Abstract
Objective
To assess the long-term efficacy of intestinal transplantation under tacrolimus-based immunosuppression and the therapeutic benefit of newly developed adjunct immunosuppressants and management strategies.
Summary Background Data
With the advent of tacrolimus in 1990, transplantation of the intestine began to emerge as therapy for intestinal failure. However, a high risk of rejection, with the consequent need for acute and chronic high-dose immunosuppression, has inhibited its widespread application.
Methods
During an 11-year period, divided into two segments by a 1-year moratorium in 1994, 155 patients received 165 intestinal allografts under immunosuppression based on tacrolimus and prednisone: 65 intestine alone, 75 liver and intestine, and 25 multivisceral. For the transplantations since the moratorium (n = 99), an adjunct immunosuppressant (cyclophos-phamide or daclizumab) was used for 74 transplantations, adjunct donor bone marrow was given in 39, and the intestine of 11 allografts was irradiated with a single dose of 750 cGy.
Results
The actuarial survival rate for the total population was 75% at 1 year, 54% at 5 years, and 42% at 10 years. Recipients of liver plus intestine had the best long-term prognosis and the lowest risk of graft loss from rejection (P = .001). Since 1994, survival rates have improved. Techniques for early detection of Epstein-Barr and cytomegaloviral infections, bone marrow augmentation, the adjunct use of the interleukin-2 antagonist daclizumab, and most recently allograft irradiation may have contributed to the better results.
Conclusion
The survival rates after intestinal transplantation have cumulatively improved during the past decade. With the management strategies currently under evaluation, intestinal transplant procedures have the potential to become the standard of care for patients with end-stage intestinal failure.
It has been only 14 years since the first scattered examples were recorded of extended survival in humans of nutrition-supporting intestinal allografts transplanted under cyclosporine-based immunosuppression. 1–6 Then, with the advent of tacrolimus in 1989 to 1990, 7,8 clinical intestinal transplantation began to emerge as a practical means of treating intestinal failure. 9–15 Multiple factors have sustained and increased these efforts, including technical innovations in surgery and improvements in nonspecific postoperative care.
The most important therapeutic achievement, however, has been the increasingly efficient prevention and/or control of rejection, exemplified by our experience reported here with 155 recipients of 165 intestinal, liver-intestinal, or multivisceral abdominal allografts whose transplantations were performed between May 2, 1990, and February 18, 2001. The emphasis in this analysis will be placed on the long-term outlook of patients who were still alive at the time of previous reports, and on attempts to improve the prognosis with new adjunct immunosuppressants or management strategies that have yielded promising results in laboratory models.
METHODS
Recipients
Sixty-two of the 155 recipients were treated between May 2, 1990, and early 1994. At the end of this time, we discontinued the program for nearly 1 year because of the seemingly fixed high death rate and excessive complication rates. In the analysis presented to the American Surgical Association in 1995, 12 six statistically significant risk factors were identified, of which three were immunosuppression-specific: excessively high blood levels of tacrolimus, the use of large intravenous boluses of prednisone, and the frequent administration of the monoclonal antilymphoid globulin OKT3. The other three risk factors were the duration of the intestinal transplant operations, inclusion in the allograft of segments of donor colon in continuity with the small bowel, and a cytomegalovirus (CMV) carrier state of the organ donor when the intestinal allografts were transplanted to CMV-negative recipients. When the moratorium was lifted and the program reopened, reforms designed to lessen the impact of these risks were instituted. To compare the results before and after the moratorium, the demographic features of the recipient populations in the two eras were determined and were found to be similar (Table 1).
Table 1. CLINICAL FEATURES
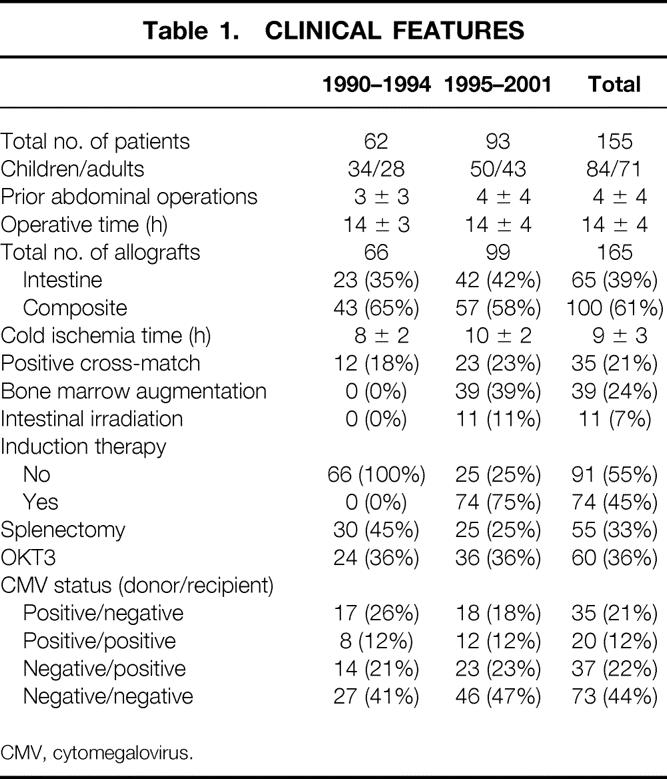
CMV, cytomegalovirus.
Allografts
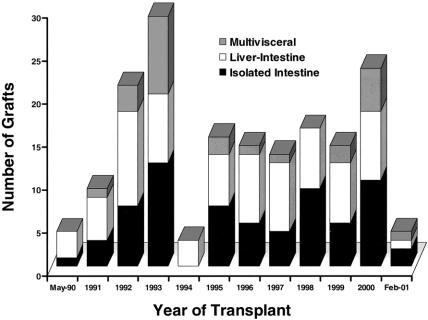
One hundred fifty-five primary transplantations and 10 retransplantations of the three different kinds of allografts shown in Figure 1 were carried out during the 11-year period. Eighty-four children with a mean age of 4.9 ± 4.8 years received 89 of the allografts, 73% of which consisted of liver plus intestine or of multiple abdominal viscera rather than intestine only. Interestingly, three of the children who received primary intestinal allografts were previous hepatic allograft recipients who had developed intestinal failure 4 to 10 years after liver transplantation because of midgut volvulus (n = 2) or the hollow visceral myopathy/neuropathy syndrome (n = 1). The 71 adult recipients of 76 allografts were 38 ± 11 years old. In contrast to the 27% incidence of pediatric recipients of intestine-only allografts, 54% of the transplantations in adult patients were of this variety.
Figure 1. The yearly number and kinds of intestinal transplant procedures performed since 1990. Note the 1994 moratorium.
Biochemical or histologic evidence of hepatic dysfunction was present in many of our 62 intestine-alone recipients. However, our policy throughout, in both adults and children, was to transplant only the intestine if damage to the liver was thought to be reversible. Retention of the damaged but salvageable native liver was not an exclusive feature of the intestine-alone recipient. In 5 of the 25 multivisceral transplantations, the native liver was not excised. Instead, the donor liver was removed from the superior end of the multivisceral allografts and used for other patients. This modification of conventional multivisceral transplantation has the advantage of permitting all of the hollow intraabdominal viscera to be transplanted in continuity and vascularized from a single vascular pedicle (Figs. 2, 3).
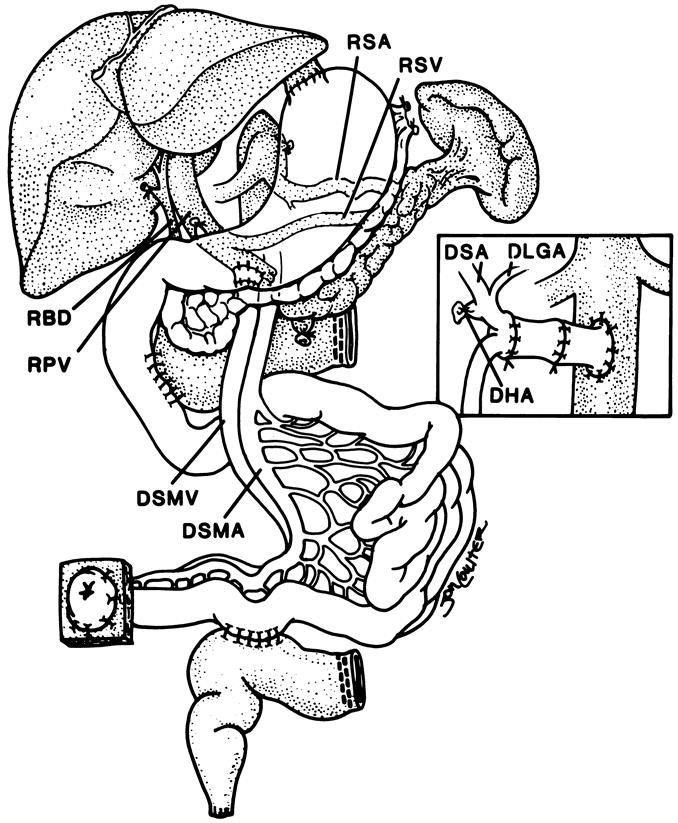
Figure 2. Transplantation of a modified multivisceral graft (unshaded organs) containing the pancreas and all of the hollow intraabdominal viscera in continuity from the esophagogastric junction to the terminal ileum. The native liver, spleen, pancreas, and a C loop of duodenum have been retained. The procedure was used to treat a patient with pseudoobstruction. Biliary drainage from the native liver as well as from both pancreases was accomplished with a side-to-side host-to-graft duodenal anastomosis. The insert shows preservation of the donor splenic (DSA) and left gastric (DLGA) arteries (with Carrel patch) with ligation of the donor hepatic artery (DHA) stump. Note that an interposition arterial graft was initially anastomosed to the recipient infrarenal aorta and before allograft implantation. RSA, recipient splenic artery; RSV, recipient splenic vein; RBD, recipient bile duct; RPV, recipient portal vein; DSMV, donor superior mesenteric vein; DSMA, donor superior mesenteric artery.
