Abstract
Objective
To evaluate the safety and efficacy of local excision in patients with T2 and T3 distal rectal cancers that have been downstaged by preoperative chemoradiation.
Summary Background Data
T2 and T3 cancers treated by local excision alone are associated with unacceptably high recurrence rates. The authors hypothesized that preoperative chemoradiation might downstage both T2 and T3 lesions and significantly expand the indications for local excision.
Methods
Local excision was performed after preoperative chemoradiation on patients with a complete clinical response or on patients who were either ineligible for or refused to undergo abdominoperineal resection. Local excision was approached transanally by removing full-thickness rectal wall and the underlying mesorectum.
Results
From 1994 to 2000, 95 patients with rectal cancers underwent preoperative chemoradiation and surgical resection for curative intent. Of these, 26 patients (28%), 19 men and 7 women, with a mean age of 63 years (range 44–90), underwent local excision. Pretreatment endoscopic ultrasound classifications included 5 T2N0, 13 T3N0, 7 T3N1, and 1 not done. Pathologic partial and complete responses were achieved in 9 of 26 (35%) and 17 of 26 (65%) patients, respectively. Two of nine partial responders underwent immediate abdominoperineal resection. The mean follow-up was 24 months (median 19, range 6–77). The only recurrence was in a patient who refused to undergo abdominoperineal resection after a partial response. There was one postoperative death from a stroke. This treatment was associated with a low rate of complications.
Conclusion
Local excision appears to be an effective alternative treatment to radical surgical resection for a highly select subset of patients with T2 and T3 adenocarcinomas of the distal rectum who show a complete pathologic response to preoperative chemoradiation.
Colorectal cancer is the third most common site for cancer in men and women in the United States. It is estimated that there will be 36,400 new diagnoses of rectal cancer and 8,600 deaths from rectal cancer in the year 2000. 1 The current standard treatment for distal rectal cancer is abdominoperineal resection (APR), low anterior resection, or resection with coloanal anastomosis. These operations are associated with significant rates of death and complications, and local or distant recurrences occur in 10% to 65% of patients. 2 The complications associated with radical rectal surgical procedures include urinary dysfunction in 10% to 70%, sexual dysfunction in 13% to 70%, and anastomotic leaks in 5% to 17%, with death rates of 2% to 6%. 3–10 Compared with a radical resection for distal rectal cancer, local excision avoids a laparotomy, permanent colostomy, and the complications associated with pelvic dissection.
The incidence of local recurrence even after radical surgery ranges from 10% to 29%. 11–14 A recent review of published series reported an 18.5% overall local recurrence rate after APR. The incidence of local recurrence increased with advancing stage: 8.5%, 16.3%, and 28.6% for Dukes A, B, and C, respectively. 11 Extrapelvic and distant recurrences occur in approximately 30% of patients. These patients likely present with occult metastatic disease and would not be expected to benefit from the more radical operations. The 5-year disease-free survival rate of patients with node-positive rectal cancers is 30% to 40%. 2 Therefore, most patients with advanced rectal cancers are not cured by radical resection of the tumor. For these reasons, treatment alternatives for distal rectal cancers are of interest.
Historically, local excision for distal rectal cancers has been approached with caution because of the high rates of local recurrence. Local therapy alone for rectal cancer has been used for patients with significant comorbid conditions that make a more radical surgery prohibitive. With newer techniques in adjuvant radiation therapy and advancements in chemotherapy, it has been possible to explore the option of multimodality treatment schemas to improve local control rates and allow better functional outcomes in a select group of patients with distal rectal cancer. In the United States, initial studies of preoperative radiation treatment for rectal cancer were influenced by the lack of efficacy of low-dose (2,000–3,000 cGy) radiation. However, data from Europe suggest that preoperative radiation alone reduces local recurrence rates and improves overall survival compared with surgery alone 15 and was more effective than postoperative radiotherapy. 16 Combination therapy using moderate-dose (4,000–4,500 cGy) and high-dose (>5,000 cGy) chemoradiation has allowed downstaging of tumors in 59% to 76% of patients, with complete pathologic response rates of 20% to 44%. 17–20 These studies have encouraged the use of preoperative chemoradiation and expanded the realm of surgical options to include sphincter preservation. We report on a highly select group of patients with advanced distal rectal cancers who had good responses to preoperative chemoradiation therapy and were treated with transanal local excisions.
METHODS
The records of 95 consecutive patients with locally advanced distal rectal adenocarcinoma who underwent preoperative chemoradiation followed by surgical resection for curative intent, from 1994 to 2000, were reviewed. Of these, 26 patients (28%) with T2 or T3 distal rectal cancers underwent local excision. Informed consent was obtained from each patient. Preoperative staging included evaluation by a surgeon, radiation oncologist, and a medical oncologist, including history and physical examination, chest x-ray, computed tomography, and serum carcinoembryonic antigen level. All patients underwent colonoscopy and the diagnosis of adenocarcinoma was confirmed on biopsy. Preradiation endoscopic rectal ultrasound (ERUS) was performed in 25 of the 26 patients, using an ultrasound endoscope initiated in the distal sigmoid colon; it included inspection of the periiliac region and proximal perirectal tissues for lymph nodes. Lymph nodes were considered positive if they had the following criteria: hypoechoic, round, larger than 5 mm, and intact borders. All patients underwent sigmoidoscopy and a digital rectal examination to evaluate for clinical response after chemoradiation. ERUS was done after chemoradiation in 12 of the 26 patients. Clinical partial response was defined as a decrease in size of the tumor by at least 50%. Clinical complete response was defined as no evidence of residual disease. Only lesions that had a complete clinical response were considered for local excision. However, we did include patients who underwent local excision because they refused to undergo radical surgery or because they had severe medical comorbidities.
