Abstract
Objective
To assess the efficacy of plasmapheresis in the treatment of children with acute hepatic failure.
Summary Background Data
Acute liver failure is expressed with severe encephalopathy, coagulopathy, and subsequent multisystem organ failure, resulting in a high death rate. Liver transplantation is considered the best option, with long-term 1-year survival rates exceeding 88%. It has been suggested that plasmapheresis may improve coagulopathy and prevent bleeding complications while maintaining adequate fluid, electrolyte, and acid–base balance.
Methods
Forty-nine patients with acute liver failure underwent a total of 243 therapeutic plasma exchanges (TPE). Indications for treatment included candidacy for liver transplant and prolonged prothrombin time. Pheresis was performed daily until the patient recovered, died, or was transplanted. Four patients were anhepatic during pheresis.
Results
Coagulation profiles after TPE significantly improved compared with mean preexchange values while maintaining euvolemia. No bleeding episodes were observed during the course of treatment. There was no sustained improvement in neurologic function. Spontaneous recovery was observed in three patients; the remaining either underwent transplantation (32/49) or were not considered transplant candidates because of irreversible neurologic insults (11/49) or sepsis (3/49).
Conclusion
For children with acute liver failure, TPE is extremely effective in preventing life-threatening bleeding while maintaining appropriate volume status in small children. This method of treatment has no effect on the neurologic complications of liver failure and has no impact on the ability of the liver to regenerate.
Acute pediatric liver failure is an uncommon yet highly fatal disease process. 1 Primary causes include fulminant hepatic failure (FHF), viral, toxic, or idiopathic. 2 It may also result from the acute decompensation of a patient with chronic liver disease, such as biliary atresia, cystic fibrosis, and other inborn errors of metabolism. 3 The critical care of patients with acute hepatic failure remains a challenge. The impaired synthetic function results in worsening coagulopathy, 4 whereas the inability to detoxify neurotoxic substances results in progressive encephalopathy, neuronal injury, increased intracranial pressure, and brain herniation. 5 With worsening disease, the patient often experiences renal failure and hemodynamic collapse. 6,7
Acute liver failure is rarely reversible, and without transplantation the survival rate is 10% to 40%, depending on the etiology of failure. In contrast, liver transplantation for FHF or acute on chronic liver failure is associated with a survival rate of more than 80% in children. 8 The inability to predict liver recovery and the need to determine whether the patient should receive a transplant when an organ becomes available may be associated with either premature or inappropriately late transplant. Ideally, the organ should be replaced when it is known that the native liver will not regenerate and before the development of irreversible neurologic injury, multisystem organ failure, or uncontrolled infection. It is obvious that the existence of a liver support device, with the ability to prevent damage from the liver dysfunction, would offer a great advantage for the correct management of these patients.
To support these patients until spontaneous recovery occurs or until a liver allograft becomes available, several groups have reported the use of bioartificial assist devices. 9,10 The preliminary data are encouraging, but the widespread clinical applicability of this technology has yet to be defined. In the interim, a therapeutic plasma exchange (TPE) may be considered to correct coagulopathy and possibly to remove various toxins from the systemic circulation. 11 An important advantage for TPE in children may be related to the ability to correct coagulopathy without risking massive fluid overload from the transfusion of blood product.
The use of plasmapheresis has been reported in the management of adults with drug-induced liver failure as well as those with delayed function of liver allografts. 12–19 Although these have been small case series, the results have been promising. It is certain that recovery of the liver in these patients is related to hepatocyte repair and regeneration. However, the mechanisms by which TPE contributes to recovery remain unclear. It has yet to be determined whether plasmapheresis is a valuable method of treatment in the maintenance of patients awaiting transplantation.
The aim of this study was to analyze outcomes of plasmapheresis in the support of children with acute liver failure. Clinical and laboratory assessments were performed before and after plasmapheresis and enabled us to dissect patterns in the correction of coagulopathy, the potential to reverse encephalopathy, and the effect of treatment on fluid status and kidney function. The existence of a small group of patients who were anhepatic during pheresis allowed us to speculate whether the presence of the failing liver interferes with treatment efficacy.
