Abstract
Objective
To determine the presence of telomerase activity in a variety of periampullary malignancies and pancreatic diseases and quantify its activity to establish any association with the stage or aggressiveness of malignancy.
Summary Background Data
Progressive shortening of telomeres, repetitive DNA sequences at the ends of chromosomes, plays a role in cell senescence. Telomerase catalyzes conservation of telomeric repeats and may promote cell immortality and hence malignancy. It is absent in normal tissues but upregulated in more than 80% of cancers.
Methods
Fresh specimens of 62 periampullary tumors were snap-frozen in liquid nitrogen and adjacent tissue was formalin-fixed for histopathology. The telomerase repeat amplification protocol (TRAP) was used to obtain telomerase DNA products. These were separated with gel electrophoresis, stained with SYBR green, and quantified by densitometry. Findings were confirmed with a fluorometric TRAP assay in which fluorescent primers specific for telomerase were selectively amplified in its presence.
Results
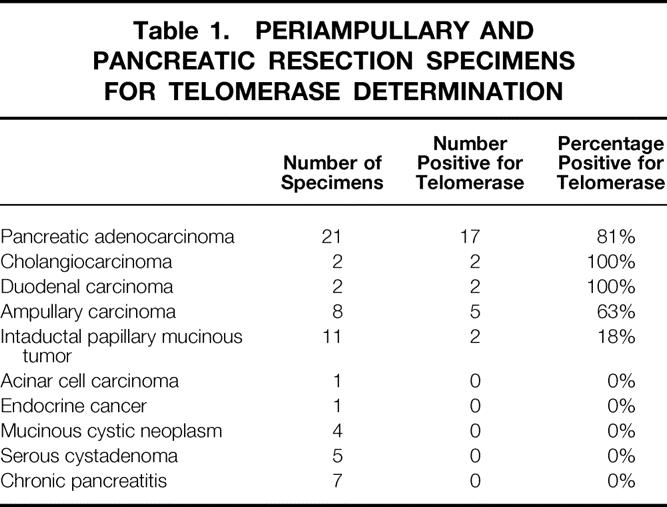
Telomerase activity was upregulated in 26 of 33 periampullary malignancies (79%): 17 of 21 pancreatic adenocarcinomas (81%), 2 of 2 cholangiocarcinomas, 2 of 2 duodenal carcinomas, and 5 of 8 ampullary carcinomas (63%). Poorly differentiated periampullary tumors had significantly higher telomerase activity than well-differentiated tumors, and tumors larger than 2 cm had significantly higher telomerase activity than those 2 cm or smaller. Pancreatic ductal adenocarcinomas with lymph node metastases had significantly greater activity than node-negative cancers. Two of 11 intraductal papillary mucinous tumors were positive for telomerase activity, but only in foci of invasive carcinoma. Chronic pancreatitis (n = 7), serous cystadenomas (n = 5), benign mucinous cystic neoplasms (n = 4), neuroendocrine cancer (n = 1), and acinar cell carcinoma (n = 1) had no detectable telomerase activity.
Conclusion
Telomerase activity is common in periampullary carcinomas. The magnitude of activity correlates with aggressiveness in pancreatic adenocarcinoma and may prove useful as a molecular index for biologic staging.
Telomeres are fragments of DNA composed of multiple repeats of the nucleotide sequence TTAGGG. Telomeres occupy the ends of eukaryotic chromosomes and are progressively denuded as individual cells undergo mitosis. 1–3 There is evidence that telomere loss may contribute to impaired cellular mitosis and function. 3–5 Thus, telomere shortening may represent a “mitotic clock” associated with cellular senescence. 6,7
Telomerase is a ribonucleoprotein enzyme that catalyzes the addition of repeats of the nucleotide sequence TTAGGG to the ends of chromosomal DNA (i.e., telomerase functions in the creation and maintenance of telomeres). 8 Telomerase activity is virtually absent from normal human somatic cells but is upregulated in germ line cells and neoplastic cells of many different types. 7,9 In fact, telomerase activity has been found to be upregulated in approximately 85% of solid tumors in humans, including cancers of the lung, breast, prostate, colon, stomach, head and neck, skin, ovary, and pancreas. 7,10 Further, investigators have transformed normal cells with the use of genetic vectors that encode and promote telomerase activity. 11,12 In terms of neoplasia, telomerase presumably grants cellular immortality through telomere maintenance and the prevention of cellular senescence. Although these molecular mechanisms are complex and have yet to be fully elucidated, it is clear that telomerase can function as an oncogene in the development of human malignancies. There is also evidence that significant upregulation of telomerase activity may be associated with advanced stage and aggressiveness in some cancers. 13–15
Periampullary malignancies, of which pancreatic ductal adenocarcinoma is by far the most common, are aggressive cancers with a poor prognosis. 16,17 Various oncogenetic mutations have been identified in pancreatic adenocarcinoma in an attempt to understand its molecular carcinogenesis and to provide more comprehensive data with regard to its diagnosis, treatment, and prognosis. Mutations of the k-ras oncogene are found in approximately 90% of pancreatic adenocarcinomas. 18,19 Although k-ras mutations are prevalent in pancreatic adenocarcinoma, they are also found in hyperplastic pancreatic tissue and other benign conditions and thus have limited specificity for malignancy. 20,21 In addition, the presence of k-ras mutations does not seem to have prognostic significance. 19 Mutations of the p53 tumor suppressor gene are found in only 50% of pancreatic adenocarcinomas; there is some evidence that patients whose tumors have mutations of p53 may have a poor prognosis. 22
Telomerase activity in pancreatic adenocarcinoma has been reported in several studies. 23–27 Hiyama et al 27 reported that 95% of 43 pancreatic adenocarcinoma specimens showed detectable telomerase activity, and Suehara et al 25 noted a higher degree of telomerase activity in pancreatic cancers versus a limited number of specimens from normal or inflamed pancreas. There are reports of telomerase activity in proximal cholangiocarcinoma 28 and malignant cystic tumors of the pancreas, 29,30 but there are otherwise few data regarding telomerase expression in other periampullary neoplasms, and none of these studies has established a quantitative relationship between telomerase activity and stage of aggressiveness in periampullary or pancreatic malignancy.