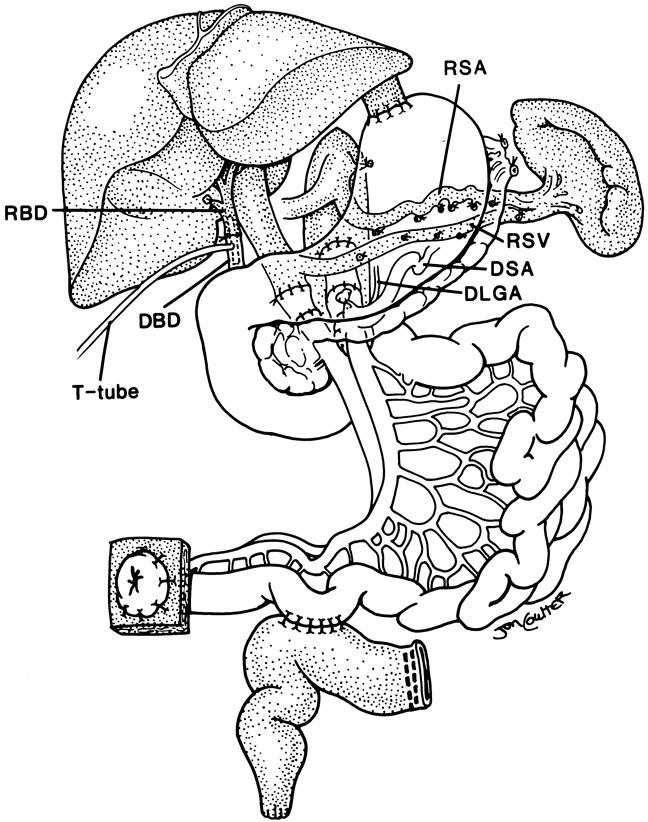
Figure 3. The use of a modified multivisceral graft (stomach, duodenum, pancreas, and small bowel) after abdominal visceral exenteration with preservation of the host liver and spleen (shaded organs). The portosplenic circulation is maintained intact during graft insertion and the preserved spleen protects the patient from the risk of posttransplant lymphoproliferative disease. This modified multivisceral transplantation has been used to treat recipients with massive gastrointestinal polyposis and extensive Crohn’s disease. Note the duct-to-duct biliary reconstruction. RSA, recipient splenic artery; RSV, recipient splenic vein; RBD, recipient bile duct; DSA, donor splenic artery; DLGA, donor left gastric artery; DBD, donor bile duct.
The 10 retransplantations in the 155 recipients were performed with isolated intestine (n = 3), liver plus intestine (n = 4), and multiple abdominal viscera (n = 3). The three intestine-alone retransplantations were performed 3, 16, and 330 days after graft enterectomy for chronic rejection. Two of the four liver-intestinal allografts were used to rescue primary intestine-only recipients, 6 and 660 days after graft enterectomy, and the other two were used to replace acutely failing primary liver-intestinal allografts. The three multivisceral grafts were retransplanted to recipients whose failing primary intestinal, liver-intestinal, and multivisceral transplants had been transplanted 94, 455, and 11 days previously.
The short gut syndrome was the cause of intestinal failure in 82% of the cases. Loss of the intestine in adults was due most commonly to thrombotic disorders, Crohn’s disease, and trauma, and in children to volvulus, gastroschisis, necrotizing enterocolitis, and intestinal atresia. Most of the thrombotic disorders were due to protein C, S, and antithrombin III deficiency, factor V/II mutation, and development of lupus anticoagulant or anticardiolipin antibodies. Indications other than the absence of bowel included dysmotility syndromes (9%), intestinal neoplasm (6%), and enterocyte failure (3%).
The most common reason for the use of composite (i.e., intestine-liver or multivisceral) allografts was liver failure induced by total parenteral nutrition (TPN; mean bilirubin 19 mg/dL). Consequently, the list of underlying etiologies of intestinal failure largely overlapped the causes of liver failure. With hepatic failure, most of the candidates for composite visceral transplantation were classified as IIA (intensive care unit-bound) or IIB in the United Network for Organ Sharing (UNOS) candidacy listing.
Donors
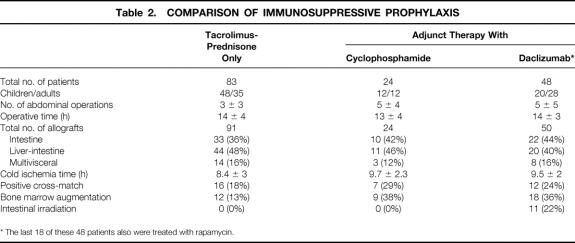
With the exception of an O-blood-type liver-intestine transplanted to an A-blood-type recipient under urgent circumstances, the cadaveric donor and recipient types were identical. Human leukocyte antigen (HLA) matching was random and uniformly poor, with no examples of zero -A, -B, -DR mismatches. The T-cell lymphocytotoxic cross-match was positive after dithiothreitol (DTT) treatment in 35 (21%) of the patients; 12 were compiled before the moratorium of 1994 (see Table 1). Of the subsequent 23 cross-match-positive recipients, 19 were treated at the time of surgery with adjunct cyclophosphamide (n = 7) or daclizumab (n = 12) in addition to the tacrolimus and prednisone therapy used throughout (Table 2).
Table 2. COMPARISON OF IMMUNOSUPPRESSIVE PROPHYLAXIS
* The last 18 of these 48 patients also were treated with rapamycin.
Because of the adverse effect of positive donor CMV serology on outcome reported in 1995, 12 an attempt was made to avoid the use of CMV-positive donors for CMV-negative intestine-alone or modified multivisceral recipients. This policy was considered impractical for patients whose need for liver-intestinal or full multivisceral grafts was generally too urgent to tolerate delays.
Donor Procedures
Details of the procurement technique for the three different types of intestinal allografts have been described before, 6,16 including the technique used for 16 of the 65 intestine-only allografts in which the liver, pancreas, and intestine from the same donor were transplanted to three different recipients. 17 To reduce postoperative graft dysmotility, 18 the enteric and celiac ganglia were preserved in the last 49 composite grafts (since February 1996). In another modification designed to avoid the potential risks of biliary reconstruction and to reduce the operating time, the duodenum was retained in continuity with the graft jejunum and biliary system in 29 liver-intestinal grafts;19,20 in 6 of these, the donor pancreas was left intact. In four intensive care unit-bound children, a left lateral segment (n = 1) or a reduced-size liver (n = 3) in combination with donor intestine were used after an in situ or ex vivo split 20,21 to overcome a mismatch between donor and recipient size.
The allografts were infused in situ with University of Wisconsin (UW) solution and immersed in UW solution for storage. Cold ischemia times ranged from 2.8 to 17 (mean 9 ± 3) hours and were significantly (P = .0001) shorter before 1994 (7.7 ± 2.1 hours) than after the moratorium (9.8 ± 2.3 hours) (see Table 1). Grafts transplanted under tacrolimus and prednisone immunosuppression only (i.e., without adjunct induction therapy) had a significantly (P = .004) shorter ischemia time than those transplanted to patients given perioperative cyclophosphamide or daclizumab (see Table 2). There was no significant (P = .9) difference between ischemia times in the adjunct cyclophosphamide versus the daclizumab subsets.
Recipient Operations
The principles 6 and details of the three intestinal transplant procedures 9–11,22–25 have been reported elsewhere. In all, an effort is made to reconstruct the vascular inflow, venous outflow, and exocrine drainage of the individual organs as normally as possible. However, compromises have been necessary. For example, although venous effluent from both the intestine and pancreas has hepatotrophic qualities and ideally should have first passage through the sinusoidal bed of the liver, 26 this was achievable with only 41 (63%) of the 65 small bowel allografts. In the other 37%, it was necessary to divert the nutrient-rich blood to the host vena cava.
Similarly, the insulin-rich effluent from the native pancreas was diverted around the transplanted liver in 60 (80%) of the liver-intestine recipients. As a temporary expedient in all patients, portacaval shunt between the host portal vein and inferior vena cava was routinely performed to avoid the acute portal hypertension caused by splanchnic venous cross-clamping during insertion of the liver-intestinal allografts. Although these shunts were systematically taken down in our initial experience to selectively deliver the insulin-rich blood to the transplanted liver, the shunts have been left in place since 1995 in preference to accepting the risk associated with their surgical disconnection and anastomosing the host portal vein to the side of the allograft portal vein.