METHODS
The records of all patients at the Children’s Hospital of Philadelphia undergoing a total of 243 TPEs (median 3) while awaiting liver transplant between 1987 and 2000 were retrospectively reviewed. Pheresis was performed more frequently before 1995, at which time an aggressive liver transplantation policy was instituted. The indication for pheresis was hepatic failure, candidacy for orthotopic liver transplant (OLT), and coagulopathy (prothrombin time [PT] > 20 seconds). Twenty (41%) patients were boys and 29 (59%) girls. Daily TPE was performed until the patient recovered, died, or underwent OLT. Age at time of treatment ranged from 10 days to 18.4 years (median 2.9 years).
The primary etiology of liver failure in these children is shown in Figure 1. Only 57% of the patients had FHF; the remainder had an acute decompensation of chronic liver disease. After OLT, 13 patients had primary graft failure (primary nonfunction, hepatic artery thrombosis, or acute rejection) and continued to receive plasmapheresis while awaiting retransplantation. Four patients were anhepatic at the time of pheresis. Survival data were obtained from hospital medical records as well as clinic notes.
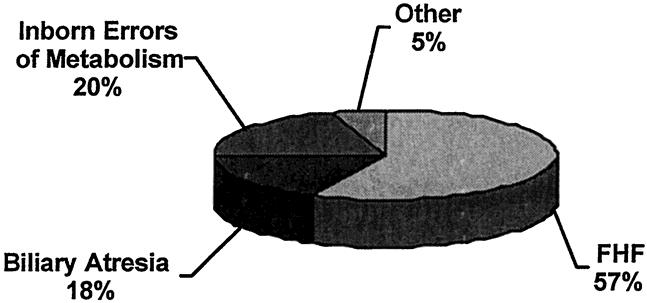
Figure 1. Primary etiology of liver failure.
Plasmapheresis was performed using the Gambro BCT (Gambro, Lakewood, CO). The cell separator was primed with red blood cells if the patient weighed less than 20 kg and/or if his or her hemoglobin level was less than 10 g/dL. Plasma volume (mean ± 1 standard deviation) removed per exchange was 121 ± 47 mL/kg (2.2 ± 0.6 plasma volume). Replacement solution consisted of 74 ± 11% fresh-frozen plasma. Cryoprecipitate was used in 63 (26%) of the TPE sessions to maintain fibrinogen levels at more than 100 mg/dL, and platelets were used in 112 (46%) of the exchanges to maintain platelet counts at more than 50 × 109/L.
Patients were examined before and immediately after plasma exchange. Neurologic status was determined by clinical assessment using a standard hepatic encephalopathy scale and was correlated with the level of blood ammonia. The pattern of coagulopathy was assessed by determination of PT (adjusted to International Normalized Ratio [INR]) and the partial thromboplastin time. Measurements of individual clotting factors were taken before and immediately after exchange and included fibrinogen, factor II, factor V, factor VII, and factor IX. Standard biochemistry panels were taken 1 hour after pheresis and included measurements of sodium, potassium, chloride, bicarbonate, and liver function tests (bilirubin, aspartate transaminase, alanine transaminase). Statistical analysis was carried out using the paired Student t test. P < 0.05 was considered significant.
RESULTS
Neurologic Status
At the initiation of treatment, all patients had stage 3 or 4 encephalopathy according to the Fogerty criteria. 20 Despite a persistent decrease in the levels of blood ammonia of more than 20 μmol/dL (mean of 102 to 83, P = .18;Table 1), there was only transient improvement in the neurologic examination results. The beneficial effect of plasmapheresis was most pronounced after the first session and was almost entirely lost in subsequent treatments. This phenomenon may be attributed to the accumulation of other nondialyzable neurotoxic substances in the systemic circulation or brain tissue. Therefore, TPE should not be expected to affect the neurologic complications secondary to acute liver failure.
