Abstract
Objective
To determine the factors affecting the outcome of orthotopic liver transplantation (OLT) for end-stage liver disease caused by hepatitis C virus (HCV) and to identify models that predict patient and graft survival.
Summary Background Data
The national epidemic of HCV infection has become the leading cause of hepatic failure that requires OLT. Rapidly increasing demands for OLT and depleted donor organ pools mandate appropriate selection of patients and donors. Such selection should be guided by a better understanding of the factors that influence the outcome of OLT.
Methods
The authors conducted a retrospective review of 510 patients who underwent OLT for HCV during the past decade. Seven donor, 10 recipient, and 2 operative variables that may affect outcome were dichotomized at the median for univariate screening. Factors that achieved a probability value less than 0.2 or that were thought to be relevant were entered into a stepdown Cox proportional hazard regression model.
Results
Overall patient and graft survival rates at 1, 5, and 10 years were 84%, 68%, and 60% and 73%, 56%, and 49%, respectively. Overall median time to HCV recurrence was 34 months after transplantation. Neither HCV recurrence nor HCV-positive donor status significantly decreased patient and graft survival rates by Kaplan-Meier analysis. However, use of HCV-positive donors reduced the median time of recurrence to 22.9 months compared with 35.7 months after transplantation of HCV-negative livers. Stratification of patients into five subgroups, based on time of recurrence, revealed that early HCV recurrence was associated with significantly increased rates of patient death and graft loss. Donor, recipient, and operative variables that may affect OLT outcome were analyzed. On univariate analysis, recipient age, serum creatinine, donor length of hospital stay, donor female gender, United Network for Organ Sharing (UNOS) status of recipient, and presence of hepatocellular cancer affected the outcome of OLT. Elevation of pretransplant HCV RNA was associated with an increased risk of graft loss. Of 15 variables considered by multivariate Cox regression analysis, recipient age, UNOS status, donor gender, and log creatinine were simultaneous significant predictors for patient survival. Simultaneously significant factors for graft failure included log creatinine, log alanine transaminase, log aspartate transaminase, UNOS status, donor gender, and warm ischemia time. These variables were therefore entered into prognostic models for patient and graft survival.
Conclusion
The earlier the recurrence of HCV, the greater the impact on patient and graft survival. The use of HCV-positive donors may accelerate HCV recurrence, and they should be used judiciously. Patient survival at the time of transplantation is predicted by donor gender, UNOS status, serum creatinine, and recipient age. Graft survival is affected by donor gender, warm ischemia time, and pretransplant patient condition. The authors’ current survival prognostic models require further multicenter validation.
Hepatitis C is a major cause of chronic liver disease worldwide. Nearly 4 million Americans and 100 million worldwide are infected with the hepatitis C virus (HCV), 1 and HCV infection causes 20% of acute and 70% of chronic hepatitis. 2 Not surprisingly, end-stage liver disease caused by HCV has become the most common indication for orthotopic liver transplantation (OLT). 3,4
The availability of second-generation antibody testing for HCV and more recently the advent of polymerase chain reaction (PCR) amplification of viral RNA 5 have greatly assisted the accurate diagnosis of HCV infection. With the application of such molecular techniques, 6,7 it has become increasingly evident that HCV recurrence after OLT, as measured by PCR detection of HCV RNA, is nearly universal 8 and may lead to progressive allograft injury and failure. 9–12 Moreover, histologic evidence of HCV recurrence is apparent in approximately 50% of transplant recipients, with ensuing graft failure in 10% of patients by the fifth postoperative year.
Despite the risk of HCV recurrence, patients undergoing OLT for HCV have been reported to exhibit comparable overall patient and graft survival rates compared with other indications for liver transplantation. 4,9,13–16 In contrast, recurrence of hepatitis B virus infection (HBV) after OLT, in the absence of antiviral prophylaxis, is associated with decreased patient and graft survival rates. 17 Thus, it is predicted that large series of OLT for HCV with long follow-up times may show the adverse effects of HCV recurrence on patient and graft survival rates.
The current era of severe donor organ shortages and the rapidly increasing demands for liver transplantation underscore the need to optimize the outcome of liver transplantation. Currently, the organ allocation system, which is based on the severity of illness, favors organ distribution to urgent recipients. 18 However, the survival benefits of transplantation in critically ill recipients are poor compared with nonurgent patients. 4 In contrast, an organ allocation system that balances disease severity with expected outcomes would maximize patients’ benefits from transplantation. Such goals can be realized only with better understanding of the factors that influence patient and graft survival and the development of prognostic models that predict OLT outcomes.
This study retrospectively evaluated the long-term clinical outcome in a large cohort of patients who underwent transplantation for end-stage liver disease caused by HCV during a 10-year period. We examined the adverse effects of HCV recurrence on the outcome of OLT and investigated the risks associated with the use of HCV-positive donor livers. By analyzing the factors that may influence patient and graft survival after OLT, we developed a model that predicts patient and graft survival rates after liver transplantation for HCV.
METHODS
Patients
From January 1990 to December 2000, 510 adults underwent OLT for end-stage liver disease secondary to HCV infection; this represents approximately 25% of all transplants performed at our center during this period. Of these patients, 80 (15.7%) required retransplantation. Only first transplants were included in this study. Fifty-nine patients (12%) received livers from HCV-positive donors and 62 (12%) exhibited concomitant hepatocellular carcinoma (HCC). Median follow-up time was 30 (range 0–130) months. Histologic HCV recurrence occurred in 212 patients and 129 died during the follow-up period. Retrospective analysis of the patients’ records was performed.
Candidates for OLT were considered as urgent or nonurgent recipients according to their medical condition before transplantation, as defined by the United Network for Organ Sharing (UNOS) categories. From 1990 to 1994 urgent recipients included status 4 patients; nonurgent patients included status 3, 2, and 1. After November 1994, the status designation was modified by UNOS on two occasions. Urgent recipients included either status 1 or 2A and nonurgent recipients included status 2, 2B, 3, or 4, according to the designated UNOS criteria at the time of transplantation.
Diagnosis and Definitions
Hepatitis C was diagnosed before OLT by anti-HCV seropositivity by enzyme-linked immunosorbent assay (ELISA 2.0) and/or PCR for detection of HCV RNA. The preoperative diagnosis was confirmed by pathologic examination of the explanted livers after surgery. Recurrent HCV was diagnosed by biochemical graft dysfunction with the presence on liver biopsy of features consistent with recurrent HCV, including portal or lobular infiltration by mononuclear cells with piecemeal necrosis in the absence of any other specific causes. Patient death or retransplantation was defined as graft failure.