Such problems have been minimized in recent modifications of the multivisceral procedure that have found a special niche in the treatment of patients in whom the native liver and possibly the pancreas and spleen can be retained with the host. After the full multivisceral graft is procured, the donor liver is removed for separate use in another recipient. Two versions of this modified operation are shown in Figures 2 and 3. The procedure has been used for the indications described in the captions.
Immunosuppression
All recipients were treated primarily with tacrolimus and steroids (Table 3). Prostaglandin E1 was infused intravenously during the first postoperative week to all but the first eight recipients. A 4-week course of 2 to 3 mg/kg/day cyclophosphamide (Cytoxan) was added to the treatment of 24 recipients of primary allografts between May 3, 1995, and November 2, 1997, and replaced for the following 2 to 3 months by mycophenolate mofetil (15–30 mg/kg/day) or azathioprine (50–100 mg/day).
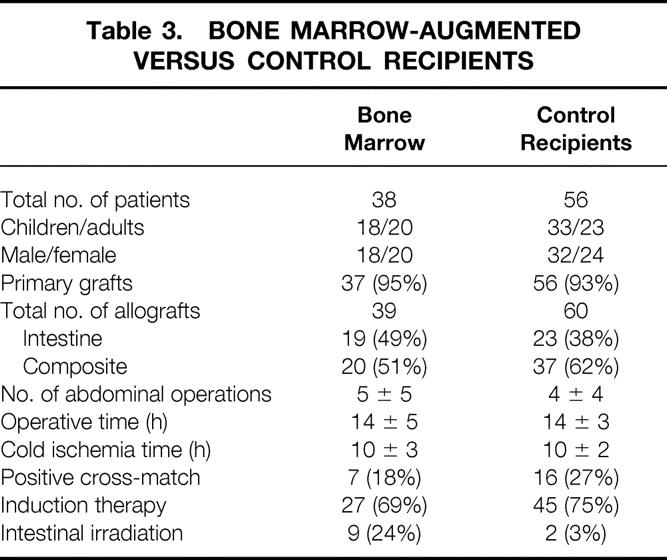
Table 3. BONE MARROW-AUGMENTED VERSUS CONTROL RECIPIENTS
Daclizumab (Zenapax), a humanized IgG1 monoclonal antibody directed at the α subunit of the human interleukin-2 receptor, was used as the third agent between May 31, 1998, and February 18, 2001, for recipients of 48 primary allografts and 2 retransplants. Five intravenous doses of 1 to 2 mg/kg were given. The first was given a few hours before surgery; the remaining four doses were given after 2, 4, 6, and 8 weeks, except in three patients in whom the drug was discontinued after two doses because of unexplained abdominal/gastrointestinal bleeding and hematuria. In addition to adjunct daclizumab, rapamycin (Rapamune) was used as a fourth drug for the first 3 to 6 months in the last 18 patients, beginning in adults with 5 mg/day for 2 weeks and 3 mg/day thereafter, and in children with 1 to 2 mg/m2/day for 2 weeks and appropriate downward adjustments thereafter.
Rejection episodes were treated with a steroid bolus and a 5-day dose taper, with adjustment of the daily tacrolimus dose to achieve higher trough levels. OKT3 or thymoglobulin was used throughout to treat steroid-resistant and severe rejection episodes. The effect of the adjunct drugs (cyclophosphamide, daclizumab) on the doses of the baseline agents (i.e., tacrolimus and prednisone) for the first year is shown in Figure 4. Patients treated with daclizumab tended to have lower doses of both tacrolimus and prednisone, an effect that may have reflected the coadministration of sirolimus in 18 of these 48 patients.
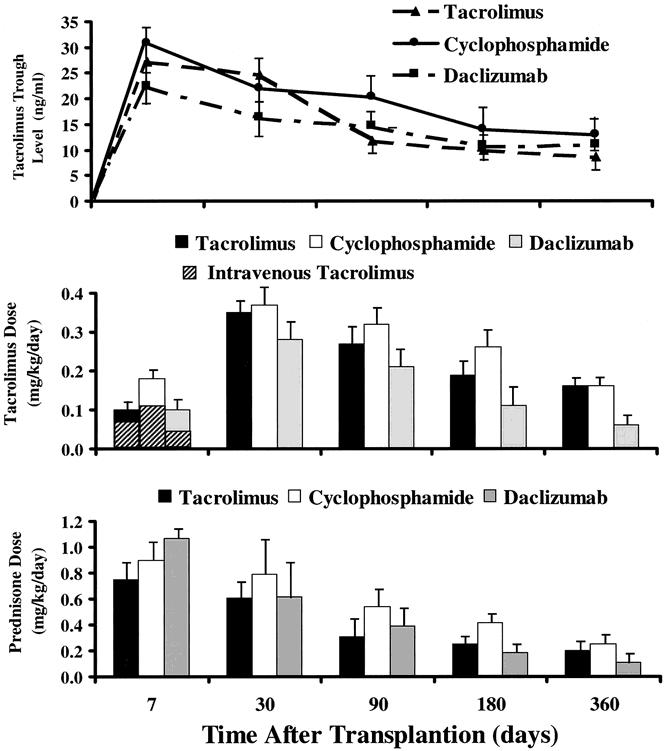
Figure 4. Tacrolimus whole blood 12-hour trough levels and tacrolimus and prednisone doses in recipients who received tacrolimus and prednisone only and those who received an adjunct induction therapy with either cyclophosphamide or daclizumab.
Bone Marrow Augmentation
With informed consent from both donor families and recipients, bone marrow cells were recovered from the thoracolumbar vertebrae of the intestinal donors and infused intravenously into the organ recipients at the time of surgery. 27–29 A single infusion of 3 to 5 × 108 donor cells/kg was given over 20 minutes within the first 12 hours after revascularization of 19 isolated intestine grafts and 20 composite grafts (see Table 3). The bone marrow cells were not available for 60 contemporaneous intestinal recipients who were considered to be prospective contemporaneous controls. Except for two patients in the bone marrow group and four controls, the bowel transplantations were primary. The clinical features of both cohorts are summarized in Table 3.
Ex Vivo Graft Irradiation
On April 20, 2000, a clinical trial of low-dose ex vivo intestinal allograft irradiation was initiated in adults. The intestine of 11 allografts (6 intestine only, 1 liver-intestine, and 4 multivisceral) given to 10 primary recipients was irradiated on the back table with a single dose of 750 cGy. Donor bone marrow cells were given to nine of these recipients (see Table 3).
Nutritional Management and Infection Prophylaxis
As described elsewhere, the achievement of full nutritional autonomy has required flexible and complex metabolic and surgical management strategies, particularly for recipients of multivisceral grafts. 9,11,30,31 Antimicrobial prophylaxis and active treatment of viral, bacterial, and fungal infections also are critical. 11,19,32 Since 1994, the newly developed technique of semiquantitative polymerase chain reaction (PCR) assay of Epstein-Barr virus (EBV) in the peripheral blood has allowed early detection, treatment, and monitoring of EBV infestation. 33 Similarly, the PP65 antigenemia test that has been used to early detect CMV reactivation or de novo infection has improved the preemptive treatment of this virus. As an adjunct to ganciclovir, CMV-specific hyperimmune globulin (Cytogam) has been added to the early postoperative antiviral prophylaxis for high-risk patients. 19,34
Immunologic Monitoring
Acute rejection of the intestinal allograft was diagnosed by histopathologic studies of endoscopically guided multiple mucosal biopsies, usually of the ileum, that were obtained when indicated by clinical judgment rather than by protocol. Chronic rejection was diagnosed on the basis of histologic examination of full-thickness enteric specimens. The criteria for the diagnoses of acute and chronic rejection have been standardized by Demetris et al. 35
To assess for graft-versus-host disease (GVHD), biopsy samples of recipient skin and native gastrointestinal tract were examined histopathologically and with immunocytochemical techniques that allowed the identification of donor leukocytes with the in situ hybridization technique using the Y-chromosome-specific probe or the immunohistologic staining of donor-specific HLA antigens. Other GVHD target organs also were closely observed and biopsy samples were taken when indicated.
Statistical Analysis
All analyses were performed using SPSS (SPSS Inc., Chicago, IL) for Windows software. Data were prospectively collected for the total population and updated to February 28, 2001. All variables were pooled at first and then stratified according to year of transplant, immunosuppressive regimen, bone marrow augmentation, and type of allograft. Data analyses in the patients treated with adjunct bone marrow were compared with the results obtained in contemporaneous controls. Continuous variables were reported as mean ± standard deviation and categorical data as proportions. Differences in group means were tested using the standard two-sample t test and one-way analysis of variance. Differences in proportions were tested by the Fisher exact test and chi-square analysis. The five multivisceral grafts that did not contain a liver were excluded from any comparative analysis between isolated intestine and composite grafts.
The mean values for the tacrolimus and steroid doses as well as the measured 12-hour tacrolimus whole blood trough levels were collected for each patient at 7 days, 1 month, 3 months, 6 months, 12 months, and every year after. The values were pooled for each cohort and the means were calculated and compared at each study point.