All patients received external beam radiation (EBRT) with concomitant chemotherapy. Radiotherapy was delivered using conventional fractionation and techniques: 4,500 cGy in 25 fractions to the primary site plus the internal iliac nodal chain and the presacral space. A three-field technique (two laterals and one posterior) was used, with the top of the field at the midpoint of the sacroiliac joints. The bottom of the field was at least 4 cm below the tumor, usually encompassing the entire anal canal. The pelvic side walls were covered by at least a 1.5-cm dosimetric margin. Chemotherapy consisted of 5-fluorouracil by continuous infusion at a dose of 300 mg/m2/day, 5 days per week on days of EBRT. 21
Local excision was done by a transanal approach under general anesthesia. The patients were placed in a prone jackknife or lithotomy position, depending on the tumor location. The tumor site was removed with electrocautery by excising the full-thickness rectal wall with a 1-cm margin around the tumor. The underlying mesorectal fat was included with the specimen. The exposed mesorectum was palpated in an effort to identify and excise any additional lymph nodes. The rectal defect was closed primarily in a transverse fashion with absorbable sutures. Patients were admitted to the hospital overnight with a Foley catheter in place and discharged home the following day with follow-up examinations at 2 weeks. Long-term follow-up was done with a digital rectal examination, endoscopy, and serum carcinoembryonic antigen level every 6 months.
The specimens were pinned and marked for orientation by the surgeon. A dedicated pathologist grossly examined each specimen. If residual carcinoma was grossly visible, at least three sections of tumor were taken. If there was no grossly identifiable tumor, usually an area of depression, ulceration, or scar was present. This area was submitted in its entirety for microscopic examination to confirm the absence of cancer. Each sample was fixed in 10% neutral buffered formalin for 6 hours. After fixation the tissue samples were processed into paraffin blocks. Four-micrometer-thick tissue sections were obtained from the paraffin blocks and stained with hematoxylin and eosin (Richard-Allan Scientific, Kalamazoo, MI) using standard histology techniques. A pathologic partial response was defined as a specimen with evidence of residual tumor. A complete pathologic response was defined as a specimen with no residual tumor on histopathologic examination.
RESULTS
Of the 26 patients who underwent chemoradiation followed by local excision for distal rectal cancers, there were 19 men and 7 women (Table 1). The mean age at presentation was 63 years (range 44–90). The most common presenting symptom was rectal bleeding. All tumors were easily palpable on digital rectal examination and were located 2 to 10 cm from the anal verge. The mean tumor size was 3 cm (range 1–6). Pretreatment ERUS classifications included 5 T2N0, 13 T3N0, 7 T3N1, and 1 not done due to poor patient tolerance.
Table 1. PATIENT CHARACTERISTICS
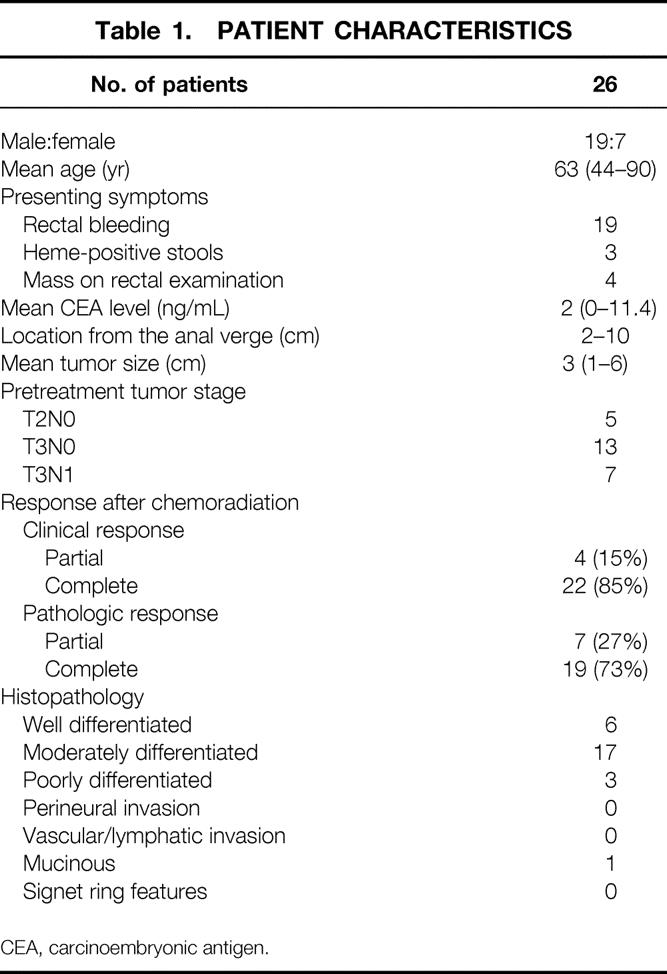
CEA, carcinoembryonic antigen.