Immunosuppression
Maintenance immunosuppression regimens consisted of either a triple cyclosporine-based drug regimen that included Sandimmune or Neoral, azathioprine, and prednisone or dual tacrolimus-based immunosuppression that used tacrolimus and prednisone. In 1996, Neoral was routinely substituted for Sandimmune. Routine use of tacrolimus was initiated at our institution in 1994. On the day of transplantation, patients were started on a rapid steroid taper according to our standard protocol. One gram of Solu-Medrol (methylprednisolone) was administered intravenously for the first day and rapidly tapered to 20 mg/day over 1 week. Oral prednisone was started on day 8 (20 mg/day) and tapered over 2 months to 5 mg/day. From 1995, steroids were discontinued at 6 months in HCV patients who did not exhibit rejection episodes.
Statistical Analysis
Survival curves were computed using Kaplan-Meier methods and compared using log-rank tests. Medians were compared via the log-rank test or the Wilcoxon test when data were not censored. Proportions were compared using the chi-square test. The log-rank test for trend was used when comparing survival curves across ordered categories. For univariate screening purposes, continuous potential predictors of patient or graft survival such as (log) creatinine were dichotomized at their overall median to form two groups of roughly equal size.
All variables found to be univariately significant at P < .20 or those thought to be important on logical and/or biomedical grounds were entered into a backward stepdown Cox proportional hazard regression analysis using a liberal P < .10 criterion for interaction variable retention. Variables with many missing values were not included. All possible two-way interactions among variables were also first considered in this Cox backward stepdown procedure. Variables that were highly skewed were transformed to the log scale, where their distribution was more symmetric. The methods of May and Hosmer were used to compute overall goodness of fit chi-square measures for the final Cox models. All analyses were carried out using the SAS system (SAS Institute Inc., Cary, NC).
RESULTS
Overall Patient and Graft Survival Rates
Kaplan-Meier patient and graft survival estimates for the entire patient population included in the study period, from 1990 to 2000, are shown in Figure 1. Of 510 patients, 80 had graft failure and required retransplantation, and 129 patients died during the follow-up period. Median follow-up was 30 (range 0–130) months. Overall patient survival rates from the date of the first transplant at 1, 5, and 10 years were 84%, 68%, and 60%, respectively. Graft survival analysis that included all causes of graft failure and/or patient deaths showed graft survival rates of 73%, 56%, and 49% at 1, 5, and 10 years, respectively.
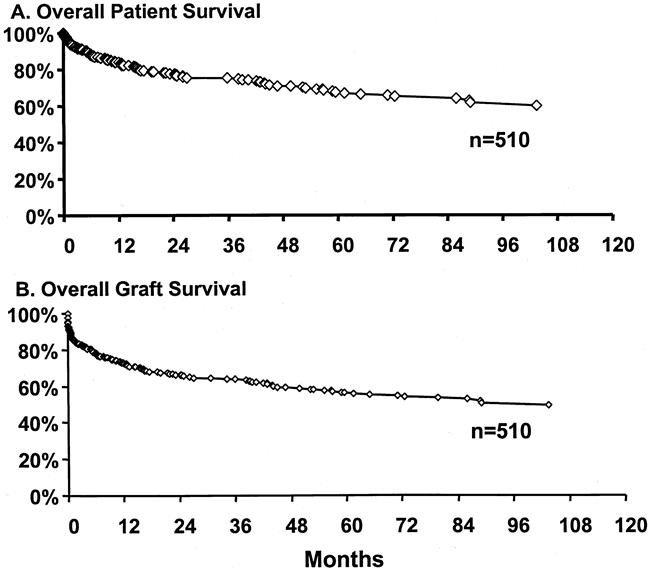
Figure 1. Kaplan-Meier patient and graft survival curves after liver transplantation for hepatitis C.
Effect of Hepatitis C Recurrence on Patient and Graft Survival
Recurrent HCV was diagnosed by graft biopsies after evidence of biochemical abnormalities during the follow-up period. Overall patient survival rates in the absence of recurrent HCV were 82%, 76%, and 68% at 1, 5, and 8 years, respectively (Fig. 2). Survival estimates of patients with HCV recurrence, with ignoring time to recurrence, were 94%, 71%, and 63% at 1, 5, and 8 years, respectively (P = NS). Similarly, recurrence of HCV did not seem to affect graft survival by Kaplan-Meier survival analysis. Overall graft survival rates without evidence of histologic recurrence were 76%, 68%, and 63% at 1, 5, and 8 years, respectively. This was not significantly different from graft survival rates with HCV recurrence: 81%, 59%, and 52% at 1, 5, and 8 years, respectively.
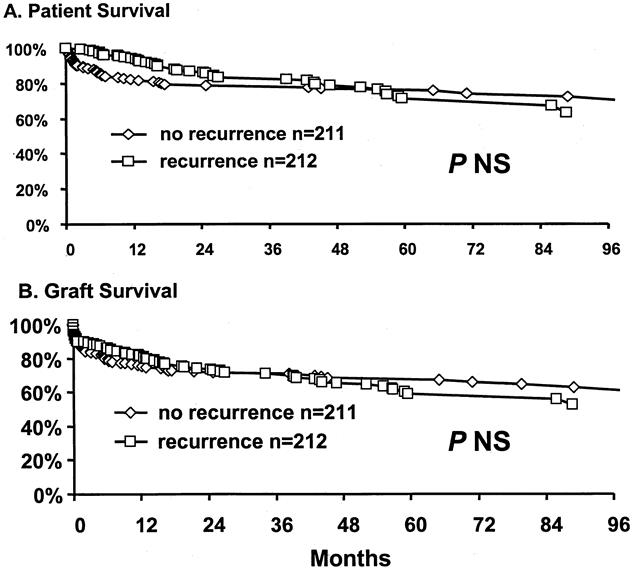
Figure 2. Outcome of liver transplantation after histologic recurrence of hepatitis C.
The effect of transplantation of HCV patients with HCV-positive donors on patient and graft survival was estimated by Kaplan-Meier survival analysis. Survival rates of patients who received a liver transplant from an HCV-positive donor were 85%, 70%, and 70% at 1, 5, and 8 years, respectively, and were not significantly different from survival rates of patents with HCV-negative donors (89%, 75%, and 66% at 1, 5, and 8 years, respectively;Fig. 3). HCV-positive donor status did not affect graft survival rates. Graft survival rates from HCV-positive donors were similar to those from HCV-negative donors (73%, 63%, and 47% vs. 79%, 64%, and 57% at 1, 5, and 8 years, respectively).