Patient and graft survival curves were generated using the Kaplan-Meier methods, 36,37 and group comparisons were done using the log-rank test. 38 The isolated intestinal recipients who were discharged from the hospital after total graft enterectomy were censored at the time of their graft removal (n = 5). The cumulative risks of graft loss from rejection, posttransplant lymphoproliferative disease (PTLD), and CMV were estimated using the Kaplan-Meier method.
RESULTS
Survival
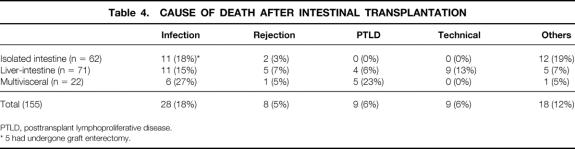
The Kaplan-Meier actuarial survival rate for the total population was 75% at 1 year, 54% at 5 years, and 42% at 10 years (Fig. 5). Most of the deaths occurred within the first 3 postoperative years, from causes summarized in Table 4, and 30 recipients (9 isolated intestine, 15 liver-intestine, 6 multivisceral) survived beyond the 5-year milestone. Eighty-three (53.6%) of the 155 recipients are currently alive, 7 of whom have returned to TPN and are waiting for either isolated intestinal (n = 6) or liver-intestinal transplants (n = 1). The remaining 76 (49%) current survivors (34 adults, 42 children) still bear functioning grafts after a mean follow-up of 43 ± 40 months (range 0.3–129). Twenty of these (4 intestine alone, 13 liver-intestine, and 3 multivisceral) have survived for more than 5 years with functioning allografts. A liver-intestine recipient at 129 months and a multivisceral recipient at 114 months have the longest surviving functional grafts of these kinds in the world. Recipients of liver-intestinal allografts had the best prognosis for continued survival beyond 5 years (Fig. 6).
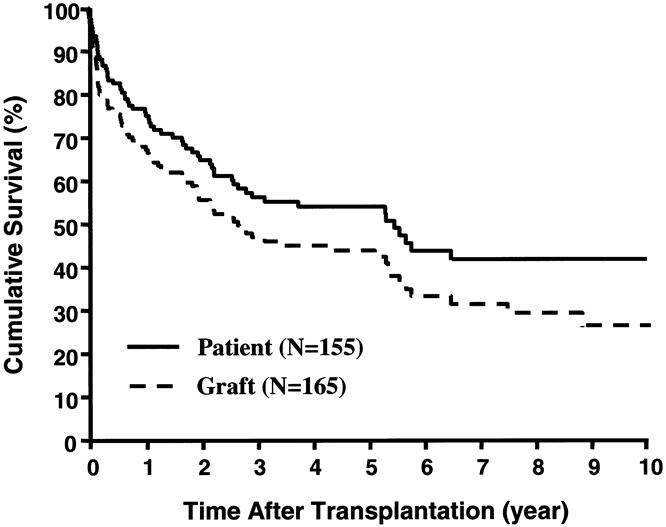
Figure 5. Kaplan-Meier patient and graft survival curves for the total population.
Table 4. CAUSE OF DEATH AFTER INTESTINAL TRANSPLANTATION
PTLD, posttransplant lymphoproliferative disease.
* 5 had undergone graft enterectomy.
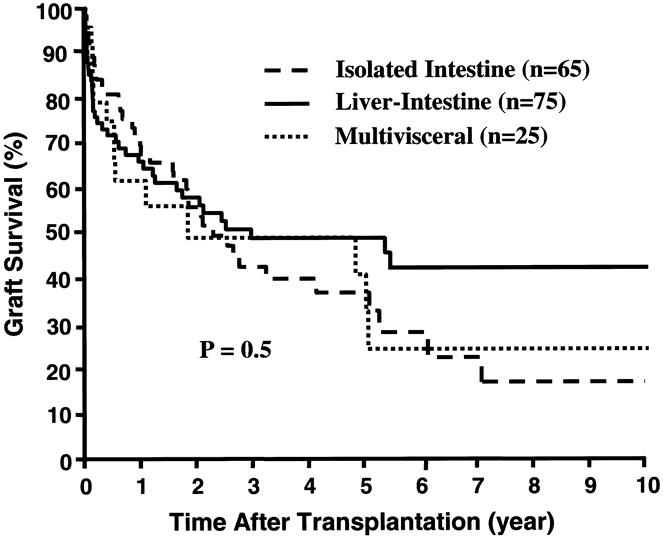
Figure 6. Kaplan-Meier survival curves of the three different types of intestinal grafts.
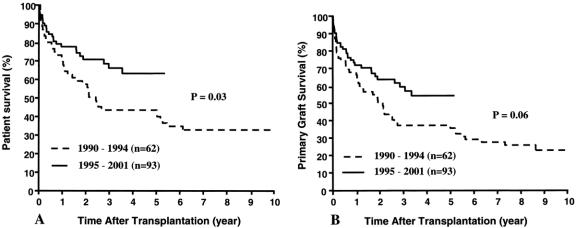
Patient as well as graft survival rates improved since 1994 compared with the premoratorium experience (Fig. 7). The 1-year actuarial patient survival rates in the early cohort of 62 recipients were 71% at 1 year and 43% at 5 years, versus 78% and 63% in the 93 recipients of the post-1994 period (P = .03). Because of the complexity of the cases and of the treatment strategies, the reasons for the improvement must be considered indeterminate. In addition to the core regimen of tacrolimus and prednisone, all but 21 of the 93 patients in the postmoratorium group were treated with adjunct immunosuppressants: cyclophosphamide (n = 24), daclizumab (n = 48), or rapamycin (n = 18). Moreover, 38 recipients of 39 intestinal allografts were infused with donor bone marrow cells. Finally, 6 of the intestine-alone allografts as well as the intestinal component of 4 multivisceral and 1 liver-intestinal grafts were subjected to ex vivo irradiation, and 9 of those 11 allografts belonged to the group of 39 infused with donor bone marrow cells (see Table 3).
Figure 7. Patient (A) and graft (B) survival rates before and after the 1994 moratorium.
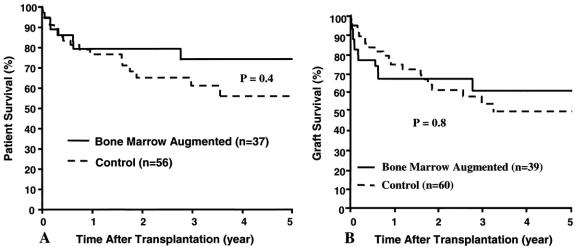
Although therapeutic efficacy cannot be definitively claimed for any of these modifications of conventional tacrolimus and prednisone immunosuppression, several observations are noteworthy. First, the 1-year actuarial survival rate in the 48 patients treated with daclizumab was 86% (P = .3) with a graft survival of 82% (P = .2). Second, the 1- and 5-year recipient survival rates in the bone marrow-augmented group were 79% and 74%, with a graft survival rate of 68% and 62% (Fig. 8), respectively. Finally, all 10 recipients of 11 irradiated allografts are well as of Feb. 28, 2001. The lost irradiated graft was because of poor donor selection.
Figure 8. Patient (A) and graft (B) survival rates for the bone marrow-augmented and control groups.
Rejection
The incidence of rejection during the first 30 postoperative days was significantly (P = .02) higher among patients who were transplanted during 1990 to 1994 (85%) than in those transplanted during 1995 to 2001 (67%). The use of daclizumab appeared to be the principal factor in reducing the incidence of early posttransplant rejection during the second era despite lower tacrolimus trough levels (Fig. 9).
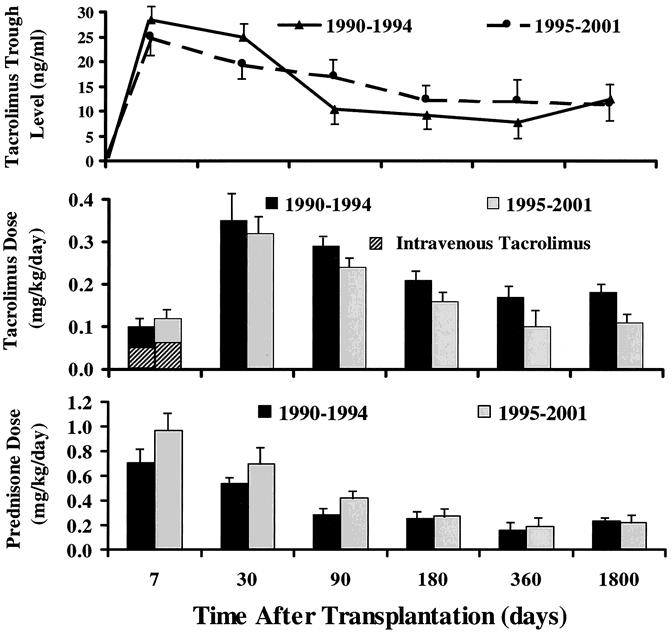
Figure 9. Tacrolimus whole blood 12-hour trough levels and tacrolimus and prednisone doses in the patients who were transplanted before and after the 1994 moratorium.