Table 1. EFFECT OF PLASMA EXCHANGE ON LIVER FUNCTION
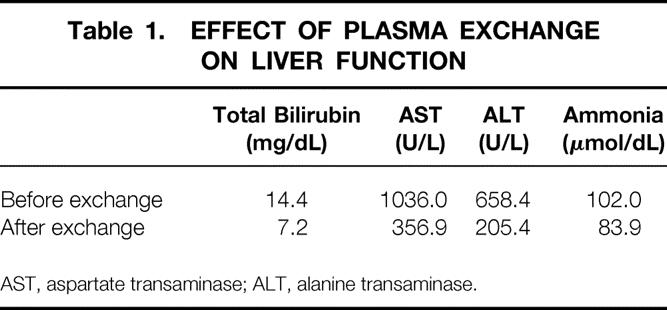
AST, aspartate transaminase; ALT, alanine transaminase.
Correction of Coagulopathy
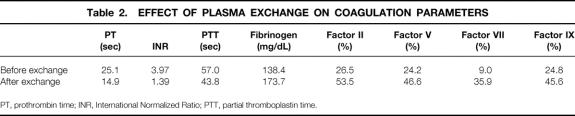
The effects of plasmapheresis on the coagulation profile are listed in Table 2. The mean postexchange coagulation values were significantly improved compared with mean preexchange values while preserving euvolemia (P < .01). Correction of coagulopathy was achieved by the twofold increase of fibrinogen and factors II, V, and IX and the fourfold increase in the coagulation factor with the shortest half-life, factor VII.
Table 2. EFFECT OF PLASMA EXCHANGE ON COAGULATION PARAMETERS
PT, prothrombin time; INR, International Normalized Ratio; PTT, partial thromboplastin time.
The pattern of PT correction for the patients undergoing at least five exchanges (n = 19) is shown in Figure 2. It appears that the reduction in PT corresponds to the correction in clotting factors that are produced by the liver. Moreover, the threefold decrease in INR was directly related to a proportional improvement in the levels of factor VII. The treatment was successful in bringing the PT level to near-normal INR; however, reduction of factor VII to less than 9% within 24 hours after exchange led to return of INR to preexchange levels. The PT in the treated population did not exceed 25 seconds as long as plasma exchange was done on a daily basis. This observation contrasts with nontreated patients with acute liver failure, where peak PT values continue to increase until the liver is replaced. Correction of coagulopathy appeared effective because none of the treated patients developed significant bleeding complications.
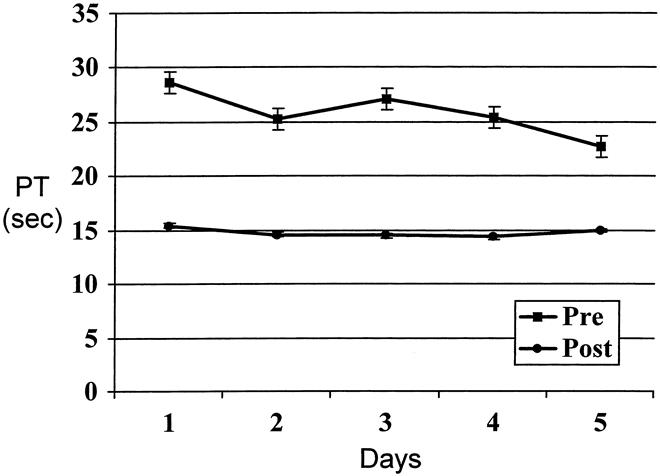
Figure 2. Prothrombin time (PT) trend in 19 patients undergoing at least five plasma exchanges.
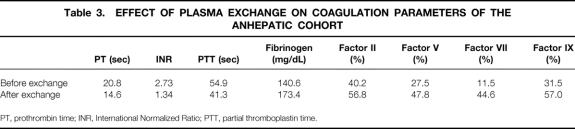
We also wished to determine whether the presence of a failing liver affects the ability of TPE to correct coagulopathy. Admittedly, the small number in the anhepatic group (n = 4) precludes a meaningful statistical analysis. However, pretreatment PT and INR were lower in the anhepatic group, and this cohort had higher levels of factors II and IX (Table 3). Anecdotal observations reveal that these patients tend to have more stable hemodynamics.