The objective of this study was to determine the presence of telomerase activity in a variety of periampullary malignancies and pancreatic diseases and quantify its activity to establish any association with the stage or aggressiveness of malignancy.
METHODS
We obtained tissue specimens from 62 patients who underwent pancreatic resection (59 pancreaticoduodenectomies and 3 distal pancreatectomies). The three distal pancreatectomies involved two benign mucinous cystic neoplasms and one serous cystadenoma. The operative specimens were transferred to the pathology frozen-section laboratory immediately after removal. There, fresh tissue was dissected from the diseased area of the specimen and snap-frozen in liquid nitrogen within 15 minutes of arrival. The samples were stored at −80°C until they were retrieved for further processing and analysis.
At the time of the preparation of protein extracts from the frozen specimens, a small (0.5 × 0.5-cm) portion of tissue was taken from a directly adjacent area and placed in formalin. This formalin-fixed tissue was embedded in paraffin and stained with hematoxylin and eosin (H&E). These H&E-stained slides were reviewed in masked fashion by a pathologist (F.G.C.). To prevent sampling error, we performed telomerase analysis on specimens in which the adjacent H&E-stained tissue histology was consistent with the final histology of the primary tumor.
The determination of telomerase activity was performed through two methods.
Classic TRAP Method
The first method of telomerase detection and quantification we used is a modification of the telomerase repeat amplification protocol (TRAP) initially described by Kim et al 7 and later by others. 25,31,32 Unless otherwise noted, all materials used in these experiments and all procedural instruction were obtained from the TRAPeze Telomerase Detection Kit and its accompanying manual, manufactured by Intergen Company, Purchase, NY. Briefly, the principle of the TRAP is based on a two-step process. In the first step, a known primer (designated TS in the TRAPeze Telomerase Detection Kit) is extended (at 30°C) with a variable number of telomeric repeats. This occurs only if the protein extract of interest contains telomerase. In the second step, the extended telomerase product is amplified by standard polymerase chain reaction (PCR). When the amplified telomerase products are separated by gel electrophoresis, a characteristic “ladder” of DNA products is visible after staining (Fig. 1). As detailed in the TRAPeze Telomerase Detection Kit manual, telomerase activity can be quantified through densitometry of the gel bands.
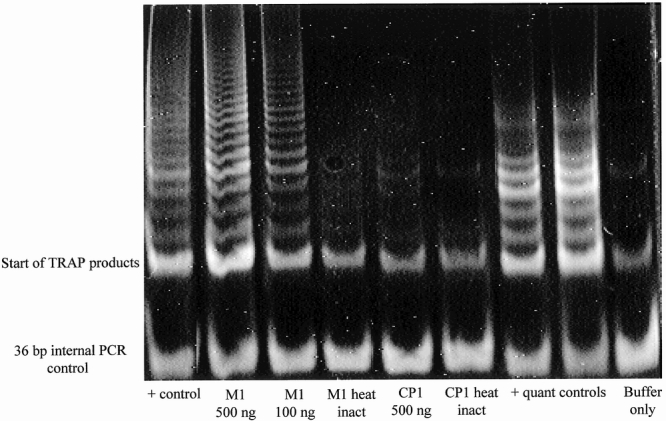
Figure 1. Results of typical polyacrylamide gel electrophoresis (PAGE) method telomerase repeat amplification protocol (TRAP). Lane 1, positive control from a cell line known to be telomerase-positive. Lane 2, pancreatic adenocarcinoma specimen designated M1, 500 ng. Lane 3, M1, 100 ng. Lane 4, M1, heat-inactivated aliquot. Lane 5, Chronic pancreatitis specimen designated CP1, 500 ng. Lane 6, CP1, heat-inactivated aliquot. Lanes 7 and 8, positive controls used for quantification purposes. Lane 9, negative control, buffer only. PCR, polymerase chain reaction.
The frozen specimens described previously were homogenized in CHAPS lysis buffer containing RNAse inhibitor (Promega Corp., Madison, WI) using an Omni TH tissue homogenizer (Omni International, Inc., Warrenton, VA). Protein extracts were obtained by centrifugation and a standardized Bradford protein assay was used to determine total protein concentrations (Sigma, St. Louis, MO). Extra volumes of protein extracts were stored at −80°C. A standardized amount of protein extract was mixed with aliquots of primers, nucleotides, water, buffer, and Taq polymerase (Fisher Scientific, Pittsburgh, PA) in PCR tubes (MJ Research, Waltham, MA). Standard three-step TRAP PCR was performed with a PTC-150 MiniCycler (MJ Research). The PCR products were loaded onto a 12.5% nondenaturing polyacrylamide gel and separated through electrophoresis (PAGE). The DNA products were stained with SYBR Green (Molecular Probes, Inc., Eugene, OR) and the intensity of bands was determined through densitometry using a ChemiImager 4400 gel imaging system with AlphaEase version 5.5 for Windows (both from Alpha Innotech Corp., San Leandro, CA). Telomerase activity was calculated as the total product generated (TPG) according to a standard formula outlined in the TRAPeze Telomerase Detection Kit manual.