Refractory rejection was the primary cause of failure of 32 (19%) of the 165 grafts: 24 intestine only, 6 liver-intestine, and 2 multivisceral for an overall incidence of 37%, 8%, and 8% for the three kinds of grafts, respectively. The cumulative risk of loss from acute and chronic rejection of the 65 intestine-only allografts was significantly greater than that of the 95 composite visceral grafts that contained liver (P = .00001) (Fig. 10). Moreover, the risk of intestine-only graft loss from rejection was not significantly reduced in the second phase of our experience compared with the first (data not shown), confirming an ominous observation that we first reported in 1998. 19
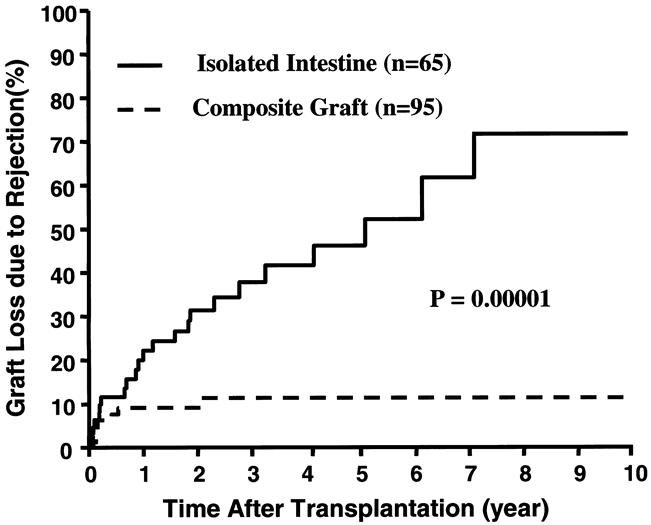
Figure 10. Cumulative risk of graft loss from rejection in the intestine-only and composite visceral grafts that contained liver.
Chronic rejection was diagnosed in 18 grafts (15 intestine alone, 3 liver-intestine) from 69 to 2,877 days after transplantation (median 236). The diagnosis was made in 15 instances by histopathologic examination of enterectomy specimens and in the 16th by examination of an autopsy liver-intestine specimen. In the remaining two recipients of liver-intestinal grafts, the diagnosis was made on the basis of obliterated submucosal blood vessels in endoscopic mucosal biopsy specimens.
The cumulative risk of chronic rejection was significantly (P = .00001) greater among the isolated intestinal grafts compared with the composite grafts that contained liver, with 1- and 5-year rates of 12% and 31% versus 1.5% and 7%, respectively. With bone marrow augmentation, chronic rejection was diagnosed in only one (2.7%) primary intestine-only graft that was removed 1,951 days after transplantation. In contrast, six (11%) of the control grafts (four isolated intestine, two liver-intestine) developed chronic rejection at 87 to 1,785 days from the date of primary transplantation. Of these six recipients, four underwent graft enterectomy, one died with graft in place, and the remaining one is currently alive with mild graft dysfunction.
Graft-Versus-Host Disease
Skin changes suggestive of GVHD were clinically observed in 13 (8.4%) of the patients but histologically documented in only 7 (4.5%). Two of the seven received intestine-only grafts and the other five received composite visceral grafts. GVHD was lethal in only one previously reported liver-intestinal recipient who had preexisting IgA deficiency. 39 Another patient, an adult multivisceral recipient, developed chronic GVHD of his tongue and buccal mucosa and eventually died of disseminated PTLD. 19
The disease was self-limited in the other five patients, three of whom had received adjunct donor bone marrow and developed mild to moderate rash 30, 188, and 312 days after liver-intestinal transplantation. The latter three patients, as well as another isolated intestinal recipient, responded to an increase in daily steroid and tacrolimus doses. The fifth recipient experienced transient GVHD 6 days after graft enterectomy and withdrawal of immunosuppression because of the development of PTLD.
Graft Enterectomy and Retransplantation
Twenty-three (35%) of the 65 intestine-only allografts were removed from 20 recipients (12 adults, 8 children) 663 ± 831 days after transplantation (range 9 days to 102 months). Irreversible acute (n = 10) and chronic rejection (n = 8) was the indication for 18 (78%) of the 23 graftectomies. In the remaining five patients, the grafts were removed after discontinuance of immunosuppression because of PTLD (n = 2), neuronal demyelinization (n = 1), refractory CMV retinitis (n = 1), and extensive bilobar Pseudomonas pneumonia (n = 1). Retransplantation was performed in 6 of the 20 patients 3 to 667 days (median 39) after their graft enterectomy. The retransplantations were with intestine alone (n = 3), liver-intestine (n = 2), and a multivisceral graft (n = 1). One of the liver-intestine recipients is well after 428 days. One of the intestine-alone recipients had graft function for 1,176 days before chronic rejection necessitated return to TPN; after another 687 days, he awaits a third transplantation. The other four retransplant recipients lost their secondary allografts within a few days to 5 months, and only one survived to return to TPN. Of the other 17 patients whose intestine-only grafts were excised without retransplantation, 4 died of sepsis soon after their enterectomies, 5 died later of TPN-related complications, and 8 are alive and receiving TPN 5 to 82 months after graft removal.
Two of the 71 primary liver-intestine recipients had their failing grafts replaced at 30 and 59 days; a third received a multivisceral graft 455 days after the primary liver-intestine procedure. The first of these patients is well 45 months after retransplantation, but the second and third died after 45 and 60 days from intractable acute rejection and PTLD, respectively.
One of the 22 primary multivisceral recipients underwent full replacement of a poorly functioning primary graft after 11 days and is currently alive with a fully functioning graft 161 days later.
Virus-Associated Syndromes
In the 1995 analysis of the first 71 intestinal recipients of this series, the high death rates associated with EBV-associated B-cell lymphomas (also known as PTLD) and with disseminated CMV infections loomed as singularly important impediments to further progress in the intestinal transplantation field. With the techniques of early detection of CMV and EBV, management of these infections has been markedly facilitated.
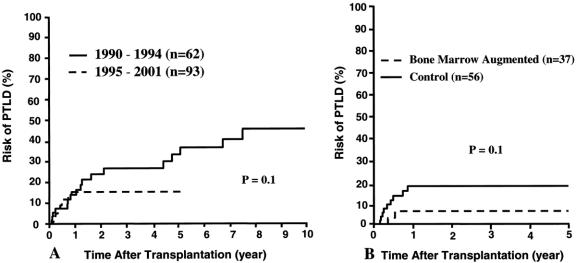
Thirty (19%) of the 155 patients developed PTLD, 18 before and 12 after the 1994 moratorium. Although the cumulative 5-year rate of PTLD was 15% in the second era (half of the earlier 33% rate;Fig. 11 A), the disease fatality was only 8% versus the earlier 44%. Young age (P = .001), type of intestinal graft (multivisceral) (P = .08), and recipient splenectomy (P = .006) continued to be significant risk factors for development of PTLD, as previously reported. 19 Contrary to predictions, 40 the 7% 1-year cumulative risk of PTLD was lower in the patients given adjunct donor bone marrow than the 20% in the control patients (Fig. 11 B). None of the patients who received irradiated intestinal allografts has developed PTLD.
Figure 11. Cumulative risk of posttransplant lymphoproliferative disease (PTLD) according to era (A) and bone marrow augmentation (B).
Similarly, the early diagnosis of CMV infection and prompt preemptive therapy has significantly (P = .05) reduced the risk of CMV disease (Fig. 12). Even with the nearly unrestricted use of CMV-positive donors (see Table 1), there have been no CMV-associated deaths since 1994.
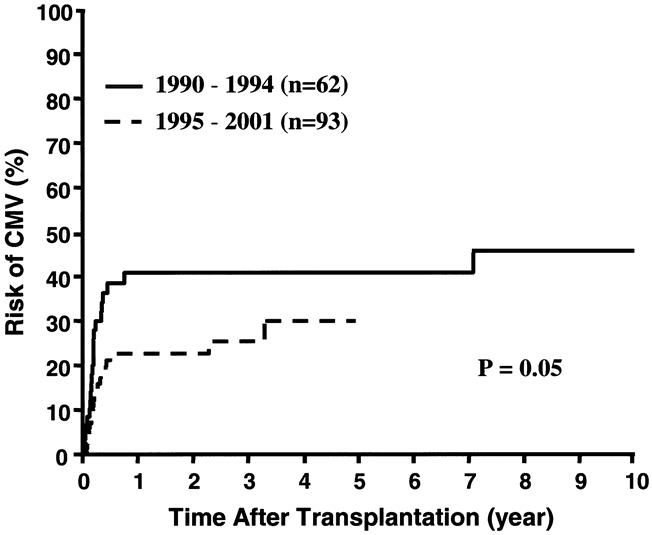
Figure 12. Cumulative risk of cytomegaloviral (CMV) disease among patients who were transplanted before and after the 1994 moratorium.
Hospitalization and Nutritional Rehabilitation
The mean duration of intensive care unit stay was significantly (P = .004) reduced from 29 ± 44 days between 1990 and 1994 to 16 ± 23 days for patients between 1995 and 2001. The total hospital stay also was significantly (P = .0001) reduced from a mean of 12 ± 8.7 to 5.7 ± 4.7 weeks.
Most of the reductions in hospital times resulted from an improved speed of recovery, including the intestinal allograft absorptive function. Complete discontinuation of TPN in the 1995-to-2001 recipients after a median of 20 days (mean 27 ± 22) was in striking contrast to the 42-day median (mean 55 ± 45) of the earlier era (P = .0001).