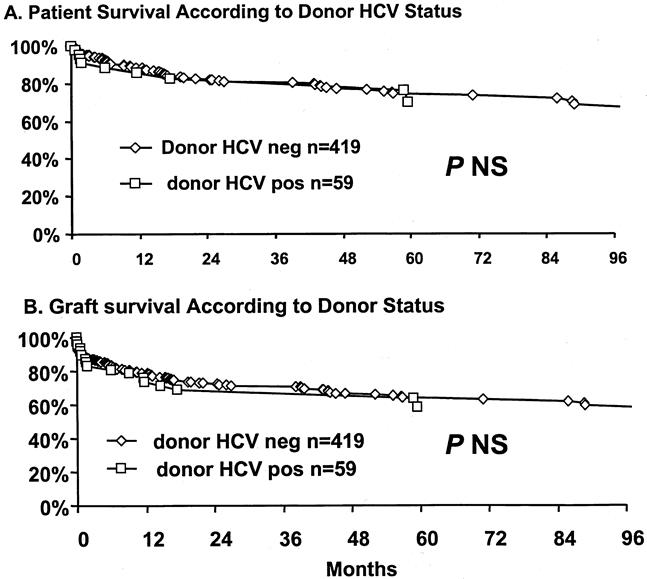
Figure 3. Overall patient and graft survival estimates based on hepatitis C (HCV) status of donor.
Effect of Hepatitis C Recurrence on Survival
Although HCV recurrence did not seem to affect overall patient or graft survival rates, there may be subgroups of patients who were adversely affected by recurrence. To identify such groups, analysis by time to recurrence was performed (Fig. 4). Estimation of overall recurrence-free survival indicates that at 1 year after transplantation, 74% of live patients were free of recurrence. However, only 43% and 32% of recipients were free of recurrence at 5 and 8 years, respectively. Thus, the overall estimated median time of recurrence was 34 months. However, in patients who received a liver transplant from an HCV-positive donor (n = 47), the median time to recurrence was shortened to 22.9 months compared with recipients with an HCV-negative donor, where the median time of recurrence was 35.7 months (P = .12).
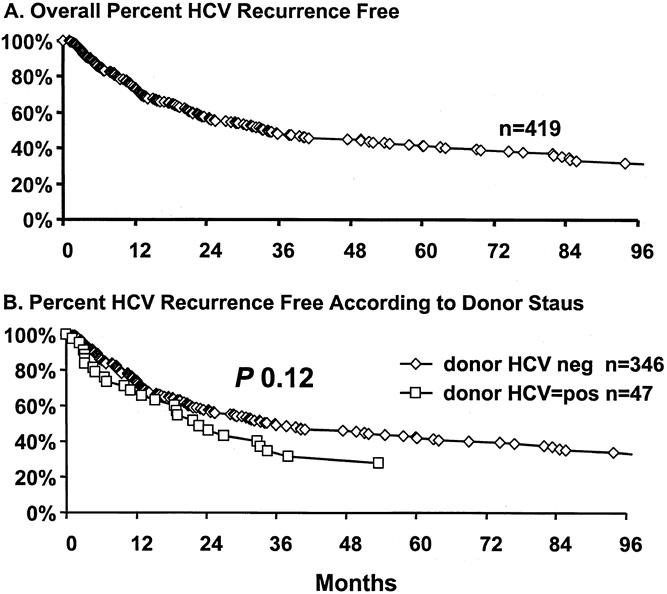
Figure 4. (A) Overall hepatitis C (HCV) recurrence-free patient survival estimates. (B) Recurrence-free patient survival estimates according to donor status; 26 patients with unknown time to recurrence and donor status were omitted.
To investigate the effect of early HCV recurrence on survival, patients were divided into five groups based on the presence or absence of histologic evidence of recurrence, as well as the time interval to recurrence. The groups included recipients who exhibited recurrence in the first 12 months (n = 95), between 12 and 24 months (n = 55), between 24 and 36 months (n = 27), or after 36 months after transplantation (n = 31). These groups were compared with 104 patients who did not exhibit histologic evidence of recurrence after 36 months of follow-up. As shown in Figure 5, the groups exhibited significantly different patient (P < .001) and graft (P < .001) survival estimates by Kaplan-Meier analysis. The worst patient and graft survival rates were seen in recipients who had recurrence within the first year after transplantation; the best survival rates were observed in patients who had recurrence only at 36 months, or who did not exhibit recurrence. The death risk ratio or graft failure risk ratio (Fig. 6) was significantly increased in patients with early recurrence. The death risk ratios were 12.4, 4.28, 5.55, and 1.76 in patients who had recurrence within the first 12, 12 to 24, 24 to 36, and more than 36 months, respectively, compared with patients who did not have recurrence after 36 months (death risk ratio = 1, P < .001). Analysis of the graft failure risk ratio, at similar time intervals to recurrence, showed significant increases to 12.59, 7.33, 5.55, and 3.53 (P < .001).
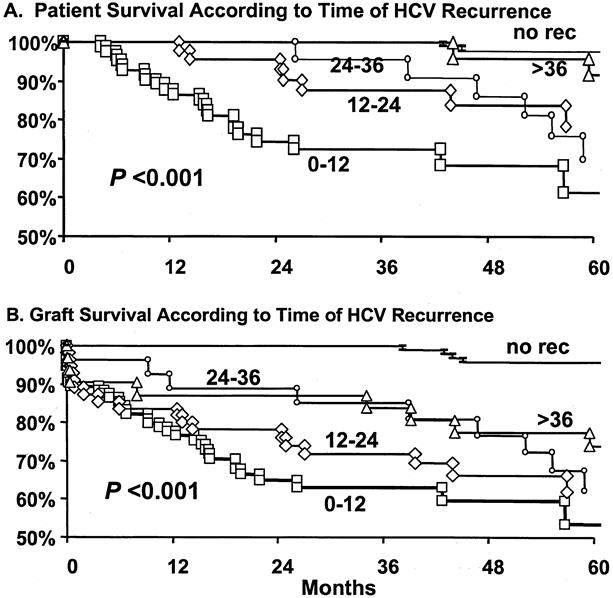
Figure 5. Patient and graft survival analysis based on time to hepatitis C (HCV) recurrence by histologic examination. Patients were classified into five subgroups: patients with recurrence within the first 12 months (▪), between 12 and 24 months (⋄), between 24 and 36 months (•), after 36 months (▴); or patients without evidence of histologic recurrence after 36 months (-).
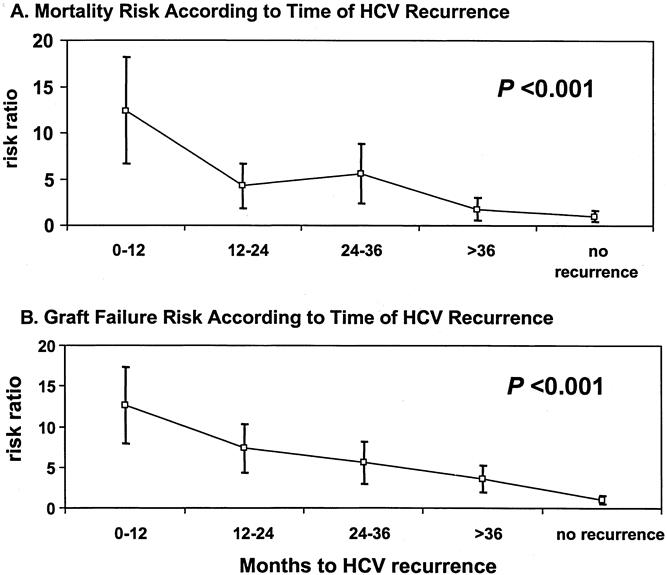
Figure 6. Risk ratios for death and graft failure based on time of histologic hepatitis C (HCV) recurrence.
