symptomatic aneurysms. The ones with moderate size aneurysms that are not symptomatic (as I mentioned in my earlier discussion) we usually observed until they grow to be huge or symptomatic.
I think all those factors contributed to the high early mortality. We agree that it is not good, and that we have to make it better. Certainly with the easier cases, with the commercially produced grafts, our mortality has been quite low and comparable to that published by other authors.
Late survival has also been poor. However, we point out in the paper that the late deaths were predominantly due to non-aneurysm related causes. This then is a reflection of the fact that these were a high risk group of patients with many systemic co-morbidities. In general they died from these co-morbidities, so we do not have too many patients followed over five or six years.
That was the reason that I made the comment earlier that I think a randomized prospective study comparing endograft treatment to no treatment is appropriate in these high-risk patients.
Dr. Anthony D. Whittemore (Boston, Massachusetts): Dr. Veith, I enjoyed hearing about your experiences, I always do, and I must say I commend you on embracing this technology with the enthusiasm and diligence you have.
One of the advantages of this technology is the associated reduction in length of stay. I wanted to address one issue Dr. Ohki raised in comparing a two-day stay of one patient to an average length of stay for open repair of nine days. Is that nine day average length of stay at your institution? Or is that a population based figure? And could you give us the comparable figure for endovascular repair, either a population based length of stay or for all your patients in your institution, in order to make a valid comparison?
Dr. Frank J. Veith: In general, we have a conservative attitude about operating on small aneurysms, and we do not see many healthy patients. Thus, the nine-day figure is an accurate one. It was our length of stay during the period that was covered by the study.
Overall, our length of stay for straight-forward endovascular AAA repair cases, unless we have a serious complication, ranges between one and three days. And the reason for the longer length of stay was the co-morbidities. Occasionally a patient will have renal failure or congestive failure and have to stay in a week, but generally our length of stay parallels that reported by some of the other endovascular enthusiasts.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association, April 26–28, 2001, the Broadmoor Hotel, Colorado Springs, Colorado.
Supported by grants from the U.S. Public Health Service (HL 02990), the James Hilton Manning and Emma Austin Manning Foundation, the Anna S. Brown Trust, The William J. Von Liebig Foundation, and the New York Institute for Vascular Studies.
Correspondence: Takao Ohki, MD, and Frank J. Veith, MD, Division of Vascular Surgery, Montefiore Medical Center, 111 E. 210th St., New York, NY 10467.
E-mail: takohki@msn.com; fjvmd@msn.com
Accepted for publication April 26, 2001.
References
- 1.Zarins CK, White RA, Schwarten D, et al. AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: multicenter prospective clinical trial. J Vasc Surg 1999; 29: 292–308. [DOI] [PubMed] [Google Scholar]
- 2.Harris PL, Vallabhaneni SR, Desgranges P, et al. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. J Vasc Surg 2000; 32: 739–749. [DOI] [PubMed] [Google Scholar]
- 3.Rhee RY, Eskandari MK, Zajko AB, et al. Long-term fate of the aneurysmal sac after endoluminal exclusion of abdominal aortic aneurysms. J Vasc Surg 2000; 32: 689–696. [DOI] [PubMed] [Google Scholar]
- 4.Zarins CK, White RA, Moll FL, et al. The AneuRx stent graft: four-year results and worldwide experience 2000. J Vasc Surg 2001; 33 (2 Pt 2):135–145. [DOI] [PubMed] [Google Scholar]
- 5.Resch T, Ivancev K, Lindh M, et al. Persistent collateral perfusion of abdominal aortic aneurysm after endovascular repair does not lead to progressive change in aneurysm diameter. J Vasc Surg 1998; 28: 242–249. [DOI] [PubMed] [Google Scholar]
- 6.Ahn SS, Rutherford RB, Johnston KW, et al. Reporting standards for infrarenal endovascular abdominal aortic aneurysm repair. J Vasc Surg 1997; 25: 405–410. [DOI] [PubMed] [Google Scholar]
- 7.Baum RA, Carpenter JP, Cope C, et al. Aneurysm sac pressure measurements after endovascular repair of abdominal aortic aneurysms. J Vasc Surg 2001; 33: 32–41. [DOI] [PubMed] [Google Scholar]
- 8.Beebe HG, Cronenwett JL, Katzen BT, et al. Results of an aortic endograft trial: impact of device failure beyond 12 months. J Vasc Surg 2001; 33 (2 Pt 2):55–63. [DOI] [PubMed] [Google Scholar]
- 9.Bush RL, Lumsden AB, Dodson TF, et al. Mid-term results after endovascular repair of the abdominal aortic aneurysm. J Vasc Surg 2001; 33 (2 Pt 2):70–76. [DOI] [PubMed] [Google Scholar]
- 10.Holzenbein TJ, Kretschmer G, Thurnher S, et al. Midterm durability of abdominal aortic aneurysm endograft repair: a word of caution. J Vasc Surg 2001; 33 (2 Pt 2):46–54. [DOI] [PubMed] [Google Scholar]
- 11.May J, White GH, Waugh R, et al. Improved survival after endoluminal repair with second-generation prostheses compared with open repair in the treatment of abdominal aortic aneurysms: a 5-year concurrent comparison using life table method. J Vasc Surg 2001; 33 (2 Pt 2):21–26. [DOI] [PubMed] [Google Scholar]
- 12.Prinssen M, Wever JJ, Mali WP, et al. Concerns for the durability of the proximal abdominal aortic aneurysm endograft fixation from a 2-year and 3-year longitudinal computed tomography angiography study. J Vasc Surg 2001; 33 (2 Pt 2):64–69. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter JP, Neschis DG, Fairman RM, et al. Failure of endovascular abdominal aortic aneurysm graft limbs. J Vasc Surg 2001; 33 (2 Pt 1):296–303. [DOI] [PubMed] [Google Scholar]
- 14.Ohki T, Veith FJ. Five-year experience with endovascular grafts for the treatment of aneurysmal, occlusive and traumatic arterial lesions. Cardiovasc Surg 1998; 6: 878–892. [DOI] [PubMed] [Google Scholar]
- 15.Ohki T, Veith FJ. Treatment of various endoleaks. Adv Vasc Surg (in press).