None of the 37 recipients who had primary chronic disease of the native intestine, including 14 with Crohn’s disease, have had any clinical or histopathologic evidence of recurrence in the intestinal transplant. Seventy-one (93%) of the 76 current survivors with graft in place are completely off TPN with full nutritional autonomy, enjoying an unrestricted oral diet. The remaining five patients are receiving partial TPN and on a temporary basis because of systemic chemotherapy (n = 1), a recent episode of rejection (n = 1), postoperative recovery from exploratory laparotomy for de novo mesenteric pseudotumor (n = 1), and recent transplant (n = 1).
DISCUSSION
Two factors accounted for the long delay in the development of intestinal transplantation relative to that of the other vital organs. First, the general perception until well into the 1990s was that GVHD mounted by the lymphoid-rich bowel was going to be a common problem, 41 similar to that which plagued clinical bone marrow transplantation. 42 Second, it was assumed that translocation of microorganisms from the intestinal lumen would constitute an overwhelming risk to the host unless rejection could be prevented or perfectly controlled with immunosuppressants that were themselves inimical to an effective defense against infections or neoplasms.
Thus, life-supporting function of a transplanted human bowel had never been achieved until this was accomplished with a multivisceral allograft transplanted in Pittsburgh in November 1987 to a child who was treated with cyclosporine-based immunosuppression, 1 using an experimental operation described in 1960. 43,44 Although the child died after 6 months of a posttransplant B-cell lymphoma, 1 enteral feeding provided adequate nutrition for most of the survival period. Then, in August 1988, Deltz et al 2 of Kiel, Germany, transplanted a small bowel segment from a live donor to a patient who ate during much of a 56-month survival. During the succeeding 15 months, Grant et al 3 from London, Ontario, and Margreiter et al 4 of Innsbruck added three more long-surviving intestine-containing composite grafts (n = 2 and 1, respectively), and on March 18, 1989, Goulet et al 5 performed a cadaveric intestine-only transplantation in a child. Although the latter patient is the only one still alive from this era, the two Canadian recipients survived for 5 years.
These cases, combined with an extensive experience with upper abdominal exenteration and reconstruction with “cluster” allografts that contained segments of stomach, duodenum, and jejunum, 45,46 established the feasibility of transplanting hollow abdominal viscera. The next step came with the report by Murase et al 47 at the 1989 European Society of Organ Transplantation meeting in Barcelona that rat bowel, transplanted alone or as part of a multivisceral complex under a short course of treatment with the new drug, tacrolimus, could be routinely engrafted. When the superior efficacy of tacrolimus in rats was promptly confirmed in other rat intestinal transplant models, 48,49 the University of Pittsburgh program of clinical intestinal transplantation was begun on May 2, 1990. 10
The linchpin of immunosuppression in all subsequent cases consisted of tacrolimus and variable-dose prednisone. During the ensuing 11 years, a variety of adjunct agents with different cellular/molecular targets have been added at the time of surgery: cyclophosphamide, mycophenolate mofetil, the interleukin-2 receptor antagonist daclizumab, and most recently sirolimus. With the possible exception of daclizumab, the additional drugs have not dramatically improved either survival or the ease of management. Consequently, we have looked to strategies beyond more potent immunosuppressive drug regimens as a means of improving the outlook for intestinal recipients.
Such strategies have been based on insight into the immunologic events leading to engraftment of all organs that began with the key observation by Murase et al 50 that the intestine-alone and multivisceral allografts of rats treated with tacrolimus become genetic composites within 14 days after transplantation. With the aid of immunocytochemical stains that differentiated donor from recipient cells, the leukocytes of the lamina propria and elsewhere, including those in the donor mesenteric lymph nodes, were identified as those of the recipient. 50 The rat findings were promptly confirmed by Iwaki et al 51 in human intestine-only and multivisceral recipients.
It had been known for nearly 20 years that this kind of leukocyte replacement occurred in human hepatic allografts, but it had been assumed to be a special quality of liver transplants. Now, it was realized that leukocyte replacement must be a generic phenomenon with the acceptance of all kinds of whole organ allografts. 6,51 Within the following year, the further studies of leukocyte migration and persistence in the host (i.e., microchimerism) that had begun with research on intestinal transplantation 50,52–54 eventually matured to the paradigm that defined events after the transplantation of all organs in terms of a mutually canceling double immune reaction in which the two cell populations reciprocally modulated immune responsiveness, inducing variable levels of mutual nonreactivity. 55–57
In appropriate inbred rat strain combinations (e.g., Brown Norway and Lewis), it has been possible to separate the contemporaneous graft-versus-host and host-versus-graft arms of the double immune reaction. 54,58 It has been shown that the quantity and lineage profiles of the passenger leukocytes contained in different kinds of organ grafts strongly influence the relative strengths of the interactive host-versus-graft and graft-versus-host reactions, the quantity and lineages of the chimerism found in the recipients, graft survival, and graft function. 54,58,59 As a practical consideration, the passenger leukocytes of the liver and bone marrow cells, both of which include large numbers of immature leukocytes and cells of myeloid origin, have been shown to be more tolerogenic, with a lower risk of GVHD, than the intestinal passenger leukocytes, which are rich in mature lymphocytes. 59
In accordance with this concept, Murase et al 60 have shown in exquisitely controlled rat studies that chronic rejection of intestinal allografts can be prevented by infusing donor bone marrow cells. Moreover, in GVHD-prone strain combinations, the GVHD is prevented with low-dose irradiation of the bowel, whereas chronic rejection of these irradiated grafts can be prevented with concomitant donor bone marrow infusion. The results in the rat experiments have guided the use of adjunct donor bone marrow in 39 of our human intestinal transplantations, including 9 recent cases in which the intestine-alone allograft or the intestinal component of multivisceral grafts was conditioned with irradiation.
The cumulative improvement in survival rates during the past 11 years has qualified intestine-alone and multivisceral transplantation for funding as a service by the Health Care Financing Administration as of Oct. 4, 2000. A good view of the still-limited world experience can be found in the report by Grant et al 61 from the International Intestinal Transplant Registry. Although it is too early to make definitive assessments of recent management changes, it is noteworthy that the rate of chronic rejection, late graft loss, and delayed death in the bone marrow-augmented recipients has been less than in the contemporaneous nonaugmented controls. Moreover, all 10 of the recipients of irradiated grafts survive to date, and none has had evidence of any irradiation-associated graft dysfunction.
DISCUSSION
Dr. Ronald W. Busuttil (Los Angeles, California): First I would like to say that I really feel privileged to have been asked to discuss this extraordinary work. Dr. Abu-Elmagd has presented a paper from Dr. Starzl’s team which I clearly consider a landmark. This body of work performed over the past ten years represents a turning point in the development of patients who have intestinal failure. Just as the Starzl team brought liver replacement from surgical curiosity to worldwide application, they have now paved the way for intestinal transplantation.
This paper is really packed with pearls as a result of the very extensive and comprehensive experience the Pittsburgh group has acquired. I really believe it is a “must” read for anyone who is interested in intestinal replacement. I have several questions that span clinical, technical, and immunological issues.
First, clinical. In the paper you discuss the issue of a salvageable liver graft. And I assume that you mean that if you have a patient who has potentially reversible liver disease that you will only do an intestinal allograft rather than the combined intestinal liver allograft. My question is, in a recipient that has portal hypertension, do you find that the portal hypertension is a deterrent to good intestinal allograft function alone? And in what percentage of those patients do you see substantial recovery in the liver function after you replace the intestine? The second question is: C-M-V has not been really a problem in our series of liver intestinal transplantation because we preemptively treat these patients with ganciclovir based on their PCR. Do you do this? Secondly, do you still not use CMV positive donors as you have written about in the past?
The technical question relates to the hook-up of the native portal vein to the portal vein of the allograft when you do a combined liver intestinal graft. Sometimes this can be problematic. When do you do that? And when do you hook up the native portal vein into the IVC?
And finally, an immunological question. Your results are very intriguing with the irradiation of the allograft and the bone marrow infusion; however, it seems to me that this may be at cross purposes since your hypothesis is that if you perform bone marrow perfusion you are increasing the amount of microchimerism and therefore decreasing the incidence of both acute and chronic rejection. If one irradiates the graft it seems that you would be decreasing the amount of passenger cells that would be allowed to migrate to the recipient lymph nodes.
Presenter Dr. Kareem Abu-Elmagd (Pittsburgh, Pennsylvania): Thank you, Dr. Busuttil, for your generous comments. Your first clinical question concerning the type of allograft that should be used for intestinal graft candidates with portal hypertension, and the outcomes in those who received intestine only is an important and practical one. Currently, there are no published data to guide the transplant physicians in making this decision. Based upon our cumulative experience, the type and degree of portal hypertension and the extent of associated liver damage are the main determining factors. Patients with extensive postmesenteric thrombosis are usually not candidates for isolated intestine and should be considered for either combined liver-intestinal or multivisceral transplantation, particularly when the thrombotic process involves the splenic vein. The degree of portal hypertension is assessed by the conventional clinical, biochemical, and endoscopic parameters including the splenic size and the platelet count. Gastroesophageal varices and ascites are less likely to develop in patients with short gut syndrome because of the reduced or absent mesenteric arterial flow. The extent of hepatocellular injury and liver damage is determined histopathologically. In general, patients with modest portal hypertension (mild splenic enlargement, platelet count >50,000, no gastroesophageal varices and minimal to moderate portal fibrosis without severe intrahepatic cholestasis) can be considered for intestinal only transplantation.