Fluorometric TRAP Method
A new TRAP method involving the use of fluorescent primers to quantitatively determine telomerase activity has been developed recently. 13,28,33 The principle of this detection system is also a two-step process. In the first step, a known primer (designated TS) is extended with a variable number of telomeric repeats. This occurs only in the presence of telomerase. In the second step, PCR amplification of the extended telomerase product results in the separation of a fluorescent moiety from its quencher at the end of the reverse primer (designated RP). The magnitude of telomerase activity then becomes a function of the fluorescence in a given sample. Because this method is more efficient in the analysis of larger sample numbers and because there is some evidence that it is more accurate in terms of quantitative determinations, 33 we adopted this method of detection after a suitable period of ensuring compatibility with the PAGE method of telomerase detection.
Protein extracts were prepared from frozen tissue and standard three-step TRAP PCR was performed as described previously. Samples were then diluted and fluorescence measurements were performed using a Spex FluoroMax fluorometer (Jobin Yvon, Inc., Edison, NJ). From the raw fluorescence measurements, telomerase activity was calculated as the TPG according to a standard procedure outlined in the TRAPeze XL Telomerase Detection Kit manual.
Pathologic Analysis
We reviewed the final pathology reports for each resection specimen and recorded the following parameters: final histologic diagnosis, size of primary tumor, presence of regional lymph node metastases, advanced local extension of primary tumor, tumor grade (grade 1, well differentiated; grade 2, moderately differentiated; grade 3, poorly differentiated; grade 4, undifferentiated), and presence of perineural invasion.
Statistical Analyses
The Pearson correlation coefficient was calculated for the correlation between TPG values generated from the PAGE and fluorometric methods. The Mann-Whitney test or the Welch corrected t test was used, where appropriate, to analyze the differences between TPGs. All statistical analyses were performed with the assistance of GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
The final histologic diagnosis of each periampullary malignancy and pancreatic tumor, with delineation of the presence or absence of telomerase activity, is provided in Table 1. A tumor was considered telomerase-positive if its TPG exceeded 0. For periampullary malignancies considered as a whole (pancreatic adenocarcinoma + duodenal carcinoma + ampullary carcinoma + cholangiocarcinoma), 26 of 33 (79%) were telomerase-positive.
Table 1. PERIAMPULLARY AND PANCREATIC RESECTION SPECIMENS FOR TELOMERASE DETERMINATION
The results from a typical PAGE-method TRAP are shown in Figure 1. Here, a specimen from a patient with pancreatic adenocarcinoma is compared with that of patient with chronic pancreatitis. The adenocarcinoma specimen clearly shows the ladder of DNA products characteristic of telomerase activity. This ladder of products is absent from the chronic pancreatitis specimen. Densitometry performed on the ladder of TRAP products can be used to determine telomerase activity quantitatively.
For 20 specimens, the PAGE and fluorometric methods of telomerase determination were both used to ensure the accuracy and reproducibility of the newer fluorometric method. The compatibility between the methods is illustrated in Figure 2. The Pearson correlation coefficient for this association was determined to be 0.92 (P < .001).
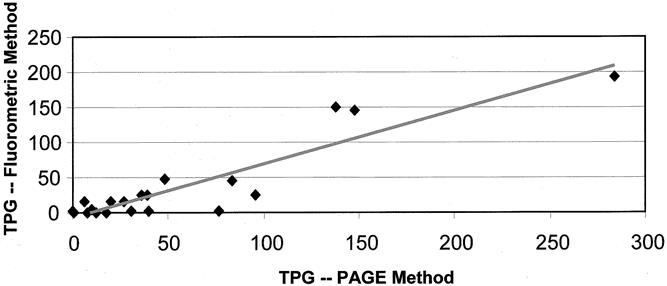
Figure 2. Fluorometric versus polyacrylamide gel electrophoresis (PAGE) method of telomerase determination. Pearson r = 0.92 (P < .001). TPG, total product generated.
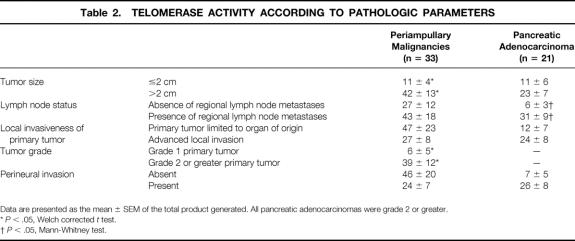
The mean TPGs for periampullary malignancies as a whole and for pancreatic adenocarcinoma are stratified according to the various pathologic parameters and compared in Table 2. For periampullary malignancies considered as a whole, tumors with regional lymph node metastases had higher telomerase activity than lymph node-negative tumors, and tumors larger than 2 cm and high-grade tumors had significantly higher telomerase activity than small or well-differentiated tumors (Fig. 3). For pancreatic adenocarcinoma considered separately, tumors larger than 2 cm, tumors with advanced local extension, and tumors with perineural invasion had greater telomerase activity than smaller, less invasive tumors, and tumors with regional lymph node metastases had significantly higher telomerase activity than node-negative tumors (Fig. 4).