Predictors of Patient and Graft Survival by Univariate Analysis
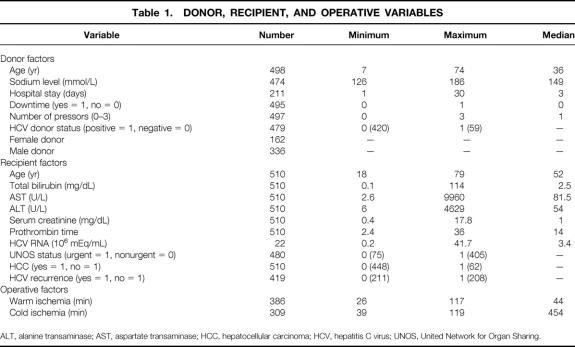
Preoperative recipient, donor, and operative variables were studied for their impact on survival outcomes. As shown in Table 1, the seven donor variables were age, sodium level, length of hospital stay before procurement, history of cardiac arrest, number of donor pressors, donor HCV status, and donor gender. The 10 recipient variables examined were age, UNOS status before transplantation, preoperative total bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), prothrombin time, serum creatinine, HCV RNA, presence or absence of HCC, and HCV recurrence. In addition, two operative variables—cold and warm ischemia times—were analyzed. These variables were dichotomized at their median value for univariate analysis.
Table 1. DONOR, RECIPIENT, AND OPERATIVE VARIABLES
ALT, alanine transaminase; AST, aspartate transaminase; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; UNOS, United Network for Organ Sharing.
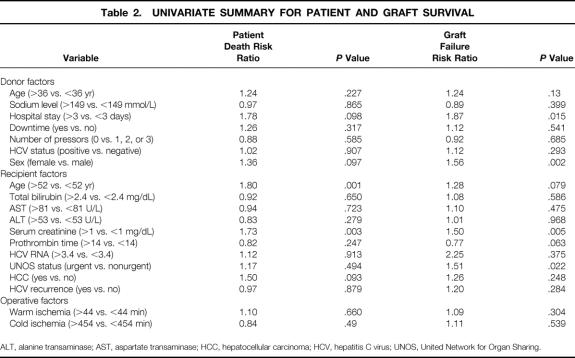
By univariate comparison, two variables were significantly associated with increased risk of patient death after transplantation (Table 2): recipient age older than 52 years (P = .001; relative risk [RR] 1.80) and preoperative serum creatinine more than 1 mg/dL (P = .003; RR 1.73). Donor length of hospital stay more than 3 days (P = .098; RR 1.78), donor female sex (P = .097; RR 1.36), and the presence of HCC (P = .093; RR 1.50) all approached statistical significance. Similarly, graft survival was affected by six variables. The four factors that exerted significant effects were donor length of hospital stay more than 3 days (P = .015; RR 1.87), donor female gender (P = .002; RR 1.56), pretransplant serum creatinine level more than 1 mg/dL (P = .005; RR 1.50), and UNOS status (urgent vs. nonurgent, P = .022; RR 1.51). Increased recipient age (older than 52 years), which approached statistical significance, appeared to reduce graft survival (P = .079; RR 1.28). Conversely, an increased pretransplant prothrombin time (>14) was protective to the graft (P = .063; RR 0.77).
Table 2. UNIVARIATE SUMMARY FOR PATIENT AND GRAFT SURVIVAL
ALT, alanine transaminase; AST, aspartate transaminase; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; UNOS, United Network for Organ Sharing.
Although many patients had pretransplant viral RNA studies to confirm HCV diagnosis, only 22 had HCV RNA estimation immediately before transplantation with similar assays of equivalent reference values to allow meaningful comparison. Nevertheless, an increase in pretransplant HCV RNA (>3.43 × 106 mEq/mL) was accompanied by a 2.25-fold increase in RR for graft failure despite the absence of a significant probability value.
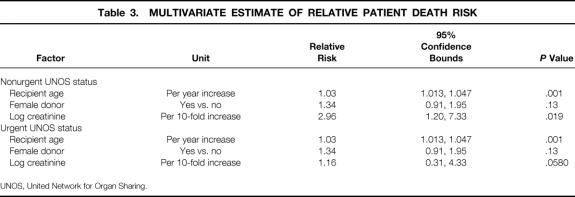
Multivariate Model for Patient Survival
Variables found to affect survival (P < .2) univariately, or those thought to be relevant, were analyzed by Cox multivariate regression analysis. Of the 15 factors considered for patient death, four were simultaneously significant: recipient age, log recipient creatinine, UNOS status (urgent vs. nonurgent), and donor gender. All factors were associated with an increased risk of death. Table 3 shows the RR (hazard) of death with the corresponding 95% confidence bounds for each factor. In this model, female donor status and recipient age imposed similar risks of deaths in urgent and nonurgent recipients. However, the RR of death caused by a log unit increase in creatinine was greater in nonurgent (RR 2.96) than urgent (RR 1.16) recipients. Nevertheless, if creatinine was held at its overall median value of 1.0 (log creatinine = 0), this model implies that the relative risk of death with an urgent UNOS status is 1.96 times that of nonurgent status (P = .013). The goodness of fit chi-square statistic for this mode was 16.6 (df = 9, P = .06).
Table 3. MULTIVARIATE ESTIMATE OF RELATIVE PATIENT DEATH RISK
UNOS, United Network for Organ Sharing.
According to this model, the estimated relative death risk = exp (death index), where the death index is given by: 0.0293 (recipient age) + 1.085 (log10 recipient cre-atinine) + 0.289 (donor female gender) + 0.675 urgent UNOS − 1.612 (log10 recipient creatinine times urgent UNOS). In the above equation the donor female gender and urgent UNOS status each are coded as 1. A nonurgent status or a male gender variable is assigned 0. For example, the estimated RR of death for a 50-year-old recipient who receives a female donor graft compared with that of an identical 40-year-old patient whose liver is from a male donor is given by: exp ( 0.0293 [50–40] + 0.289 [1–0]) = exp (0.592) = 1.78. Thus, the risk of death of the first recipient is approximately 1.8 times that of the second.