In 62 (68%) of our primary intestine only recipients, the pretransplant liver biopsy showed mild to moderate portal fibrosis with serum bilirubin ranging from normal to 21 mg/dl. In all of these recipients, the serum bilirubin normalized within the first 4-8 weeks after surgery and the implanted grafts functioned well except for those that were lost to intractable rejection. It is important to mention that in patients with significant portal hypertension, the venous out flow of the isolated intestinal allografts was drained into the recipient systemic circulation via the inferior vena cava. The histopathologic reversibility of the TPN induced hepatic damage in these recipients, particularly those with long-term full gastrointestinal nutritional autonomy, is currently under evaluation.
As for the second question about CMV, we monitor all of our transplant patients including the intestinal recipients as described in the manuscript, with weekly pp65 antigenemia test, at very frequent intervals during the first 8-12 postoperative weeks. The use of CMV positive grafts was avoided only in CMV negative intestine-alone or modified multivisceral recipients unless there was an urgent need for transplantation.
The technical question relating to the hook-up of the native portal vein to the portal vein of the liver-intestinal graft is also a valid one. In all of the liver-intestinal recipients, a portocaval shunt was initially performed before dissecting the residual native abdominal viscera. the purpose was to decompress the mesenteric collaterals and minimize blood loss. In our early experience, we routinely disconnected the shunt shortly after reperfusion and anastomosed the end of the native portal vein to the side of the allograft portal vein. After the development of portal vein thrombosis in one of our pediatric recipients who had a small portal vein (1995), we have accepted the physiologic compromise of potential diversion of the insulin rich blood away from the transplanted liver and have left the portocaval shunt permanently.
The final question concerning the concept of simultaneous bone marrow infusion and intestinal allograft irradiation is an important one. In a series of exquisitely controlled rat studies, my colleague Dr. Murase and others have shown that the quantity and lineage profiles of the passenger leukocytes contained in different organ grafts strongly influence the quantity and lineage of microchimerism, graft survival, and function. In these experiments, the intestinal passenger lymphocytes have been shown to be less tolerogenic with a higher risk of GVHD than the passenger leukocytes of the liver and bone marrow cells, both of which include large numbers of immature leukocytes and cells of myeloid origin. Accordingly, cytoreduction of the intestinal allograft with low-dose ex-vivo irradiation, which removes large numbers of mature T cells, combined with simultaneous donor bone marrow cell replacement should improve the clinical outlook of intestinal transplantation. This has been shown to reduce the risk of rejection without risk of GVHD in our preclinical studies.
Dr. Andreas G. Tzakis (Miami, Florida): I would like to thank Dr. Abu-Elmagd for allowing me to review the manuscript and also congratulate him for his fine work and presentation. I would like to acknowledge that it was Dr. Abu-Elmagd’s initiative that brought federal funding to intestinal transplantation recently. I have one comment and one question.
The presentation reflects the continuous innovations and intensity which have characterized the Pittsburgh program from the outset. It also shows the confusing nature of the results in such an elaborate project.
There are two clear messages in the paper which I reviewed. The first message is that for whatever reason, and probably for a variety of reasons, the results in intestinal transplantation are improving over time. And I wholeheartedly agree with that.
The second message in the paper was that the presence of a contemporaneously transplanted liver conveys a protective effect in the combined liver-intestinal graft. It is offered as an explanation of two findings: the superior long-term results of the combined liver-intestinal grafts in Pittsburgh, and the observed increased incidence of acute and chronic rejection of isolated grafts.
Nevertheless, in the same paper, the liver failed to offer a similar protection in the liver containing multivisceral grafts. In addition, isolated intestinal transplants had the same patient and graft survival at five years, which is the last time point where there are meaningful numbers to make comparisons. Our own results at the University of Miami indicate that liver-intestinal and multivisceral transplants behave similarly and we cannot distinguish the results between them.
In regard to isolated intestinal grafts, we have marginally higher incidence of acute rejection, a difference which is disappearing with improving immunosuppression. In fact, the overall results are better in our program with the isolated intestinal transplants because the patients are less ill, the operation is smaller, and the incidence of severe complications is lower.
Clearly, the combination of bone marrow infusions with radiation treatment of the graft is a revolutionary new approach, and its universal success to date may have far-reaching implications. For that reason, I would like to elaborate on Dr. Busuttil’s question and ask Dr. Abu-Elmagd if he has any facts from the human experience regarding chimerism or any other immunological studies to show what actually happens in humans. To my knowledge, the only reported relevant data have only been in small animals.
Dr. Kareem Abu-Elmagd: Thank you, Dr. Tzakis, for acknowledging my role in the recent approval by the Health Care Financing Administration (HCFA) of intestinal and multivisceral transplantation for funding as a clinical service. Concerning your comment about the immunoprotective effect of the liver allograft on the simultaneously transplanted intestine, I would like to address a few important points that may help eliminate the present confusion and controversy. First, it is obvious from our Kaplan-Meier (cumulative) survival curves shown in Figure 6 of our manuscript that long-term follow-up (beyond 5 years) is needed to demonstrate any difference in survival of the three different kinds of the intestinal allografts. The lower long-term survival rate of the multivisceral grafts (n=25) compared to the combined liver-intestinal grafts (n=75) despite inclusion of the liver in 20 of the 25 in the first group and all 75 of the second group, could be explained simply by the documented higher risk of PTLD and lethal infections among the multivisceral recipients as shown in Table 4 of the manuscript and the relatively small number of the multivisceral grafts (only 11) that survived beyond the 5-year milestone. Second, we agree with Dr. Tzakis, we as previously published, that combined liver-intestinal and multivisceral recipients belong to a high-risk population because of the disease gravity prior to transplantation, added to the complexity of the multivisceral operations, and the difficult perioperative management.
These non-immunologic risk factors partially erode the immunoprotective advantage of the liver and its positive impact, both in the early and overall graft survival. To resolve this debate, it will be necessary for Dr. Tzakis to identify those grafts that were lost due to rejection in his series and calculate the difference in the cumulative risk from rejection between the non-liver and liver cohorts as we showed in Figure 10 of our manuscript. I believe that with longer follow-up Dr. Tzakis and others will come to the same conclusion about the liver’s protective effect as we have. However, the recent management strategy of combined graft immune-modulation and induction immunosuppressive therapy with monoclonal antibodies may eliminate the difference or even achieve better survival after isolated intestinal transplantation by reducing the risk of rejection in this low operative risk and more healthy population.
As to the question about the comparative results of the chimerism and other immunologic studies in our recipients who received irradiated intestine and simultaneous bone marrow infusion versus those treated conventionally, we can only say at the present time that the clinical results have been encouraging. An analysis of the chimerism, immunologic, and other biologic studies will be published when we have more data.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association on April 26, 2001, the Broadmoor Hotel, Colorado Springs, Colorado.
Supported in part by research grants from the Veterans Administration and Project Grant No. DK-29961 and R01 DK54232 from the National Institutes of Health, Bethesda, MD.
Correspondence: Kareem Abu-Elmagd, MD, PhD, University of Pittsburgh, Thomas E. Starzl Transplantation Institute, Falk Medical Building, 4th Floor, 3601 Fifth Ave., Pittsburgh, PA 15213.
E-mail: kae@med.pitt.edu
Accepted for publication April 26, 2001.