Table 2. TELOMERASE ACTIVITY ACCORDING TO PATHOLOGIC PARAMETERS
Data are presented as the mean ± SEM of the total product generated. All pancreatic adenocarcinomas were grade 2 or greater.
*P < .05, Welch corrected t test.
†P < .05, Mann-Whitney test.
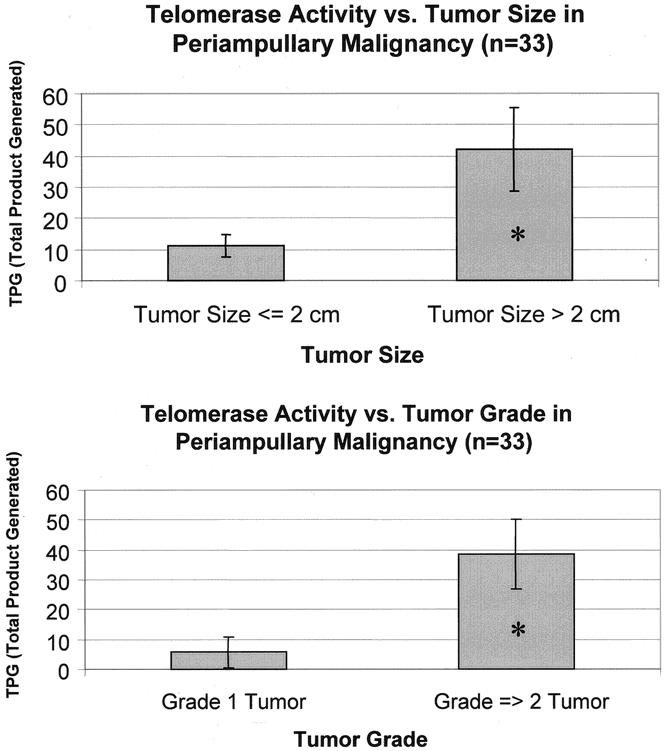
Figure 3. Telomerase activity in periampullary malignancies on stratification by tumor size and grade. *P < .05 by Welch corrected t test.
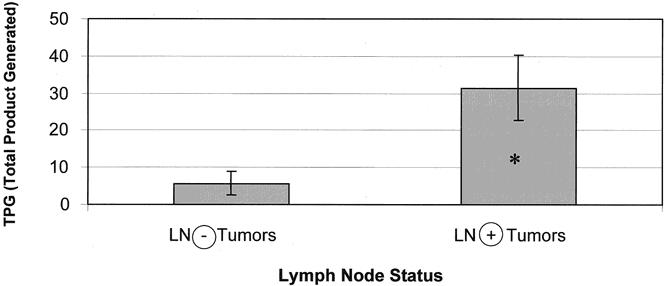
Figure 4. Telomerase activity in pancreatic adenocarcinoma on stratification by presence of regional lymph node metastases. *P < .05 by Mann-Whitney test.
DISCUSSION
Telomerase activity is upregulated in a wide variety of human cancers. 13,34–38 In agreement with the results of other investigators, 27,28 we have shown that telomerase activity is upregulated in malignant periampullary tumors and pancreatic adenocarcinoma. Notably, the frequency of telomerase-positive testing for periampullary malignancies and pancreatic adenocarcinoma in our series (79% and 81%, respectively) compares favorably with the rate (80–85%) reported for other solid human tumors. 7,25 All cholangiocarcinomas (n = 2) and duodenal carcinomas (n = 2) that we tested were positive for telomerase. Interestingly, the rate of telomerase-positive ampullary carcinoma was 63% in our series of eight cases. Intraductal papillary mucinous tumors (IPMTs) tested positive for telomerase in 2 of 11 cases; both positive specimens were obtained directly from foci of invasive carcinoma within the tumor. These last observations probably reflect two facts: first, telomerase is not universally reactivated in all human cancers, and second, as will be discussed further below, telomerase upregulation may be a relatively late event in carcinogenesis.
Understandably, part of the interest in telomerase from a clinical perspective is its potential utility as a diagnostic adjunct. Several investigators have proposed the use of telomerase assays in the preoperative investigation of various malignancies, 10,23,28,39,40 including fine-needle aspiration of masses suspicious for cancer. Most of these reports highlight the fact that although the assays have limited sensitivity (as evidenced by the fact that only 80–85% of human cancers seem to be telomerase-positive), the detection of telomerase does seem to be rather specific for cancer. One report, based on a large series of breast fine-needle aspiration specimens, goes on to suggest that telomerase testing may ultimately be used to scrutinize equivocal biopsies. 39 Our data support this endorsement of the specificity inherent in telomerase detection. In our series, seven chronic pancreatitis specimens, five serous cystadenomas, and four benign mucinous cystic neoplasms were telomerase-negative. There were, in fact, no false-positive results (i.e., all specimens with benign diagnoses tested negative for telomerase).