There were 4 (15%) partial and 22 (85%) complete clinical responses. The four patients with partial clinical responses were confirmed to have residual tumor on pathologic examination. There were 9 (35%) partial and 17 (65%) complete pathologic responses. Five patients who were considered to have a complete clinical response had microscopic residual adenocarcinoma in the pathologic specimen (Table 2). In 25 of 26 (96%) patients, the margins of resection were negative after local excision. The histology included moderately to well-differentiated adenocarcinoma in 23 of 26 patients (89%) and poorly differentiated adenocarcinoma in 3 of 26 (12%). No tumors had evidence of vascular, neural, or lymphatic invasion. Lymph nodes were identified in 6 of 26 (23%) specimens, and none contained metastases.
Table 2. TUMOR RESPONSE AFTER CHEMORADIATION
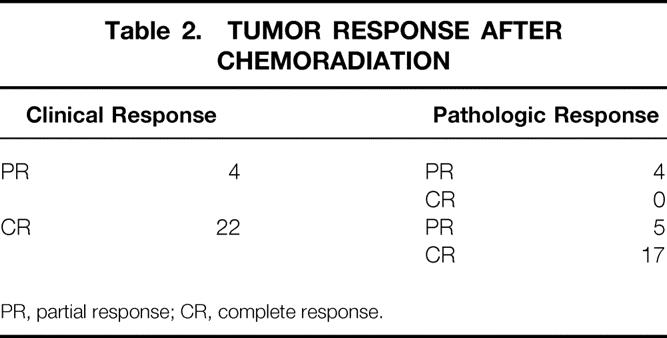
PR, partial response; CR, complete response.
We recommended radical resection for patients with partial responses (gross or pathologic). Only two of nine patients had additional surgery, and both required an APR. One patient had no evidence of residual carcinoma at the primary site, but lymph node metastases were found in three of six nodes. The other patient had no residual tumor or lymph node involvement. The remaining seven patients did not undergo APR because they refused to have a colostomy (two patients) or because medical comorbidities precluded them from undergoing a major operation (five patients).
One patient died on postoperative day 7 from a stroke. He was known to have severe coronary artery disease and was considered too ill to undergo radical resection. His tumor was a T3N0 rectal cancer that had a partial clinical response to chemoradiation. The local excision specimen had residual T3 disease. One patient developed a rectal stricture that resolved after serial dilations. Patients frequently reported reduced rectal sphincter function in the early postoperative period. This resolved in nearly all patients by the 6-month follow-up. There were no patients with urinary incontinence or wound infections. Sexual function was not assessed. Complications during and up to 4 weeks after chemoradiation were mild, with patients experiencing bowel incontinence (1/25) and perianal dermatitis (7/25).
The mean follow-up was 24 months (median 19, range 6–77). One patient, age 90, died of myocardial infarction at 3 months; this patient had a partial response to chemoradiation for a T3N0 tumor but was not a candidate for local excision because of severe coronary artery disease. One patient developed an intramural recurrence in the anal sphincter and a synchronous distant recurrence in the inguinal lymph nodes at 13 months. This patient had a partial clinical response after chemoradiation but refused to undergo an APR. He underwent salvage APR for the recurrence and an inguinal lymph node dissection. Pathologic findings included a poorly differentiated adenocarcinoma with squamous differentiation (whereas the initial lesion was a moderately differentiated adenocarcinoma). Metastatic poorly differentiated carcinoma was found in two of seven mesorectal lymph nodes and four of eight inguinal lymph nodes. None of the patients with complete pathologic responses have had recurrence. Currently, there are no patients with evidence of recurrence.
DISCUSSION
Local excision for distal rectal tumors is an accepted treatment for T1 adenocarcinomas that have favorable prognostic features, such as small size (<4 cm), mobile, and moderately to well-differentiated histology without vascular, lymphatic, or perineural invasion. 22–25 Local excision as a surgical option in these patients is possible because the cure rate is high (>90%), the risk of recurrence is low (<10%), and the patient is spared the complications of a more radical operation. Most studies looking at local excision in patients with T2 or T3 distal rectal cancers are difficult to interpret because they are retrospective, have small sample size, include a heterogeneous patient population, use a variety of surgical techniques (piecemeal snaring, fulguration, transsphincteric, transsacral, or transanal resections, and endoscopic microsurgery), and use varying regimens of adjuvant chemotherapy and radiation therapy.