References
- 1.Starzl TE, Rowe MI, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA 1989; 261: 1449–1457. [PMC free article] [PubMed] [Google Scholar]
- 2.Deltz E, Schroeder P, Gebhardt H, et al. Successful clinical small bowel transplantation: Report of a case. Clin Transplant 1989; 3: 89–91. [Google Scholar]
- 3.Grant D, Wall W, Mimeault R, et al. Successful small-bowel/liver transplantation. Lancet 1990; 335: 181–184. [DOI] [PubMed] [Google Scholar]
- 4.Margreiter R, Konigsrainer A, Schmid T, et al. Successful multivisceral transplantation. Transplant Proc 1992; 24: 1226–1227. [PubMed] [Google Scholar]
- 5.Goulet O, Revillon Y, Brousse N, et al. Successful small bowel transplantation in an infant. Transplantation 1992; 53: 940–943. [DOI] [PubMed] [Google Scholar]
- 6.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet 1991; 172: 335–344. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Todo S, Fung J, et al. FK 506 for human liver, kidney and pancreas transplantation. Lancet 1989; 2: 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg 1990; 212: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todo S, Tzakis AG, Abu-Elmagd K, et al. Intestinal transplantation in composite visceral grafts or alone. Ann Surg 1992; 216: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todo S, Tzakis AG, Abu-Elmagd K, et al. Cadaveric small bowel and small bowel-liver transplantation in humans. Transplantation 1992; 53: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Elmagd K, Todo S, Tzakis A, et al. Three years clinical experience with intestinal transplantation. J Am Coll Surg 1994; 179: 385–400. [PMC free article] [PubMed] [Google Scholar]
- 12.Todo S, Reyes J, Furukawa H, et al. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg 1995; 222: 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzakis AG, Todo S, Starzl TE. Intestinal transplantation. In: Coggins CH, Hancock EW, eds. Annual review of medicine: selected topics in the clinical sciences. Palo Alto, CA: Annual Reviews Inc.; 1994: 79–91. [DOI] [PMC free article] [PubMed]
- 14.Grant D. International Intestinal Transplant Registry: current results of intestinal transplantation. Lancet 1996; 347: 1801–1803. [DOI] [PubMed] [Google Scholar]
- 15.Abu-Elmagd K, Reyes J, Fung JJ, et al. Evolution of clinical intestinal transplantation: improved outcome and cost effectiveness. Transplant Proc 1999; 31: 582–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casavilla A, Selby R, Abu-Elmagd K, et al. Logistics and technique for combined hepatic-intestinal retrieval. Ann Surg 1992; 216: 605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu-Elmagd K, Reyes J, Bueno J, et al. Logistics and technique for simultaneous intestinal, pancreatic, and hepatic retrieval. Ann Surg 2000; 232: 680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirose R, Taguchi T, Hirata Y, et al. Immunohistochemical demonstration of enteric nervous distribution after syngeneic small bowel transplantation in rats. Surgery 1995; 117: 560–569. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Elmagd K, Reyes J, Todo S, et al. Clinical intestinal transplantation: new perspectives and immunologic considerations. J Am Coll Surg 1998; 186: 512–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bueno J, Abu-Elmagd K, Mazariegos G, et al. Compossite liver-small bowel allografts with preservation of donor duodenum and hepatic biliary system in children. J Pediatr Surg 2000; 35: 291–296. [DOI] [PubMed] [Google Scholar]
- 21.Reyes J, Fishbein T, Bueno J, et al. Reduced-size orthotopic composite liver-intestinal allograft. Transplantation 1998; 66: 489–492. [DOI] [PubMed] [Google Scholar]
- 22.Todo S, Tzakis A, Reyes J, et al. Small intestinal transplantation in humans with or without colon. Transplantation 1994; 57: 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Todo S, Tzakis A, Abu-Elmagd K, et al. Abdominal multivisceral transplantation. Transplantation 1995; 59: 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzakis AG, Nour B, Reyes J, et al. Endorectal pullthrough of transplanted colon as part of intestinal transplantation. Surgery 1995; 117: 451–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzakis AG, Todo S, Reyes J, et al. Piggyback orthotopic intestinal transplantation. Surg Gynecol Obstet 1993; 176: 297–298. [PMC free article] [PubMed] [Google Scholar]
- 26.Starzl TE, Watanabe K, Porter KA, et al. Effects of insulin, glucagon, and insulin/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet 1976; 1: 821–825. [DOI] [PubMed] [Google Scholar]
- 27.Fontes P, Rao A, Demetris AJ, et al. Augmentation with bone marrow of donor leukocyte migration for kidney, liver, heart, and pancreas islet transplantation. Lancet 1994; 344: 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starzl TE, Demetris AJ, Rao AS, et al. Spontaneous and iatrogenically augmented leukocyte chimerism in organ transplant recipients. Transplant Proc 1994; 26: 3071–3076. [PMC free article] [PubMed] [Google Scholar]
- 29.Rao AS, Fontes P, Zeevi A, et al. Augmentation of chimerism in whole organ recipients by simultaneous infusion of donor bone marrow cells. Transplant Proc 1995; 27: 210–212. [PMC free article] [PubMed] [Google Scholar]
- 30.Abu-Elmagd K, Fung JJ, Reyes J, et al. Management of intestinal transplantation in humans. Transplant Proc 1992; 24: 1243–1244. [PMC free article] [PubMed] [Google Scholar]
- 31.Reyes J, Tzakis AG, Todo S, et al. Nutritional management of intestinal transplant recipients. Transplant Proc 1993; 25: 1200–1201. [PMC free article] [PubMed] [Google Scholar]
- 32.Reyes J, Bueno J, Kocoshis S, et al. Current status of intestinal transplantation in children. J Pediatr Sur 1998; 33: 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green M, Bueno J, Rowe D, et al. Predictive negative value of persistent low EBV viral load after intestinal transplantation in children. Transplantation 2000; 70: 593–596. [DOI] [PubMed] [Google Scholar]
- 34.Bueno J, Green M, Kocoshis S, et al. Cytomegalovirus infection after intestinal transplantation in children. Clin Infect Dis 1997; 25: 1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee RG, Nakamura K, Tsamandas AC, et al. Pathology of human intestinal transplantation. Gastroenterology 1996; 110: 2009–2012. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan EL, Meier P. Nonparametric estimation from complete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 37.Matthews DE, Farewell VT. Using and understanding clinical statistics. New York: Karger; 1985: 67–87.
- 38.Mantel N. Evaluation of survival data and two new range statistics arising in its consideration. Cancer Chemo 1966; 50: 163–170. [PubMed] [Google Scholar]
- 39.Reyes J, Todo S, Green M, et al. Graft-versus-host disease after liver and small bowel transplantation in a child. Clin Transplant 1997; 11: 345—348. [PMC free article] [PubMed] [Google Scholar]
- 40.Gruessner RWG, Uckun FM, Pirenne J, et al. Recipient preconditioning and donor-specific bone marrow infusion in a pig model of total bowel transplantation. Transplantation 1997; 63: 12–20. [DOI] [PubMed] [Google Scholar]
- 41.Monchik GJ, Russell PS. Transplantation of the small bowel in the rat: technical and immunologic considerations. Surgery 1971; 70: 693–702. [PubMed] [Google Scholar]
- 42.Billingham RE. Reactions of grafts against their hosts: transplantation immunity works both ways: hosts destroy grafts and grafts may harm hosts. Science 1959; 130: 947. [DOI] [PubMed] [Google Scholar]
- 43.Starzl TE, Kaupp HA Jr. Mass homotransplantation of abdominal organs in dogs. Surg Forum 1960; 11: 28–30. [PMC free article] [PubMed] [Google Scholar]
- 44.Starzl TE, Kaupp HA Jr, Brock DR, et al. Homotransplantation of multiple visceral organs. Am J Surg 1962; 103: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starzl TE, Todo S, Tzakis A, et al. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg 1989; 210: 374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alessiani M, Tzakis A, Todo S, et al. Assessment of 5-year experience with abdominal organ cluster transplantation. J Am Coll Surg 1995; 180: 1–9. [PMC free article] [PubMed] [Google Scholar]
- 47.Murase N, Kim D, Todo S, et al. Induction of liver, heart and multivisceral graft acceptance with a short course of FK 506. Transplant Proc 1990; 22: 74–75. [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffman A, Makowka L, Cai X, et al. The effect of FK 506 on small intestine allotransplantation in the rat. Transplant Proc 1990; 22: 76–77. [PMC free article] [PubMed] [Google Scholar]
- 49.Lee K, Stangl MJ, Todo S, et al. Successful orthotopic small bowel transplantation with short-term FK 506 immunosuppressive therapy. Transplant Proc 1990; 22: 78–79. [PMC free article] [PubMed] [Google Scholar]
- 50.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small bowel allotransplantation under FK 506. Surgery 1991; 110: 87–98. [PMC free article] [PubMed] [Google Scholar]
- 51.Iwaki Y, Starzl TE, Yagihashi A, et al. Replacement of donor lymphoid tissue in human small bowel transplants under FK 506 immunosuppression. Lancet 1991; 337: 818–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murase N, Demetris AJ, Kim DG, et al. Rejection of the multivisceral allografts in rats: a sequential analysis with comparison to isolated orthotopic small bowel and liver grafts. Surgery 1990; 108: 880–889. [PMC free article] [PubMed] [Google Scholar]
- 53.Murase N, Lieberman I, Nalesnik M, et al. FK 506 prevents spontaneous diabetes in the BB rat. Transplant Proc 1991; 23: 551–555. [PMC free article] [PubMed] [Google Scholar]
- 54.Murase N, Demetris AJ, Woo J, et al. Graft versus host disease (GVHD) after BN to LEW compared to LEW to BN rat intestinal transplantation under FK 506. Transplantation 1993; 55: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet 1992; 339: 1579–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology 1993; 17: 1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 57.Starzl TE, Zinkernagel R. Antigen localization and migration in immunity and tolerance. N Engl J Med 1998; 339: 1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanabe M, Murase N, Demetris AJ, et al. The influence of donor and recipient strains in isolated small bowel transplantation in rats. Transplant Proc 1994; 26: 4325–4332. [PMC free article] [PubMed] [Google Scholar]
- 59.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft versus host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to Brown-Norway rats. Transplantation 1995; 60: 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murase N, Ye Q, Nalesnik MA, et al. Immunomodulation for intestinal transplantation by allograft irradiation, adjunct donor bone marrow infusion, or both. Transplantation 2000; 70: 1632–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grant D. Intestinal transplantation: 1997 Report of the International Registry. Transplantation 1999; 67: 1061–1064. [DOI] [PubMed] [Google Scholar]