Multiple investigators have now shown that the magnitude of telomerase activity may be useful in predicting the biologic behavior of different cancers. 13–15,35 In pancreatic adenocarcinoma, various clinicopathologic and molecular factors have been proposed to hold prognostic importance. Among others, the Johns Hopkins group has shown that the most important factors influencing long-term survival in pancreatic adenocarcinoma are the size of the primary tumor, the presence of advanced local invasion/positive surgical margins, and the presence of regional lymph node metastases. 41 Other important but slightly more controversial factors include high tumor grade, presence of perineural invasion, and nondiploid DNA content in the primary tumor. 41–43 Similarly, several factors have been found to be important in predicting a worse prognosis for periampullary malignancies, including large tumor size, advanced local invasion/positive surgical margins, presence of regional lymph node metastases, and high tumor grade. 17 For pancreatic adenocarcinoma, we have shown that tumors with regional lymph node metastases have significantly higher telomerase activity than node-negative tumors. We have also shown that for periampullary malignancies considered as a whole, tumors larger than 2 cm and high-grade tumors have significantly higher telomerase activity than smaller, better differentiated tumors. Thus, telomerase activity may be an important marker for aggressive cancer and worse prognosis in pancreatic adenocarcinoma and periampullary malignancy in general.
As a final consideration, we propose that telomerase upregulation may be a relatively late event in the molecular carcinogenesis of periampullary and pancreatic malignancies. We deliver this premise based on several observations. First, as detailed previously, telomerase activity is significantly upregulated in late-stage cancers, as evidenced by its higher activity in large tumors, poorly differentiated tumors, and tumors with regional lymph node metastases. Second, several specimens from patients with chronic pancreatitis, benign mucinous cystic neoplasms, and benign IPMTs contained preneoplastic changes consistent with pancreatic intraepithelial neoplasia level 1 and 2. These changes are thought to be precursors to the development of carcinoma in situ and invasive adenocarcinoma of the pancreas. 44,45 Despite the presence of pancreatic intraepithelial neoplasia in these specimens, they were uniformly telomerase-negative. Lastly, only 2 of 11 (18%) of IPMTs were telomerase-positive, and these specimens were obtained only from foci of invasive carcinoma in two malignant IPMTs. Importantly, adjacent areas of noncancerous ductal epithelium in these tumors tested negative for telomerase. We also showed that the four benign mucinous cystic neoplasms in our series tested negative for telomerase activity. Thus, for IPMTs and mucinous cystic neoplasms, our data seem to indicate that areas of noncancerous dysplasia lack telomerase activation, whereas focally malignant IPMTs show upregulated telomerase activity. Our IPMT data are consistent with a recent report by Inoue et al 29 of a small series of patients with IPMT who underwent endoscopic retrograde pancreatic juice aspiration with subsequent telomerase analysis. Eleven of 13 (85%) patients with malignant IPMT were correctly diagnosed through a combination of cytology and telomerase analysis, and zero of 15 patients with benign IPMT had telomerase-positive aspirations. 29 There is evidence from multiple investigators experimenting with gastrointestinal, oral, and breast cancer that telomerase activation may be a late event in carcinogenesis. 46–49 Taken together, this body of evidence may point to a later role for telomerase in neoplasia.
In summary, telomerase activation is present in pancreatic and other periampullary adenocarcinomas and is absent in benign and premalignant tumors. Increased telomerase activity correlates with factors traditionally associated with poor prognosis.
DISCUSSION
Dr. Charles J. Yeo (Baltimore, Maryland): I rise to congratulate Drs. Balcom, Warshaw, and co-authors from the MGH for a well presented, well written study of telomerase activity in periampullary malignancies.
As many in this room are aware, the telomerase story is fascinating, scientifically exciting, and not yet fully understood. In particular, one broad, unanswered question is whether telomerase and its intracellular machinery can be harnessed to generate cellular immortality in normal cells, to prevent senescence and apoptosis — i.e., is it a “Fountain of Youth” surrogate? I have several questions for the authors.
Number 1. Have you correlated telomerase activity with survival and outcome independent of the pathologic variables? While 79% of periampullary malignancies were positive for telomerase activity, 21% were negative. How did these groups sort as regards to survival?
Question Number 2. In your studies a tumor was considered telomerase activity positive if its total product generated — i.e., TPG — exceeded zero. How diffuse or focal is the expression? Does the degree of telomerase expression matter? Does it correlate with the adverse pathologic criteria that you mentioned?
Question 3. Your data and discussion imply that telomerase activity is a marker for aggressive behavior and a late event in the neoplastic progression paradigm. Could you speculate what this means as regards the biology of telomerase and its presence in progenitor cells — i.e., stem cells — and late tumors?
Number 4. Your data in the manuscript showed no telomerase activity in PanIN-1 or PanIN-2 lesions and no activity in non-invasive IPMNs. How useful can telomerase activity be for screening, particularly in high risk groups? That is, can it identify PanIN-3 lesions, which are the immediate precursor of invasive tumors?
Like many fine studies this work has generated further important issues. I commend the authors for their contribution.
Presenter Dr. James H. Balcom (Boston, Massachusetts): Obviously, an examination of prognosis and telomerase activity demands some examination of survival; and this has been limited by a couple of things: Number 1, the assay uses fresh tissue, so we are not able to retrospectively examine telomerase activity. Number 2, the specimens that we collect had their telomerase activity degraded over the span of about a year. We actually had specimens that were positive that over the year lost some or all of their activity.