Reports have shown that patients with T2 or T3 lesions have a 17% to 50% incidence of local recurrence with local excision alone. 23,26,27 This may be partly explained by the higher incidence of nodal metastases found with progressively higher T classification, ranging from 15% for tumors confined to the rectal wall and up to 60% with extrarectal invasion. 24,26 To reduce the high local recurrence rates for more advanced rectal cancers after standard surgical resection, combined adjuvant chemotherapy and radiation have shown efficacy in clinical trials. 28–30 Retrospective studies of local excision followed by adjuvant radiation alone 25,31–33 or chemoradiation 34 for selected tumors have shown that they can reduce the expected rate of local recurrence for selected low rectal cancers and include large and more advanced tumors (T2–3N0–1). The only prospective multiinstitutional trial of local excision for select T2 rectal cancers (<4 cm, <40% circumference, node-negative) with postoperative adjuvant chemoradiation showed that cancer control could be achieved with sphincter preservation with 6-year survival and disease-free survival rates of 85% and 71%, respectively. 35 In this study, comparison of outcomes at 48 months with historical data from the National Cancer Database showed no significant disadvantage to this treatment approach, although a plateau in the disease-free survival had not been observed.
Preoperative radiation followed by local excision for select T3 rectal cancers was reported by Mohiuddin et al. 17 Select patients who had T3 tumors that were downsized by radiation alone, without sensitizing chemotherapy, and who met the criteria for full-thickness local excision (≤T2 and <3 cm size of primary tumors) had 5-year survival rates of 88% and a local recurrence rate of 10%. Patients who had a pathologic postradiation classification of T3 had a much poorer prognosis (50% 5-year survival) despite initial treatment with radical surgical resection. Moderate-dose preoperative chemoradiation has been shown to be effective in downstaging rectal cancers, including T3 tumors, 21,36 and allows sphincter-preserving surgery in 75% to 86% of patients. 21,37–41 Complete pathologic responses can be achieved in up to 9% to 31% of patients after preoperative chemoradiation. Improved survival is seen in patients who had no gross residual disease. 38,42–44
The potential advantages to preoperative combination chemoradiation are that it downstages tumors so that patients may undergo less radical surgery, with decreased complication rates and sphincter preservation; involves a smaller treatment field with less small bowel toxicity; delivers radiation to well-oxygenated tissue for improved efficacy; and eradicates any micrometastatic disease (locoregional and distant) early in the treatment course. We reasoned that by evaluating the effect of preoperative chemoradiation, we can select patients who have the most favorable tumor response (i.e., complete pathologic response).
In our series, no patients with pathologic complete responses who underwent local excision have had recurrence to date. We reasoned that the patients who had a complete response to preoperative chemoradiation should have had a similar response in the pelvis (mesorectal lymph nodes). In patients who might bear micrometastatic disease in the pelvic lymph nodes, the preoperative chemoradiation can potentially sterilize the pelvis. For this highly select group of patients, no further surgical intervention was recommended; however, we recommended that all patients receive postoperative chemotherapy. We realize that some patients in this group will ultimately have recurrent disease because they initially presented with advanced cancers. It remains to be seen whether cure rates are comparable to those of patients treated with radical surgery after preoperative therapy.
For patients who have residual carcinoma in the local excision specimen, we recommended that they undergo a more definitive operation such as an APR. We reasoned that with an incomplete microscopic ablation of the primary tumor site, a similar incomplete response might be seen in the regional lymph nodes. Of the nine patients with residual tumor after local excision, two underwent APR within 30 days of local excision, one of whom had residual tumor. The one recurrence in our series was in a patient who had a partial pathologic response after local excision. This patient developed local and extrapelvic disease at 13 months. The recurrent tumor was a poorly differentiated mixed adenosquamous carcinoma in the anal canal with inguinal and mesorectal lymph node metastases. For patients with residual disease who were too ill to undergo major surgery, local excision offered a reasonable alternative to no treatment.
The technique of local excision is critical in the adequate assessment of the response to chemoradiation. Although some patients will have a complete clinical response, evaluation of the deep sections of the bowel wall may show residual tumor. Careful inspection of the bowel lumen will show a scar, ulceration, or indentation at the site of the ablated tumor. Simple biopsies of the mucosal surface or deep piecemeal biopsies of the rectal wall are inaccurate methods of detecting residual cancer cells because they may miss microscopic disease that may be found in deeper tissue layers. In our study, of the 22 patients deemed to have a complete clinical response, 5 (23%) had residual tumor on the pathologic specimen after complete excision of the primary tumor site. For this reason, we strongly emphasize full-thickness excision of the rectal wall, including the underlying perirectal fat, with at least a 1-cm margin of normal-appearing rectum around the lesion.
A limitation of local excision is that mesenteric lymph nodes are not adequately sampled. Only 6 of 26 (23%) of our surgical specimens contained identifiable lymph nodes, including three tumors that had been staged as T3N1 by ERUS. None of the specimens with lymph nodes had metastasis. Three of the specimens with lymph nodes were found in patients with incomplete pathologic responses. Two of these patients underwent APR, and one was found to have lymph node metastasis. The other patient initially refused to undergo APR and had recurrence 13 months later. Thus, the finding of a negative lymph node in a patient with a partial pathologic response does not rule out the possibility of lymph node metastasis.
The finding of lymph node metastasis in patients who have complete pathologic responses at the primary tumor site has not been previously reported. 38,43,44 However, in a recent abstract, of 30 patients who had complete responses at the primary tumor site after preoperative chemoradiation, 3 were found to have positive N1 lymph nodes in the specimen after radical resection. 45 There have been no recurrences in these three patients. Thus, the lymph node positivity rate in complete pathologic responders after chemoradiation is low (0–10%). Therefore, it seems that few these patients would benefit from a major radical operation.