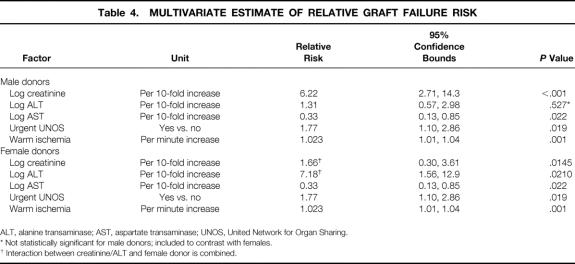
Multivariate Model for Graft Failure
Six simultaneous factors significantly affected graft survival: log recipient creatinine, log AST, log ALT, urgent UNOS status, female donor, and warm ischemia time (Table 4). According to this model, the association of AST (RR 0.33), UNOS status (RR 1.7), and warm ischemia (RR 1.023) with the risk of graft failure is not affected by donor gender. Although a given increase in log pretransplant creatinine is associated with an increased risk of graft failure regardless of the donor gender, a given increase in pretransplant serum creatinine is associated with a higher risk in male (RR 6.22) as opposed to female donors (RR 1.66). Conversely, an increase in ALT imposes a larger increase in graft failure risk with female (RR 7.18) than male (RR 1.31) donors. Holding creatinine and ALT at their median values of 1.0 and 54, respectively, a female donor increases the risk of graft failure by a factor of 1.6. The graft failure model achieved a goodness of fit chi-square statistic of 7.8 (df = 9, P = .56).
Table 4. MULTIVARIATE ESTIMATE OF RELATIVE GRAFT FAILURE RISK
ALT, alanine transaminase; AST, aspartate transaminase; UNOS, United Network for Organ Sharing.
* Not statistically significant for male donors; included to contrast with females.
† Interaction between creatinine/ALT and female donor is combined.
In accordance with this model, the estimated relative graft failure risk = exp (graft failure index). The estimated graft failure index, in patients who receive a male donor, is given by: 1.828 (log10 recipient creatinine) + 0.2668 (log10 recipient ALT) - 1.10 (log10 recipient AST) + 0.574 (urgent UNOS status) + 0.02287 (warm ischemia time). In contrast, the graft failure index for female donors is estimated by: 0.041 (log10 recipient creatinine) + 1.503 (log10 recipient ALT) - 1.10 (log10 recipient AST) + 0.574(urgent UNOS status) + 0.02287 (warm ischemia time - 1.554). Thus, the relative risk of graft failure for a patient with a pretransplant creatinine of 1.5 (log creatinine = 0.176) who receives a male donor, compared with an identical patient with a serum creatinine of 1.2 (log creatinine = 0.079) who had a female donor, is given by: exp ( 1.828 [0.176] - 0.041 [0.079] - 1.554) = exp (0.3217 + 1.551) = 6.50.
DISCUSSION
The first aspect of this study investigated the overall outcome of OLT for HCV. Transplantation of 510 patients at our institution showed overall patient survival rates of 84%, 68%, and 60% and overall graft survival rates of 73%, 56%, and 49% at 1, 5, and 10 years, respectively. These results confirm our previous report, which showed that survival outcomes after OLT for HCV were similar to other indications for liver transplantation. 4 Other investigators reported similar findings. 13–16 Only patients who underwent transplants for cholestatic liver diseases achieved better long-term survival. 16 Our current study showed no difference in overall survival estimates in patients with histologic evidence of HCV recurrence compared with those without evidence of recurrence. Thus, despite previous expectations that HCV recipients may experience poor outcomes with long-term follow-up, we and others have shown that OLT for HCV achieves good medium- and long-term patient and graft survival rates in large patient cohorts.
To analyze further the possible adverse effects of HCV recurrence after transplantation, we estimated the median time to histologic recurrence. Such estimation was based on graft biopsies obtained after evidence of biochemical graft dysfunction. The overall estimated median time to recurrence was 34 months. In contrast, other studies that used serial graft biopsies showed evidence of histologic recurrence within the first year after transplantation. 9 Because the time of histologic recurrence 9 or onset of increased viral load 19 may predict the long-term progression of allograft hepatitis, we analyzed the association between the onset of histologic recurrence and survival outcomes. Early recurrence, particularly that occurring within the first year after transplantation, was associated with significantly poor patient and graft survival rates. Survival estimates exhibited a stepwise improvement with delayed onset of recurrence. Patients with or without recurrence after 36 months of transplantation showed excellent patient and graft survival rates. In addition, risk ratios for death and graft failure were sharply elevated with early recurrence. Thus, early HCV recurrence is associated with rapid graft destruction and lower patient survival rates.
We are currently analyzing the group of patients who developed rapid HCV recurrence within the first 12 months after transplantation to detect risk factors that may predispose to early recurrence. 20–26 Some studies implicated elevated pretransplant HCV RNA levels 16 and infection with HCV genotype 1b 14 with poor outcome and increased rate of graft damage. However, other reports indicated no difference in HCV genotypes, basal immunosuppression, or incidence of rejection episodes between patients who ultimately develop graft failure and cirrhosis versus those with milder hepatitis. 9 Thus, it is increasingly apparent that although graft reinfection with HCV is almost universal by PCR analysis, graft loss and death from recurrent HCV is less common and affects a small group of patients. In one study that included 149 patients who underwent transplantation for HCV, graft loss occurred in 27 of 149 patients, but only 8 patients suffered graft loss secondary to HCV. 14 Recurrent HCV with ensuing graft failure, in a series of 166 HCV-infected transplant recipients, was the cause of death in 11 of 39 patients who died during the follow-up period. 16 Further, only a small subset of patients appear to develop severe allograft injury that prompts retranplantation. 27–29 Taken together, the above data may account, in part, for the good overall patient and graft survival rates despite HCV recurrence.
The current study addressed the effects of transplantation in HCV-infected patients with HCV-positive livers obtained from cadaveric donors. Based on previous reports that showed no adverse effects of HCV-positive livers, 30 we have used such livers to expand a severely depleted organ pool. However, the impact of viral superinfection on HCV-infected patients is largely unknown. 31 Our results show that patient and graft survival rates in recipients of HCV-positive donors was not significantly different from those of patients who received a liver transplant from an HCV-negative donor. However, HCV-infected patients who received HCV-positive livers exhibited a shorter median time to recurrence of 22.9 months versus 35.7 months in recipients of HCV-negative livers (P = .12). Although statistically borderline, such findings may indicate that HCV-positive livers may shorten the time to recurrence, thereby increasing the incidence of graft failure. Thus, the use of HCV-positive donors in urgent recipients should be advocated, whereas use in nonurgent recipients should be judiciously used until the issue is further analyzed.