- 16.Holzenbein TJ, Kretschmer G, Dorffner R, et al. Endovascular management of endoleaks after transluminal infrarenal abdominal aneurysm repair. Eur J Vasc Endovasc Surg 1998; 16: 208–217. [DOI] [PubMed] [Google Scholar]
- 17.Laheij RJF, Buth J, Harris PL, et al. Need for secondary interventions after endovascular repair of abdominal aortic aneurysms. Intermediate-term follow-up results of a European collaborative registry (EUROSTAR). Br J Surg 2000; 87: 1666–1673. [DOI] [PubMed] [Google Scholar]
- 18.Zarins CK, White RA, Fogarty TJ. Aneurysm rupture after endovascular repair using the AneuRx stent graft. J Vasc Surg 2000; 31: 960–970. [DOI] [PubMed] [Google Scholar]
- 19.Parodi JC, Marin ML, Veith FJ. Transfemoral, endovascular stented graft repair of an abdominal aortic aneurysm. Arch Surg 1995; 130: 549–552. [DOI] [PubMed] [Google Scholar]
- 20.Ohki T, Veith FJ. Endovascular grafts and other image guided catheter based adjuncts to improve the treatment of ruptured aortoiliac aneurysms. Ann Surg 2000; 232: 466–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohki T, Veith FJ. Standard and new treatments for abdominal aortic aneurysms: the value of the Montefiore endovascular grafts for difficult aneurysms. Jpn Circ J 1999; 63: 829–837. [DOI] [PubMed] [Google Scholar]
- 22.Marin ML, Veith FJ, Cynamon J, et al. Initial experience with transluminally placed endovascular grafts for the treatment of complex vascular lesions. Ann Surg 1995; 222: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohki T, Marin ML, Veith FJ, et al. Endovascular aorto-uni-femoral grafts and femorofemoral bypass for bilateral limb-threatening ischemia. J Vasc Surg 1996; 24: 984–997. [DOI] [PubMed] [Google Scholar]
- 24.Mehta M, Ohki T, Veith FJ, et al. All sealed endoleaks are not the same: a treatment strategy based on an ex vivo analysis. Eur J Vasc Endovasc Surg 2001; 21: 541–545. [DOI] [PubMed] [Google Scholar]
- 25.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991; 5: 491–499. [DOI] [PubMed] [Google Scholar]
- 26.Sahgal A, Veith FJ, Lipsitz E, et al. Diameter changes in isolated iliac artery aneurysms 1 to 6 years after endovascular graft repair. J Vasc Surg 2001; 33: 289–295. [DOI] [PubMed] [Google Scholar]
- 27.Dake MD, Miller DC, Semba CP, et al. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. N Engl J Med 1994; 331: 1729–1734. [DOI] [PubMed] [Google Scholar]
- 28.Moore WS, Rutherford RB. Transfemoral endovascular repair of abdominal aortic aneurysm: results of the North American EVT phase 1 trial. J Vasc Surg 1996; 23: 543–553. [DOI] [PubMed] [Google Scholar]
- 29.Moore WS, Kashyap VS, Vescera CL, et al. Abdominal aortic aneurysm: a 6-year comparison of endovascular versus transabdominal repair. Ann Surg 1999; 230: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ermis C, Kramer S, Tomczak R, et al. Does successful embolization of endoleaks lead to aneurysm sac shrinkage? J Endovasc Ther 2000; 7: 441–445. [DOI] [PubMed] [Google Scholar]
- 31.Wesselink W, Cuesta MA, Berends PJ, et al. Retroperitoneal endoscopic ligation of lumbar and inferior mesenteric artery as a treatment of persistent endoleak after endovascular aortic aneurysm repair. J Vasc Surg 2000; 31: 1240–1244. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez LA, Faries PL, Marin ML, et al. Chronic intraaneurysmal pressure measurement: an experimental method for evaluating the effectiveness of endovascular aortic aneurysm exclusion. J Vasc Surg 1997; 26: 222–230. [DOI] [PubMed] [Google Scholar]
- 33.Marty B, Sanchez LA, Ohki T, et al. Endoleak after endovascular graft repair of experimental aortic aneurysms: does coil embolization with angiographic “seal” lower intraaneurysmal pressure? J Vasc Surg 1998; 27: 454–462. [DOI] [PubMed] [Google Scholar]
- 34.Plate G, Hollier LA, O’Brien P, et al. Recurrent aneurysms and late vascular complications following repair of abdominal aortic aneurysms. Arch Surg 1985; 120: 590–594. [DOI] [PubMed] [Google Scholar]
- 35.Ruberti U, Scorza R, Biasi G, et al. Nineteen-year experience on the treatment of aneurysms of the abdominal aorta: a survey of 832 consecutive cases. J Cardiovasc Surg 1985; 26: 547–553. [PubMed] [Google Scholar]
- 36.Hallet JW, Marshall DM, Gray DT, et al. Graft-related complications after abdominal aortic aneurysm repair: reassurance from a 36-year population-based experience. J Vasc Surg 1997; 25: 277–286. [DOI] [PubMed] [Google Scholar]