Table 3. EFFECT OF PLASMA EXCHANGE ON COAGULATION PARAMETERS OF THE ANHEPATIC COHORT
PT, prothrombin time; INR, International Normalized Ratio; PTT, partial thromboplastin time.
Liver Function
The dilutional effect of plasmapheresis was well demonstrated by the decrease in total bilirubin and transaminases (see Table 1, P < .01). The artificial reduction in both bilirubin and liver enzymes may have masked real improvement of liver function. Recovery of liver function was characterized by the ability to maintain lower bilirubin levels after TPE, whereas progressive liver failure was expressed by worsening bilirubin. Clinical correlation is imperative. The relatively low incidence of spontaneous recovery is similar to that documented in nontreated patients with acute liver failure and suggests that TPE does not affect the natural course of the liver disease or promote regeneration.
Renal and Metabolic Function
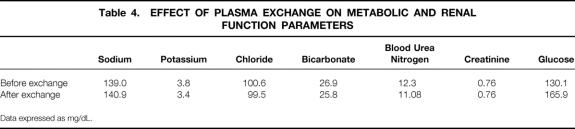
Renal dysfunction is fairly common in acute on chronic liver failure. The mechanisms for the development of low urine output and subsequent renal shutdown in the presence of normal hemodynamics are not clear. However, this phenomenon contributes to the ongoing electrolyte abnormalities and metabolic acidosis. As seen in Table 4, delivery of TPE was achieved without significant electrolyte or acid–base abnormalities. Moreover, blood urea nitrogen and creatinine levels remained normal in 46 (94%) patients. Of those with abnormal renal function, two patients had previously received liver allografts and had chronic renal insufficiency secondary to cyclosporine toxicity. These results suggest a possible protective effect of TPE on renal function.
Table 4. EFFECT OF PLASMA EXCHANGE ON METABOLIC AND RENAL FUNCTION PARAMETERS
Data expressed as mg/dL.
Complications of Plasmapheresis
Anticipated complications for TPE were defined as those related to either placement of large-bore intravenous access, infections acquired from the central line, and transfusion reactions. Despite severe coagulopathy, the presence of a large intravenous line was not associated with any internal bleeding or significant bleeding at the site of catheter insertion. There was an infrequent need for either replacement or repositioning of the catheter in 17 of the 243 sessions of TPE. In only 5 of the 243 sessions were positive blood or catheter tip cultures documented. Transfusion reactions, including fever, rigor, and urticaria, were seen in 11 of the 243 sessions. No hemodynamic instability or systemic organ failure was associated with any of these episodes.
Clinical Outcome
A total of 32 patients received transplants between 1987 and 2000; of those, 17 are alive today. All the anhepatic patients were successfully bridged to transplant. In the past 5 years, expedited use of the left lateral segment from a live or in situ split cadaveric donor resulted in far better survival with transplantation.
Of the 17 patients not transplanted, spontaneous recovery was observed in 3; the remainder were excluded from transplant candidacy and died of severe sepsis (n = 3) or irreversible neurologic insults resulting from liver failure and/or inborn errors of metabolism (n = 11) (Fig. 3).
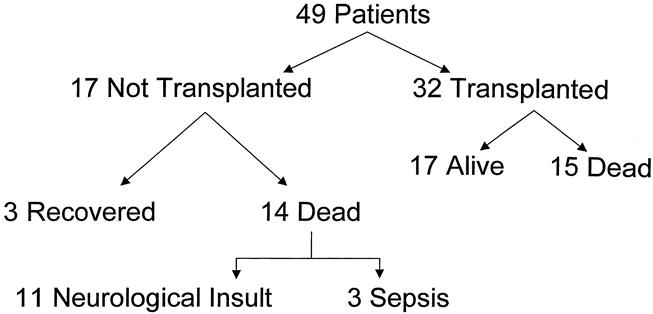
Figure 3. Clinical outcomes.