Univariate analysis of recipient, donor, and operative factors identified two significant variables that reduced patient survival rates after OLT for HCV: age of the recipient (older than 52 years) and preoperative serum creatinine level (>1 mg/dL). Factors that significantly affected graft survival included donor length of hospital stay (>3 days), donor female gender, urgent UNOS status of the recipient, and preoperative serum creatinine level (>1 mg/dL). HCV recurrence did not affect either patient or graft survival. Such an effect was not surprising given our previous findings that HCV recurrence did not affect the overall outcome of OLT for HCV. Surprisingly, an increase in pretransplant prothrombin time afforded protection against graft loss (P = .063, RR 0.77). There were only 22 patients with pretransplant RNA levels that were appropriate for statistical analysis. However, our data, similar to previous findings, 16 showed that increased pretransplant RNA levels were univariately associated with a marked increase of the risk ratio for graft failure. The absence of a significant probability value was almost certainly due to the small sample size. Variables not considered in the current study that may affect patient and graft survival included ABO compatibility, 32 recipient gender, and split liver transplantation. We have previously reported that in vivo split liver transplantation did not affect patient or graft survival. 33 Also, we rarely used transplantation of mismatched organs to allow meaningful analysis. By multivariate analysis, factors that simultaneously predicted patient survival were UNOS status, donor female gender, recipient age, and serum creatinine level. Risk of graft failure was predicted by donor gender, pretransplant creatinine, AST, ALT, UNOS status, and warm ischemia time.
The variables identified in our study to affect patient and graft survival in HCV patients, particularly urgent UNOS status and pretransplant serum creatinine level, have been shown to influence the outcome of OLT in multiple studies. Cuervas-Mons et al 34 found that preoperative serum creatinine level predicted survival in 79% of patients. Similarly, pretransplant creatinine level was shown to be related to early postoperative sepsis and hospital death. 35,36 Preoperative recipient physiology, assessed by the UNOS scoring system, is not only a primary determinant for survival and cost, but also a predictor of complications after liver transplantation. 37–39 Recipient age, number of transplants, and UNOS status were identified as independent risk factors for patient death in a study that evaluated 250 patients who underwent retransplantation at UCLA. 40 The deleterious effect of prolonged warm ischemia on the outcome of transplantation is well established. 39 Similarly, a prolonged donor hospital stay has been clearly shown to reduce graft and patient survival rates after OLT. 39,41,42 It therefore appears that factors affecting patient survival after OLT for HCV are similar to those previously identified for whole-organ transplantation.
An important aspect of this paper is the development of a prognostic model for patient and graft survival that can be applied at the time of transplantation. We have developed a single model for patient survival and two models for graft survival. These models contain variables that are readily obtainable from the medical records and are easy to use by transplant personnel. The patient survival model is based on recipient age, creatinine level, UNOS status, and donor gender. The graft survival models in addition use recipient ALT, AST, and warm ischemia time. Ricci et al 43 have developed a prognostic model for patients with cholestatic liver disease to predict complications after OLT. Interestingly, the prognostic indicators for patients with cholestatic liver disease are similar to those detected in our study for HCV patients. Such indicators included recipient age, serum creatinine level, UNOS status, and Child class. Similarity to our model may indicate the feasibility of developing prognostic models that accurately predict OLT outcomes regardless of the etiology of end-stage liver disease. The development and application of such prognostic models may affect the current organ distribution system, which is based solely on disease severity regardless of the outcome. 18 However, additional validation, as with any prognostic model, is needed to establish this methodology.
DISCUSSION
Dr. Charles M. Miller (New York, New York): I would like to congratulate you and your team at UCLA for this superb paper. Thanks for allowing me to review your manuscript in advance. As you point out, HCV is epidemic. It is one of the largest hurdles in liver transplantation and the research and discovery directed at preventing HCV recurrence after transplant is now a cottage industry. But progress has been very slow.
Unlike the revolutionary impact of hepatitis B immunoglobulin prophylaxis for patients with hepatitis B in terms of their disease recurrence and overall survival, there has been no outstanding magic bullet that has evolved out of research for hepatitis C. So we are really left trying to do better for our patients without that single magic bullet. I have the following questions:
Somewhat contradictory to what I might have expected, when you stratify your entire cohort with respect to recurrence versus no recurrence, you don’t find any differences with respect to overall patient graft survival at five years. However, as you pointed out very nicely, when you further analyze the sub-group with respect to time to recurrence, recurrence at less than a year, does have a significant impact on patient graft survival?
My question is, to fully assess the impact of the recurrence of the entire cohort, don’t you need to analyze patient graft survival at 8 to 10 years? And have you done that?
Patricia Shelver from our group has published findings that suggest that the use of prophylactic interferon delays the onset of histological occurrence, especially in patients with low to moderate viral load. It is certainly not the magic bullet that I described, but have you used any kind of prophylaxis in this cohort of patients and/or have you used any kind of treatment once you have a recurrence?
Finally, I am intrigued by your predictive model. Actually I like it a lot. It suggests that we might want to select, as you pointed out, male donors to transplant non-urgent patients with normal renal function and with minimal ischemia. That is pretty hard to do in a cadaveric situation. Do you think that the living donor will give us the flexibility to accomplish this?
And finally I have a concern, and I wonder if you would share it? If you transplant less and less urgent patients don’t we open up the possibility of premature transplantation? Isn’t that quite dangerous for patients with Hep C?
Presenter Dr. Rafik M. Ghobrial (Los Angeles, California): Dr. Miller, thank you very much. Those are all excellent questions and they bring up very important points in transplantation for hepatitis C.
With regards to your first question, as you pointed out, our previous study, as well as, the current study, demonstrate that overall patient and graft survival after transplantation for hepatitis C is equivalent to other indications for transplantation. However, if we look at different patient groups, as defined by the time of recurrence, we see a drop in patient and graft survival in those who recurred early. The question is how we reconcile these two seemingly opposing facts?
First, I believe that there is a small group of patients out of the entire population that is adversely affected by recurrence, and this group is only about 10 to 20% of the series. Those patients, who did poorly, when they are entered into the entire population, they do not significantly affect the overall survival analysis of the whole population. Second, the timing to recurrence appears to be very important. Overall survival analysis does not take the time of recurrence into account. The effect of time to recurrence can only be seen if you compare groups of patients based on the onset of recurrence.
With regard to your second question concerning the prophylaxis or treatment of hepatitis C. We have not used prophylaxis because we cannot tell who are the patients who will need it — and from our studies here we show it is only a small group of patients that require it. The price that patients pay for interferon therapy is quite high, in term of morbidity, so we have not employed prophylactic therapy.
As for treatment, we have had about 45 patients that were treated at UCLA. We did not have consistent treatment protocols to evaluate it statistically. I think that question should be answered by a prospective study, and I believe that some studies are underway right now.
The last question is about the prognostic model in patient and graft survival at the time of transplantation. We are very excited at UCLA about this model, because we all know that there is a time where patients are too sick to be transplanted. However, there are no established criteria that guide our decision in saying that a patient is too sick, and that a transplant will not be beneficial. So I think the prognostic model would allow us to make this decision accurately.