Accurate preoperative staging is important for the selection of patients undergoing preoperative chemoradiation. With technologic advancements in imaging (ERUS and high-quality computed tomography scans), tumors can now be very accurately staged before treatment. 46,47 The accuracy rate of ERUS for staging in rectal cancer is 90% for T stage and 80% for predicting lymph node involvement. 48 However, ERUS can assess only structural change and cannot differentiate between tumor, inflammation, and scar. Therefore, the staging accuracy is severely restricted after therapeutic intervention. 49 ERUS was performed after chemoradiation in the initial 12 patients (46%) in our series. We found that the T-classification accuracy rate dropped to approximately 30% after chemoradiation therapy compared with the pathologic classification at surgery. Therefore, we no longer routinely perform ERUS after chemoradiation.
Reports have shown that the overall surgical complication rate, including the incidence of wound infection and anastomotic leak, is not increased after preoperative chemoradiation. 50,51 The toxicity associated with chemoradiation treatment is generally mild, with 30% of patients developing grade 1 or 2 stomatitis, diarrhea, and hematologic toxicity. Local excision is associated with a low death rate and a very low complication rate compared with a major resection. 26,34 Although we performed local excision in a previously irradiated rectum, the incidence of treatment complications from the surgery was low in our study. Many patients reported bowel urgency or incontinence in the perioperative period, yet no patients reported incontinence 6 months after local excision. One patient developed a rectal stricture that resolved after serial dilatations.
In summary, local excision after preoperative chemoradiation is a well-tolerated procedure that allows for the selection of prognostically favorable tumors before surgical treatment. Our data suggest that patients with a partial tumor response to preoperative chemoradiation should undergo radical surgical resection. Local excision may be a reasonable alternative to radical operation, or no treatment, in medically debilitated patients who would not be expected to tolerate major surgery. In this study, patients with a complete pathologic response after preoperative chemoradiation had no evidence of recurrence with a median follow-up of 19 months. Although this treatment regimen seems promising, the length of follow-up is short. Based on this early experience, we are cautiously proceeding with a prospective study of additional patients.
DISCUSSION
Dr. Alfred M. Cohen (Lexington, Kentucky): I enjoyed this provocative presentation. From my perspective, the main issue is the potential paradigm shift in the treatment of rectal cancer, perhaps becoming analogous to our current treatment strategy for squamous cell cancer of the anus, radical surgery only for chemoradiation therapy and local excision failures.
There is no question that local excision for exophytic very early cancers works very well. But for T2 and T3 lesions, other published series suggest surgery alone is not acceptable. Individual reports suggest that local excision and radiation — the lumpectomy and radiation approach — works, but the multi-center trial that Glenn Steele reported, although it reports a 71% survival, local failure is 20%, and the salvage was only possible in 50%.
We know for patients who are undergoing radical surgery, preoperative radiation may be more effective than postoperative. And in fact, as you mention in your manuscript, there has been a prior study like yours reported by Jerry Marks from Thomas Jefferson suggesting preoperative radiation followed by local excision is safe and effective.
You use the term “downstaging.” I think we would all agree that preoperative radiation is capable of “downsizing,” whether it is capable of downstaging is unclear.
You talked about excising with 1 centimeter margins. I am still not quite sure how you do that when you are dealing with a 4 or 5 centimeter cancer. It also can be very difficult to identify the original tumor site when you have a complete response. I have two questions relevant to this issue: Did you mark or tattoo the tumor pre-radiation with India ink? How big was your excision when you had a complete response?
Focusing on the issue of downstaging itself, let me make a few comments about radiation dose interval to surgery, and post-op chemotherapy. The 4,500 rads, even with infusional 5FU, is a relatively low dose. Did any patient get higher doses than that? Do you have any plans to boost the dosage so perhaps you don’t even need the local excision?
There are some data on the use of radiation therapy alone for rectal cancer that indicate the time to complete clinical response is not always 4 to 6 weeks, but can be as long as 6 months. Did you look at the interval from the completion of radiation therapy to when you actually did your excision? Was there a correlation between that interval and complete response?
Lastly, did you give postoperative chemotherapy? And if not, do you plan to in the future?
We should all bear in mind the median follow-up in this series is >2 years. I will look forward to an analysis of more mature data.
Presenter Dr. Jorge Marcet (Tampa, Florida): Thank you, Dr. Cohen. I will try to answer all of your questions.
We did not tattoo the tumor in any of these patients. We localized the site of the tumor prior to treatment based on digital rectal exam. In all cases we are able to find the area of the previous tumor even when there has been a complete clinical response as there is either a scar or an umbilication at the site. So we did not need to tattoo the tumor.
The trans-anal local excision is a full thickness excision of the rectal wall and includes underlying mesorectum. incise the rectum along a 1 centimeter margin around the previous tumor site.
Regarding radiation, all our patients received 4,500 centigrades of radiation. Some of our radiation therapy colleagues may feel that a higher dose is perhaps more optimal and future studies may include a higher dose of radiation.