However, I have one slide to show. This is very limited data, obviously, but this is from 18 patients with pancreatic adenocarcinoma at a median follow-up of 12 months. There is a suggestion of higher telomerase activity in the non-survivors, but the difference is not yet statistically significant. Without more patients and longer follow-up, we cannot make any definitive comments about survival.
Dr. Yeo’s second question dealt with the focal or diffuse nature of telomerase activity in the specimens. We did not look at foci of telomerase activity on a cellular level within a given tumor. I think to do that you would have to use a technique such as in situ hybridization. We are assuming the activity is coming from the malignant cells, but we have not specifically addressed that in our study.
Dr. Yeo’s next question dealt with PanIN-3 and 4 and the use of telomerase in screening a high risk population. We didn’t see any changes consistent with PanIN-3 and 4 in our specimens when we reviewed them with pathology, so it is hard for me to comment on that. Certainly it seems from the collective data that we have that this is a relatively late change seen in invasive carcinomas. I would guess, although I don’t have any direct evidence, that PanIN-3 and 4 specimens would not exhibit telomerase activity.
Dr. Yeo also hinted at the possibility of using telomerase as a screening biopsy technique. There has been some interest, with a published report by Dr. Evans’ group at M. D. Anderson, in using telomerase determination to screen pancreatic biopsy specimens in preoperative candidates. At M. D. Anderson they were able to show that there are some positive samples from the FNA specimens, and that this positive tissue can be obtained from material that is normally discarded after an FNA. Therefore, it could be useful in preoperative diagnosis. We are examining the use of endoscopic ultrasound and fine needle aspiration in the preoperative differentiation of malignant cystic neoplasms from benign cystic masses.
Dr. Yeo also asked about the biology of telomerase. As he said at the beginning of his discussion, there is still a lot that is not known about the molecular biology of this enzyme. There is molecular evidence, however, that it is a late change. For instance, DePinho’s group and Weinberg’s group in Boston have shown that if cells are able to survive senescence, or normal aging (which they think that might be mediated through the P-53 pathway), telomerase activation may occur, resulting in unchecked proliferation and a more aggressive phenotype. Our data would be consistent with these observations. Further work needs to be done to examine the presence or absence of other mutations in neoplastic lesions with telomerase activity. This would give us a definitive picture of the role of the enzyme in carcinogenesis.
Dr. Michael G. Sarr (Rochester, Minnesota): Dr. Yeo asked questions about the prognosis. I am going to ask questions about the diagnosis and pathogenesis.
You have shown nicely that telomerase activity is present in most periampullary malignant neoplasms but not in chronic pancreatitis nor with benign pancreatic neoplasms. Thus, your implications are that you might use this in the diagnosis in patients who have an equivocal biopsy or in the pathogenesis of carcinoma. So I have two questions for you.
First, however, the real use of this might even be in managing treatment or in determining its role in the progression of neoplasm. For instance, in a patient with a periampullary villous adenoma, it would be a huge benefit if we knew that benign periampullary villous adenoma do not express telomerase activity while malignant periampullary villous tumors do express telomerase activity. Have you looked at any periampullary villous tumors?
Second, you have told us about telomerase activity — that is, the activity of the enzyme. Do you have any data on expression for message of telomerase — that is, messenger RNA — either looking at message itself or using in situ hybridization within these tumors? This would tell us if this was a specific effect of the neoplasm or, as you have previously shown for other markers, whether this is a field effect similar to what we heard about yesterday with IPMN. And the second part of this is, you took a sample of adjacent tissues for fixed histopathy, why didn’t you evaluate telomerase activity in these adjacent areas as well?
Dr. James H. Balcom: Thank you, Dr. Sarr. I think the example of the villous adenoma is great, because that is exactly what this assay may be useful for. Although we did not test any tubular villous adenomas, if indeed in the future we find that they do test negative you would have a tool with which you could scrutinize equivocal biopsies. In other words, if a gastroenterologist tells you, “I can’t really tell if it is a tubular villous adenoma or if it is a cancer” and the biopsy specimen tests positive for telomerase, that might swing the pendulum toward calling it a cancer and being more aggressive about treatment. And, as I mentioned previously, there is ongoing interest in using the assay for the preoperative diagnosis of pancreatic masses.
As I said before, we did not use in situ hybridization in these experiments. And we did not look at messenger RNA. Those are both techniques that would complement and increase the significance of these studies.
Lastly, Dr. Sarr asked about adjacent areas, particularly in IPMT specimens, that were submitted for histology. When the tissue was available, we would examine areas of IPMTs that contained cancer along with adjacent areas of normal duct or less dysplastic ductal epithelium. We found that the specimens that contained benign epithelium or mildly dysplastic epithelium tested negative. Again, the only specimens that were positive were from areas of frankly invasive cancer. I think this also supports the notion that telomerase activation is a relatively late neoplastic event.
Dr. David C. Allison (Toledo, Ohio): I really enjoyed this paper. Abnormal chromosome numbers, or tumor aneuploidy, occur in about 80% of pancreatic carcinomas, and is a very important and independent prognostic determinant for this disease. It is very interesting that the proportion of tumors in this study with increased telomerase activity was also 80%, and I think it would be very important for you to correlate DNA content and telomerase activities for the same tumors, if you have not already done this.
On another note, when one looks at aneuploid tumor chromosomes under the microscope, approximately 1 to 2% of them are joined at their telomeres as large fusion chromosomes. Telomerase activity may play a role in this chromosomal fusion process, and these large chromosomes could conceivably unbalance the mitotic spindle and drive the genetic instability of these malignancies.