I do agree with you that the living donor is the ideal transplant. But as you had mentioned, how early should we transplant hepatitis C patients and how long should we wait? I think the conventional wisdom would indicate once the patient has been accepted as a candidate for liver transplantation, and there is a living donor, we should go ahead with the transplant. Delaying the transplant because there is a living donor may not be beneficial because the recipient may suffer a fatal complication at any time while waiting.
Dr. Abraham Shaked (Philadelphia, Pennsylvania): There were two messages that I got from this manuscript. One is to the patient and transplant physician demonstrating that survival after transplantation for chronic active hepatitis C is not too bad. The results are good, despite recurrence of the disease. The second message is that we need to identify specific sub-groups, as mentioned before by you, that can be treated immediately after transplantation with some kind of prophylaxis aiming to prevent recurrence of the disease and to prolong survival. With this in mind, I have a few questions to you.
What we are discussing here, although in your model are predictive for hepatitis C, are really variables that are predictive of outcome after transplantation in any given patient suffering from end stage liver disease. So how do you compare it to other non-hepatitis C patients? I think that the proper thing to do is maybe to take a cohort of PBC to compare to this cohort and see whether the variables are different and whether it does affect the proposed formula that calculate risk of recurrence and death.
The second issue is, that although hepatitis recurrence did not affect the ultimate survival, the recurrence does effect the quality of life. These patients don’t live so good. Do you think that quality of life by itself is an indication for some prophylaxis therapy?
The third question is related to an interesting dichotomy presented in your findings. You have indicated that recurrence in those who get livers were infected with hepatitis C experience earlier recurrence, yet in this sub-group the survival is not affected. However, in those receiving “clean” livers, recurrence is associated with poor long-term results and higher mortality. So how can you explain this dichotomy between the infected or non-infected liver?
And the last question is a question that I struggle with in my own practice. What do you do for those with severe recurrent disease? Do you retransplant them? And if you do, what are the results?
Dr. Rafik M. Ghobrial: Dr. Shaked, thank you very much. You raise very important questions.
The first question pertains to the model, did that model have other indications of liver transplantation? And as you just pointed out, this model does not have in it the effects of recurrent disease. So I think at this moment it can be applicable to other indication of liver transplantation. And I believe that this is only the beginning, and we are looking at the predictability of this model with other indications. There has been a paper that came out from Mayo that has a model that predicts the complications after transplantation. And the factors that are in that paper are very similar to the ones that we have here.
Your second question relates to the quality of life. The quality of life for patients after recurrence is very poor and some of them do suffer a lot especially from side effects of medications. So I believe that treatment for hepatitis C is a very important question. But I think when you initiate the treatment, you initiate it in relation to histological findings. These are questions that we have to answer by prospective study.
The third question relates to the hepatitis C positive donors. We have used hepatitis C positive donors. In our study there were about 45 patients that received hepatitis C positive donors. And as time goes on there would be more hepatitis C positive donors because of the continuing increases in the population. Our data suggests that they reduce the time to recurrence and that early recurrence may have reduced patient graft survival. So what we advocate now is that these livers should be used for urgent recipients. We also advocate utilization by patient-to-patient analysis at the time of the transplant for non-urgent recipients.
The fourth question is what we do with hepatitis C patients who have recurred. We have studied those patients in our center, and what we have shown is that if you retransplant these patients earlier in the course of the recurrence, they do very well. But if we wait until these patients with recurrence deteriorate and become urgent recipients and are transplanted when they are on life support in the intensive care, their survival with liver transplantation drops significantly to about 50%, whereas if they are transplanted early the survival is about 85%. So we do advocate early retransplantation for patients with hepatitis C.
Dr. Haile T. Debas (San Francisco, California): The absolute increased incidence in hepatocellular carcinoma in this country has been ascribed to hepatitis C. Are you concerned that the immunosuppressed patient who has hepatitis C after transplantation may be more susceptible to carcinoma? Is there any evidence?
Dr. Rafik M. Ghobrial: We looked at the sub-populations of the patients that had hepatocellular carcinoma. There are some factors that can predict early recurrence.
Immunosuppression was not one. The size and stage of the tumor, as well as the presence of intravascular invasion, were the factors that predicted early recurrence of hepatocellular carcinoma after transplantation.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association, April 26–28, 2001, the Broadmoor Hotel, Colorado Springs, Colorado.
Supported in part by the Dumont Foundation, the Torino Foundation, and the Joanne Barr Foundation.
Correspondence: Ronald W. Busuttil, MD, PhD, Dumont-UCLA Transplant Center, UCLA School of Medicine, 10833 LeConte Ave., 77-132 CHS, Los Angeles, CA 90095.
E-mail: rbusultil@mednet.ucla.edu.
Accepted for publication April 26, 2001.
References
- 1.Alter MJ. Epidemiology of hepatitis C. Hepatology 1997; 26 (suppl 1): 62S–65. [DOI] [PubMed] [Google Scholar]
- 2.Murphy EL, Bryzman S, Williams AE, et al. Demographic determinants of hepatitis C virus seroprevalence among blood donors. JAMA 1996; 275: 995–1000. [PubMed] [Google Scholar]
- 3.Wright TL. Liver transplantation in patients with chronic hepatitis B and C. In: Maddrey WC, Sorrell MF, eds. Transplantation of the liver, 2d ed. Norwalk, CT: Appleton & Lange; 1995: 477.