Regarding chemotherapy, we did recommend that patients be treated with standard postoperative adjuvant therapy, 5-Fluorouracil and leucovorin. However, many of the patients did not have postoperative treatment either because the medical oncologists did not feel treatment was warranted or patients did not take treatment. So the role of adjuvant therapy after complete tumor ablation is inconclusive from this study.
Dr. David A. Rothenberger (St. Paul, Minnesota): I would like to congratulate the group from Tampa on an excellent and provocative preliminary report. There are many questions that come to mind.
As I understand it, your hypothesis is basically that downstaging to the point of a complete pathologic response effected by preoperative chemoradiation can be used to select out a favorable group of distal rectal cancers which in turn can be treated by local excision rather than undergoing traditional radical resection so you can minimize morbidity. Embedded within that hypothesis are a number of other hypotheses which I think I would like to question.
First of all, do you really believe that you can reliably determine complete response to chemoradiation by local excision of the residual scar or inflammation? This sort of ignores the possibility of leaving behind residual occult metastases in the adjacent lymph nodes or adjacent tissues. How do you know that T-stage responders are also end-stage responders?
Why is it that your complete response rate is 65% when most of the rest of the literature is much lower than that? I am concerned about this because in our own analysis of 114 patients with stage 2 and 3 rectal cancers followed up for a mean of 48 months, having had radical rectal surgery where you can look at all the specimen our complete response rate was only 66%.
Secondly, local excision will not compromise subsequent radical resection seems to be a part of your hypothesis. I wonder if we have data that implies that this is truly safe.
Similarly, you seem to imply that the morbidity related to chemoradiation will be early. But I think we all recognize that radiation can produce long-term terrible problems. How do we know this is really a safe regimen?
Dr. Jorge Marcet: Thank you, Dr. Rothenberger. This study represents a very select group of patients. These are patients that appear to have a very favorable response to the preoperative treatment.
A complete pathologic response rate of 65% was found in patients who underwent local excision. This was in a select group, that is, patients who demonstrated a very favorable response to neoadjuvant treatment, and so the percentage of patients with complete pathologic response is high. When we look at complete pathologic response rate in the total group of 95 patients who had neoadjuvant therapy, there were 21 patients, or 22%. This is consistent with published reports.
We know that patients who have complete response or very favorable downstaging by preoperative therapy are those who also demonstrate improved survival when compared to those patients who do not have as favorable a response to neoadjuvant radiation. So what we are attempting to do is select those patients with very favorable treatment responses and treat with a less morbid operative procedure.
We do not know what the lymph node status is following chemoradiation. Only 6 of the 26 patients that underwent local excision had nodes recovered in the specimen. A very low number. None of these had cancer, by the way. Local excision does not reliably sample lymph nodes. It is also possible that some lymph nodes are destroyed by the radiation.
In all the studies that I reviewed looking at neoadjuvant therapy and patients with complete response, there are no recorded incidents of nodal metastases after a complete response in the original specimen.
Dr. John H. Pemberton (Rochester, Minnesota): Thank you for the opportunity to review this paper. As the other discussants have mentioned, we need to wait a while longer for more complete follow-up before judging the merits of this approach to the management of colorectal cancer.
Changing tack, we have found problems with our patient population when we combined radical resections with chemoradiation in terms of functional outcomes. Have you had the opportunity to look at functional outcomes in your group of patients either retrospectively or prospectively?
Second, in follow up to that question, were the fields used to apply the radiation malleable at all, depending on the size and location of the tumor, perhaps attempting to spare the anus and pelvic floor from the effects of radiation? Thank you again for the opportunity to review and comment on the paper.
Dr. Jorge Marcet: Thank you, Dr. Pemberton. In answer to your last question, yes, the radiation fields are mounted, the radiation therapists attempt to cover a field that is at least 4 centimeters below the distal margin of the tumor. Now, because we are dealing with distal rectal cancers, in many instances this does include irradiation of the anal canal.
We did not evaluate the anorectal function prior to treatment, so it is hard to comment on their function after treatment. Fecal incontinence occurs frequently during the radiation treatments. I can tell you from personal experience that in the perioperative period patients frequently do complain of troubling incontinence following local excision. However, at the six-month follow-up, incontinence is not a major issue for patients. Some do report minor incontinence, but no one has required a colostomy to treat their incontinence.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association, April 26–28, 2001, the Broadmoor Hotel, Colorado Springs, Colorado.
Correspondence: Jorge Marcet, MD, Department of Surgery, GI Tumor Program, H. Lee Moffitt Cancer Center, 12902 Magnolia Drive, Tampa, FL 33612.
E-mail: jmarcet@hsc.usf.edu
Accepted for publication April 26, 2001.
References
- 1.Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer 2000; 88: 2398–2424. [DOI] [PubMed] [Google Scholar]
- 2.Cohen AM, Minsky B, Schilsky R. Cancer of the rctum. In: Hellman S, Rosenberg SA. Cancer: principles and practice. Philadelphia: Lippincott; 1997: 1197–1234.