This was a great paper and I look forward to hearing more of your future work.
Dr. James H. Balcom: We have not looked at chromosome fusions or aneuploidy in these same specimens. I agree that that would be very interesting examine. What I do know is that shortening of the telomeres sometimes results in the very fusions of which you speak, and can create genetic instability. Depending on the further genetic context, cellular death or unchecked proliferation may result.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association, April 26–28, 2001, the Broadmoor Hotel, Colorado Springs, Colorado.
Supported by the Edward D. Churchill, MD, Resident Research Fellowship, Harvard Medical School, Boston, Massachusetts.
Correspondence: Carlos Fernández-del Castillo, MD, Massachusetts General Hospital, WACC 336, 55 Fruit St., Boston, MA 02114.
E-mail: cfernandez@partners.org
Accepted for publication April 26, 2001.
References
- 1.Blackburn EH. Structure and function of telomeres. Nature 1991; 350: 569–573. [DOI] [PubMed] [Google Scholar]
- 2.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature 1990; 345: 458–460. [DOI] [PubMed] [Google Scholar]
- 3.Allsopp RC, Vaziri H, Patterson C, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci USA 1992; 89: 10114–10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaziri H, Schachter F, Uchida I, et al. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet 1993; 52: 661–667. [PMC free article] [PubMed] [Google Scholar]
- 5.Wright WE, Shay JW. Telomere positional effects and the regulation of cellular senescence. Trends in Genetics 1992; 8: 193–197. [DOI] [PubMed] [Google Scholar]
- 6.Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res 1991; 256: 271–282. [DOI] [PubMed] [Google Scholar]
- 7.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science 1994; 266: 2011–2015. [DOI] [PubMed] [Google Scholar]
- 8.Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell 1989; 59: 521–529. [DOI] [PubMed] [Google Scholar]
- 9.Rhyu MS. Telomeres, telomerase, immortality. J Natl Cancer Inst 1995; 87: 884–894. [DOI] [PubMed] [Google Scholar]
- 10.Burger AM, Bibby MC, Double JA. Telomerase activity in normal and malignant mammalian tissues: feasibility of telomerase as a target for cancer chemotherapy. Br J Cancer 1997; 75: 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin L, Artandi SE, Shen Q, et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 1999; 97: 527–538. [DOI] [PubMed] [Google Scholar]
- 12.Hahn WC, Counter CM, Lundberg AS, et al. Creation of human tumour cells with defined genetic elements. Nature 1999; 400: 464–468. [DOI] [PubMed] [Google Scholar]
- 13.Shoji Y, Yoshinaga K, Inoue A, et al. Quantification of telomerase activity in sporadic colorectal carcinoma: association with tumor growth and venous invasion. Cancer 2000; 88: 1304–1309. [DOI] [PubMed] [Google Scholar]
- 14.Hoos A, Hepp HH, Kaul S, et al. Telomerase activity correlates with tumor aggressiveness and reflects therapy effect in breast cancer. Int J Cancer 1998; 79: 8–12. [DOI] [PubMed] [Google Scholar]
- 15.Taga S, Osaki T, Ohgami A, et al. Prognostic impact of telomerase activity in non-small cell lung cancers. Ann Surg 1999; 230: 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med 1992; 326: 455–465. [DOI] [PubMed] [Google Scholar]
- 17.Yeo CJ, Cameron JL, Sohn T, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s. Ann Surg 1997; 226: 248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motojima K, Urano T, Nagata Y, et al. Detection of point mutations in the Kirsten-ras oncogene provides evidence for the multicentricity of pancreatic carcinoma. Ann Surg 1993; 217: 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hruban RH, van Mansfeld A, Offerhaus G, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol 1993; 143: 545–554. [PMC free article] [PubMed] [Google Scholar]
- 20.Yanagisawa A, Ohtake K, Ohashi K, et al. Frequent c-Ki-ras oncogene activation in mucous cell hyperplasias of pancreas suffering from chronic inflammation. Cancer Res 1993; 53: 953–956. [PubMed] [Google Scholar]
- 21.Yanagisawa A, Kato Y, Ohtake K, et al. c-Ki-ras point mutations in ductectatic-type mucinous cystic neoplasms of the pancreas. Jpn J Cancer Res 1991; 82: 1057–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redston MS, Caldas C, Seymour AB, et al. p53 mutations in pancreatic carcinoma and evidence of common involvement in homopolymer tracts in DNA microdeletions. Cancer Res 1994; 54: 3025–3033. [PubMed] [Google Scholar]
- 23.Pearson AS, Chiao P, Zhang L, et al. The detection of telomerase activity in patients with adenocarcinoma of the pancreas by fine-needle aspiration. Int J Oncol 2000; 17: 381–385. [DOI] [PubMed] [Google Scholar]