- 4.Ghobrial RM, Farmer DG, Baquerizo A, et al. Orthotopic liver transplantation for hepatitis C: outcome, effect of immunosuppression and causes of retransplantation during an eight-year single center experience. Ann Surg 1999; 6: 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gretch DR. Diagnostic tests for hepatitis C. Hepatology 1997; 26: 43S. [DOI] [PubMed] [Google Scholar]
- 6.Feray C, Samuel D, Thiers V, et al. Reinfection of liver grafts by hepatitis C virus after liver transplantation. J Clin Invest 1992; 89: 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chazouilleres O, Kim M, Combs C, et al. Quantitation of hepatitis C virus RNA in liver transplant recipients. Gastroenterology 1994; 106: 994–999. [DOI] [PubMed] [Google Scholar]
- 8.Wright TL, Donegan E, Hsu HH, et al. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology 1992; 103: 317–322. [DOI] [PubMed] [Google Scholar]
- 9.Rosen H, Gretch DR, Oehlke M. Timing and severity of initial hepatitis C recurrence as predictors of long-term liver allograft injury. Transplantation 1998; 65: 1178–1182. [DOI] [PubMed] [Google Scholar]
- 10.Bizollon T, Ducerf C, Trepo C. Hepatitis C virus recurrence after liver transplantation. Gut 1999; 44: 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samuel D, Feray C. Recurrent hepatitis C after liver transplantation: clinical and therapeutic issues. J Viral Hepatitis 2000; 7: 87–92. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira R, Pastacaldi S, Papatheodoridis GV, et al. Recurrent hepatitis C after liver transplantation. J Med Vir 2000; 61: 443–454. [DOI] [PubMed] [Google Scholar]
- 13.Boker KHW, Dalley G, Bahr MJ, et al. Long-term outcome of hepatitis C virus infection after liver transplantation. Hepatology 1997; 25: 203–210. [DOI] [PubMed] [Google Scholar]
- 14.Gane EJ, Portmann BC, Naoumov NV, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med 1996; 334: 815–820. [DOI] [PubMed] [Google Scholar]
- 15.Casavilla FA, Rakela J, Kapur S, et al. Clinical outcome of patients infected with hepatitis C virus infection on survival after primary liver transplantation under Tacrolimus. Liver Transplant Surg 1998; 4: 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlton M, Seaberg E, Wiesner R, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology 1998; 28: 823–830. [DOI] [PubMed] [Google Scholar]
- 17.Todo S, Demetris AJ, Van Thiel D, et al. Orthotopic liver transplantation for hepatitis B virus-related liver disease. Hepatology 1991; 13: 619–626. [PMC free article] [PubMed] [Google Scholar]
- 18.Federal Register (FR Doc 98–8191) 42 CFR Part 121, April 2:16296, 1998.
- 19.Feray C, Gigou M, Samuel D, et al. The course of hepatitis C virus infection after liver transplantation. Hepatology 1994; 20: 1137–1143. [DOI] [PubMed] [Google Scholar]
- 20.Rosen HR, Shackleton CR, Higa L, et al. Use of OKT3 is associated with early and severe recurrence of hepatitis C after liver transplantation. Am J Gastroenterol 1997; 92: 1453–1457. [PubMed] [Google Scholar]
- 21.Singh N, Gayowski T, Ndimbie OK, et al. Recurrent hepatitis C virus in liver transplant recipients receiving tacrolimus: association with rejection and increased immunosuppression after transplantation. Surgery 1996; 119: 452–456. [DOI] [PubMed] [Google Scholar]
- 22.Sheiner PA, Schwartz ME, Mor E, et al. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology 1995; 21: 30–34. [PubMed] [Google Scholar]
- 23.Herrero JI, De La Pena A, Quiroga J, et al. Risk factors for recurrence of hepatitis C after liver transplantation. Liver Transplant Surg 1998; 4: 265–270. [DOI] [PubMed] [Google Scholar]
- 24.Freeman RB, Tran S, Lee YM, et al. Serum hepatitis C RNA titer after liver transplantation are not correlated with immunosuppression or hepatitis. Transplantation 1996; 61: 542–546. [DOI] [PubMed] [Google Scholar]
- 25.Gane EJ, Naoumov NV, Qian KP, et al. A longitudinal analysis of hepatitis C virus replication folowing liver transplantation. Gastroenterology 1996; 110: 167–177. [DOI] [PubMed] [Google Scholar]
- 26.Schluger LK, Sheiner PA, Thung SN, et al. Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology 1996; 23: 971–976. [DOI] [PubMed] [Google Scholar]
- 27.Rosen HR, O’Reilly PM, Shackleton CR, et al. Graft loss following liver transplantation in patients with chronic hepatitis C. Transplantation 1996; 62: 1773–1776. [DOI] [PubMed] [Google Scholar]
- 28.Ghobrial RM, Colquhoun S, Rosen H, et al. Retransplantation for recurrent hepatitis C following tacrolimus or cyclosporine immunosuppression. Transplant Proc 1998; 30: 1470–1471. [DOI] [PubMed] [Google Scholar]
- 29.Sheiner PA, Schluger LK, Emre S, et al. Retransplantation for hepatitis C. Liver Transplant Surg 1997; 3: 130–136. [DOI] [PubMed] [Google Scholar]
- 30.Mulligan DC, Goldstein RM, Crippin JS, et al. Use of anti-hepatitis C virus seropositive organs in liver transplantation. Transplant Proc 1995, 27: 1204–1205. [PubMed] [Google Scholar]
- 31.Fishman JA, Rubin RH, Koziel MJ, et al. Hepatitis C virus and organ transplantation. Transplantation 1996; 62: 147–154. [DOI] [PubMed] [Google Scholar]
- 32.Figueras J, Busquets J, Grande L, et al. The deletrious effect of donor high plasma sodium and extended preservation in liver transplantation. A multivariate analysis. Transplantation 1996; 61: 410–413. [DOI] [PubMed] [Google Scholar]
- 33.Ghobrial RM, Yersiz H, Farmer DG, et al. Predictors of survival after in vivo split liver transplantation: analysis of 110 consecutive cases. Ann Surg 2000; 232: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuervas-Mons V, Millan J, Gavaler JS, et al. Prognostic value of preoperatively obtained clinical and laboratory data in predicting survival following orthotopic liver transplantation. Hepatology 1986; 6: 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baliga P, Merion RM, Turcotte JG, et al. Preoperative risk factor assessment in liver transplantation. Surgery 1992; 112: 704–710. [PubMed] [Google Scholar]
- 36.Doyle HR, Morelli F, McMichael J, et al. Hepatic retransplantation—an analysis of risk factors associated with outcome. Transplantation 1996; 61: 1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spanier TB, Klein RD, Nasrawy SA, et al. Multiple organ failure after liver transplantation. Crit Care Med 1995; 23: 466–473. [DOI] [PubMed] [Google Scholar]
- 38.Wong T, Devlin J, Roland N, et al. Clinical characteristics affecting the outcome of liver retransplantation. Transplantation 1997; 64: 878–882. [DOI] [PubMed] [Google Scholar]
- 39.Strasberg SM, Howard TK, Molmenti EP, et al. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology 1994; 20: 829–838. [DOI] [PubMed] [Google Scholar]
- 40.Markmann JF, Markowitz JS, Yersiz H, et al. Long-term survival after retransplantation of the liver. Ann Surg 1997; 226: 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mor E, Klintmalm GB, Gonwa TA, et al. The use of marginal donors for liver transplantation. Transplant Proc 1992; 53: 383–386. [DOI] [PubMed] [Google Scholar]
- 42.Ploeg RJ, D’Alessandro AM, Knechtle SJ, et al. Risk factors for primary dysfunction after liver transplantation: a multivariate analysis. Transplantation 1993; 55: 807–813. [DOI] [PubMed] [Google Scholar]
- 43.Ricci P, Therneau TM, Malinchoc M, et al. A prognostic model for the outcome of liver transplantation in patients with cholestatic liver disease. Hepatology 1997; 25: 672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]