- 3.Havenga K, Enker WE, McDermott K, et al. Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. J Am Coll Surg 1996; 182: 495–502. [PubMed] [Google Scholar]
- 4.Zaheer S, Pemberton JH, Farouk R, et al. Surgical treatment of adenocarcinoma of the rectum. Ann Surg 1998; 227: 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neal DE, Williams NS, Johnston D. A prospective study of bladder function before and after sphincter-saving resections for low carcinoma of the rectum. Br J Urol 1981; 53: 558–564. [DOI] [PubMed] [Google Scholar]
- 6.Longo WE, Virgo KS, Johnson FE, et al. Outcome after proctectomy for rectal cancer in Department of Veterans Affairs Hospitals: a report from the National Surgical Quality Improvement Program. Ann Surg 1998; 228: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enker WE, Thaler HT, Cranor ML, et al. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 1995; 181: 335–346. [PubMed] [Google Scholar]
- 8.Hojo K, Sawada T, Moriya Y. An analysis of survival and voiding, sexual function after wide iliopelvic lymphadenectomy in patients with carcinoma of the rectum, compared with conventional lymphadenectomy. Dis Colon Rectum 1989; 32: 128–133. [DOI] [PubMed] [Google Scholar]
- 9.Rothenberger DA, Wong WD. Abdominoperineal resection for adenocarcinoma of the low rectum. World J Surg 1992; 16: 478–485. [DOI] [PubMed] [Google Scholar]
- 10.Balslev I, Harling H. Sexual dysfunction following operation for carcinoma of the rectum. Dis Colon Rectum 1983; 26: 785–788. [DOI] [PubMed] [Google Scholar]
- 11.McCall JL, Cox MR, Wattchow DA. Analysis of local recurrence rates after surgery alone for rectal cancer. Int J Colorectal Dis 1995; 10: 126–132. [DOI] [PubMed] [Google Scholar]
- 12.Nymann T, Jess P, Christiansen J. Rate and treatment of pelvic recurrence after abdominoperineal resection and low anterior resection for rectal cancer. Dis Colon Rectum 1995; 38: 799–802. [DOI] [PubMed] [Google Scholar]
- 13.Pilipshen SJ, Heilweil M, Quan SH, et al. Patterns of pelvic recurrence following definitive resections of rectal cancer. Cancer 1984; 53: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 14.Rosen L, Veidenheimer MC, Coller JA, et al. Mortality, morbidity, and patterns of recurrence after abdominoperineal resection for cancer of the rectum. Dis Colon Rectum 1982; 25: 202–208. [DOI] [PubMed] [Google Scholar]
- 15.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med 1997; 336:980–987. [DOI] [PubMed]
- 16.Frykholm GJ, Glimelius B, Pahlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum 1993; 36: 564–572. [DOI] [PubMed] [Google Scholar]
- 17.Mohiuddin M, Marks G, Bannon J. High-dose preoperative radiation and full thickness local excision: a new option for selected T3 distal rectal cancers. Int J Radiat Oncol Biol Phys 1994; 30: 845–849. [DOI] [PubMed] [Google Scholar]
- 18.Chen ET, Mohiuddin M, Brodovsky H, et al. Downstaging of advanced rectal cancer following combined preoperative chemotherapy and high dose radiation. Int J Radiat Oncol Biol Phys 1994; 30: 169–175. [DOI] [PubMed] [Google Scholar]
- 19.Janjan NA, Khoo VS, Abbruzzese J, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys 1999; 44: 1027–1038. [DOI] [PubMed] [Google Scholar]
- 20.Chari RS, Tyler DS, Anscher MS, et al. Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann Surg 1995; 221: 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janjan NA, Crane CN, Feig BW, et al. Prospective trial of preoperative concomitant boost radiotherapy with continuous infusion 5-fluorouracil for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2000; 47: 713–718. [DOI] [PubMed] [Google Scholar]
- 22.Morson BC, Bussey HRJ, Samoorian S. Policy of local excision for early cancer of the colorectum. Gut 1977; 18: 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biggers OR, Beart RW, Ilstrup DM. Local excision of rectal cancer. Dis Colon Rectum 1986; 29: 374–377. [DOI] [PubMed] [Google Scholar]
- 24.Minsky BD, Rich T, Recht A, et al. Selection criteria for local excision with or without adjuvant radiation therapy for rectal cancer. Cancer 1989; 63: 1421–1429 [DOI] [PubMed] [Google Scholar]
- 25.Minsky BD, Enker WE, Cohen AM, et al. Local excision and postoperative radiation therapy for rectal cancer. Am J Clin Oncol 1994; 17: 411–416. [DOI] [PubMed] [Google Scholar]
- 26.Killingback M. Local excision of carcinoma of the rectum: indications. World J Surg 1992; 16: 437–446. [DOI] [PubMed] [Google Scholar]
- 27.Graham RA, Hackford AW, Wazer DE. Local excision of rectal carcinoma: a safe alternative for more advanced tumors? J Surg Oncol 1999; 70: 235–238. [DOI] [PubMed] [Google Scholar]
- 28.Prolongation of the disease-free interval in surgically treated rectal carcinoma. Gastrointestinal Tumor Study Group. N Engl J Med 1985; 312:1465–1472. [DOI] [PubMed]