- 24.Uehara H, Nakaizumi A, Tatsuta M, et al. Diagnosis of pancreatic cancer by detecting telomerase activity in pancreatic juice: comparison with K-ras mutations. Am J Gastroenterol 1999; 94: 2513–2518. [DOI] [PubMed] [Google Scholar]
- 25.Suehara N, Mizumoto K, Muta T, et al. Telomerase elevation in pancreatic ductal carcinoma compared to nonmalignant pathological states. Clin Cancer Res 1997; 3: 993–998. [PubMed] [Google Scholar]
- 26.Iwao T, Hiyama E, Yokoyama T, et al. Telomerase activity for the preoperative diagnosis of pancreatic cancer. J Natl Cancer Inst 1997; 89: 1621–1623. [DOI] [PubMed] [Google Scholar]
- 27.Hiyama E, Kodama T, Shinbara K, et al. Telomerase activity is detected in pancreatic cancer but not in benign tumors. Cancer Res 1997; 57: 326–331. [PubMed] [Google Scholar]
- 28.Itoi T, Shinohara Y, Takeda K, et al. Detection of telomerase activity in biopsy specimens for diagnosis of biliary tract cancers. Gastrointest Endosc 2000; 52: 380–386. [DOI] [PubMed] [Google Scholar]
- 29.Inoue H, Tsuchida A, Kawasaki Y, et al. Preoperative diagnosis of intraductal papillary-mucinous tumors of the pancreas with attention to telomerase activity. Cancer 2001; 91: 35–41. [DOI] [PubMed] [Google Scholar]
- 30.Yeh T, Cheng A, Chen T, et al. Telomerase activity is a useful marker to distinguish malignant pancreatic cystic tumors from benign neoplasms and pseudocysts. J Surg Res 1999; 87: 171–177. [DOI] [PubMed] [Google Scholar]
- 31.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282: 1145–1147. [DOI] [PubMed] [Google Scholar]
- 32.Blasco MA, Lee HW, Hande MP, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 1997; 91: 25–34. [DOI] [PubMed] [Google Scholar]
- 33.Uehara H, Nardone G, Nazarenko I, et al. Detection of telomerase activity utilizing energy transfer primers: comparison with gel- and ELISA-based detection. Biotechniques 1999; 26: 552–558. [DOI] [PubMed] [Google Scholar]
- 34.Umbricht CB, Sherman ME, Dome J, et al. Telomerase activity in ductal carcinoma in situ and invasive breast cancer. Oncogene 1999; 18: 3407–3414. [DOI] [PubMed] [Google Scholar]
- 35.Albanell J, Lonardo F, Rusch V, et al. High telomerase activity in primary lung cancers: association with increased cell proliferation rates and advanced pathologic stage. J Natl Cancer Inst 1997; 89: 1609–1615. [DOI] [PubMed] [Google Scholar]
- 36.Jong HS, Park YI, Kim S, et al. Up-regulation of human telomerase catalytic subunit during gastric carcinogenesis. Cancer 1999; 86: 559–565. [PubMed] [Google Scholar]
- 37.Zhang W, Kapusta LR, Slingerland JM, et al. Telomerase activity in prostate cancer, prostatic intraepithelial neoplasia, and benign prostatic epithelium. Cancer Res 1998; 58: 619–621. [PubMed] [Google Scholar]
- 38.Wu A, Ichihashi M, Ueda M. Correlation of the expression of human telomerase subunits with telomerase activity in normal skin and skin tumors. Cancer 1999; 86: 2038–2044. [DOI] [PubMed] [Google Scholar]
- 39.Hiyama E, Saeki T, Hiyama K, et al. Telomerase activity as a marker of breast carcinoma in fine-needle aspirated samples. Cancer 2000; 90: 235–238. [PubMed] [Google Scholar]
- 40.Arinaga M, Shimizu S, Gotoh K, et al. Expression of human telomerase subunit genes in primary lung cancer and its clinical significance. Ann Thorac Surg 2000; 70: 401–405. [DOI] [PubMed] [Google Scholar]
- 41.Cameron JL. Long-term survival following pancreaticoduodenectomy for adenocarcinoma of the head of the pancreas. In: Cameron JL, ed. Pancreatic neoplasms. Surg Clin North Am 1995:939–951. [DOI] [PubMed]
- 42.Mannell A, van Heerden JA, Weiland LH, et al. Factors influencing survival after resection for ductal adenocarcinoma of the pancreas. Ann Surg 1986; 203: 403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993; 165: 68–73. [DOI] [PubMed] [Google Scholar]
- 44.Luttges J, Kloppel G. Precancerous conditions of pancreatic carcinoma. J Hepatobiliary Pancreat Surg 2000; 7: 568–574. [DOI] [PubMed] [Google Scholar]
- 45.Brat DJ, Lillemoe KD, Yeo CJ, et al. Progression of pancreatic intraductal neoplasia to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol 1998; 22: 163–169. [DOI] [PubMed] [Google Scholar]
- 46.Bachor C, Bachor OA, Boukamp P. Telomerase is active in normal gastrointestinal mucosa and not up-regulated in precancerous lesions. J Cancer Res Clin Oncol 1999; 125: 453–460. [DOI] [PubMed] [Google Scholar]
- 47.Mutirangura A, Supiyaphun P, Trirekapan S, et al. Telomerase activity in oral leukoplakia and head and neck squamous cell carcinoma. Cancer Res 1996; 56: 3530–3533. [PubMed] [Google Scholar]
- 48.Jiang C, Juo L, Said TK, et al. Immortalized mouse mammary cells in vivo do not exhibit increased telomerase activity. Carcinogenesis 1997; 18: 2085–2091. [DOI] [PubMed] [Google Scholar]
- 49.de Lange T, Jacks T. For better or worse? Telomerase inhibition and cancer. Cell 1999; 98: 273–275. [DOI] [PubMed] [Google Scholar]