- 29.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991; 324: 709–715. [DOI] [PubMed] [Google Scholar]
- 30.Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Medical Research Council Rectal Cancer Working Party. Lancet 1996; 348:1605–1610. [PubMed]
- 31.Taylor RH, Hay JH, Larsson SN. Transanal local excision of selected low rectal cancers. Am J Surg 1998; 175: 360–363. [DOI] [PubMed] [Google Scholar]
- 32.Chakravarti A, Compton CC, Shellito PC, et al. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg 1999; 230: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balani A, Turoldo A, Braini A, et al. Local excision for rectal cancer. J Surg Oncol 2000; 74: 158–162. [DOI] [PubMed] [Google Scholar]
- 34.Graham RA, Atkins MB, Karp DD, et al. Local excision of rectal carcinoma. Early results with combined chemoradiation therapy using 5-fluorouracil and leucovorin. Dis Colon Rectum 1994; 37: 308–312. [DOI] [PubMed] [Google Scholar]
- 35.Steele GD, Herndon JE, Bleday R, et al. Sphincter-sparing treatment for distal rectal adenocarcinoma. Ann Surg Oncol 1999; 6: 433–441. [DOI] [PubMed] [Google Scholar]
- 36.Grann A, Minsky BD, Cohen AM, et al. Preliminary results of preoperative 5-fluorouracil, low-dose leucovorin, and concurrent radiation therapy for clinically resectable T3 rectal cancer. Dis Colon Rectum 1997; 40: 515–522. [DOI] [PubMed] [Google Scholar]
- 37.Marks G, Mohiuddin MM, Masoni L, et al. High-dose preoperative radiation and full-thickness local excision. A new option for patients with select cancers of the rectum. Dis Colon Rectum 1990; 33: 735–739. [DOI] [PubMed] [Google Scholar]
- 38.Rich TA, Skibber JM, Ajani JA, et al. Preoperative infusional chemoradiation therapy for stage T3 rectal cancer. Int J Radiat Oncol Biol Phys 1995; 32: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 39.Wagman R, Minsky BD, Cohen AM, et al. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis: long-term follow-up. Int J Radiat Oncol Biol Phys 1998; 42: 51–57. [DOI] [PubMed] [Google Scholar]
- 40.Rouanet P, Fabre JM, Dubois JB, et al. Conservative surgery for low rectal carcinoma after high-dose radiation. Functional and oncologic results. Ann Surg 1995; 221: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hyams DM, Mamounas EP, Petrelli N, et al. A clinical trial to evaluate the worth of preoperative multimodality therapy in patients with operable carcinoma of the rectum: a progress report of National Surgical Breast and Bowel Project Protocol R-03. Dis Colon Rectum 1997; 40: 131–139. [DOI] [PubMed] [Google Scholar]
- 42.Bozzetti F, Baratti D, Andreola S, et al. Preoperative radiation therapy for patients with T2-T3 carcinoma of the middle-to-lower rectum. Cancer 1999; 86: 398–404. [DOI] [PubMed] [Google Scholar]
- 43.Bosset JF, Magnin V, Maingon P, et al. Preoperative radiochemotherapy in rectal cancer: long-term results of a phase II trial. Int J Radiat Oncol Biol Phys 2000; 46: 323–327. [DOI] [PubMed] [Google Scholar]
- 44.Kaminsky-Forrett MC, Conroy T, Luporsi E, et al. Prognostic implications of downstaging following preoperative radiation therapy for operable T3-T4 rectal cancer. Int J Radiat Oncol Biol Phys 1998; 42: 935–941. [DOI] [PubMed] [Google Scholar]
- 45.Onatis M, Ludwig K, Mantyh C, et al. Complete response to neoadjuvant chemoradiation for rectal cancers does not influence survival. Cancer Symposium, Society of Surgical Oncology, 2001. [DOI] [PubMed]
- 46.Orrom WJ, Wong WD, Rothenberger DA, et al. Endorectal ultrasound in the preoperative staging of rectal tumors. A learning experience. Dis Colon Rectum 1990; 33: 654–659. [DOI] [PubMed] [Google Scholar]
- 47.Beynon J, Mortensen NJ, Foy DM, et al. Pre-operative assessment of local invasion in rectal cancer: digital examination, endoluminal sonography or computed tomography? Br J Surg 1986; 73: 1015–1017. [DOI] [PubMed] [Google Scholar]
- 48.Boyce GA, Sivak MV, Jr., Lavery IC, et al. Endoscopic ultrasound in the pre-operative staging of rectal carcinoma. Gastrointest Endosc 1992; 38: 468–471. [DOI] [PubMed] [Google Scholar]
- 49.Rau B, Hunerbein M, Barth C, et al. Accuracy of endorectal ultrasound after preoperative radiochemotherapy in locally advanced rectal cancer. Surg Endosc 1999; 13: 980–984. [DOI] [PubMed] [Google Scholar]
- 50.Enker WE, Merchant N, Cohen AM, et al. Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service. Ann Surg 1999; 230: 544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bannon JP, Marks GJ, Mohiuddin M, et al. Radical and local excisional methods of sphincter-sparing surgery after high-dose radiation for cancer of the distal 3 cm of the rectum. Ann Surg Oncol 1995; 2: 221–227. [DOI] [PubMed] [Google Scholar]