DISCUSSION
Acute liver failure is a consequence of either fulminant liver injury appearing in an otherwise noncirrhotic organ, or an acute decompensation in the presence of preexisting chronic liver disease. It is a life-threatening process characterized by progressive hepatic synthetic and metabolic dysfunction resulting in hypoglycemia, severe coagulopathy, jaundice, and worsening encephalopathy. Ongoing destruction of liver tissue and the inability to recover hepatocyte function leads to various metabolic derangements and ultimately the development of multisystem organ failure, as expressed with renal and pulmonary compromise. The outcomes are directly related to the etiology and chronicity of the disease, as well as the development of extrahepatic complications. 8 The ability to arrest the progression of the liver injury and prevent complications related to the poor synthetic and detoxifying capacity of the failing organ may allow the time necessary for regeneration and recovery.
The etiology of FHF in children is mostly related to acute viral hepatitis, injury from hepatotoxic drugs, and other idiopathic causes. The prognosis in this group is directly related to the primary cause of the liver injury: most cases of acetaminophen toxicity may resolve with a complete regeneration of the liver tissue, whereas hepatitis of viral or of unknown cause has a less favorable outcome, with a death rate exceeding 85%. Acute decompensation of preexisting liver failure is seen in metabolic liver diseases and biliary atresia and is often the result of acute bleeding or infectious episodes. The prognosis in these patients depends on the residual liver reserve and the ability to treat the primary event leading to the acute decompensation. Most of these children are expected to recover after appropriate resuscitation and treatment of the systemic insult. 21
At present, the greatest chance for survival is by replacement of the diseased organ through liver transplantation. The refinement of OLT techniques and posttransplant management has resulted in 1-year survival rates of greater than 85% in pediatric recipients. 8 Organ scarcity, however, has led to increased deaths in patients awaiting liver transplantation. Although infants younger than 1 year of age make up a relatively small percentage of those awaiting transplant, a disproportionately large number (17%) die before an organ becomes available. 22 These excellent results are far better then those seen in nontransplanted historical controls of patients with FHF. The potential for reversing acute liver failure with a timely transplant procedure was the primary indication for instituting broader sharing of organs across large geographic regions in the country. Urgent transplantation with the left lateral segment from a cadaveric split procedure or a living donor has been explored as a treatment option when a full-size liver graft is unavailable, and this should be an integral part of the armamentarium in any program performing pediatric transplantation. 23,24
Transplantation may not be immediately available, and other treatment modalities should be considered with the goal of managing the complications of severe hepatic failure. These therapeutic options should be applied as a bridge to transplantation, as a definitive treatment for reversible liver failure, or in the treatment of patients who are not candidates for transplantation at the time of presentation. Admittedly, the need for the application of these technologies in the setting of pediatric liver transplantation has significantly decreased with the progress that has been achieved with surgical techniques of segmental liver transplantation. Indeed, most of the experience presented in this series was gained before application of segmental transplantation and wide sharing of cadaveric organs for FHF in our region.
Various modalities have been developed to temper the complications of liver failure, attempting to stabilize the patient until recovery and regeneration or in anticipation of a liver allograft. These liver bridging techniques include ex vivo whole-organ perfusion of the swine liver, bioartificial liver assist devices using freshly isolated pig hepatocytes and well-differentiated human hepatocellular cancer cell lines, and detoxification strategies such as plasmapheresis and charcoal absorption. Correction of coagulopathy, reversal of encephalopathy, and prevention of additional end-organ damage are the shared goals of these strategies.
Whole-organ perfusion techniques have been fraught with complications, such as clotting and acute tissue rejection. These shortcomings have served as an impetus for the development of bioreactor technologies. 9 These bioartificial systems also have various drawbacks, including incompatibility of cell cultures derived from nonhuman cells, insufficient cell proliferation, rapid deterioration of cellular function because of an impoverished cellular environment, and lack of system scalability. 25–30 During the past decade these devices have been refined and have been evaluated in limited clinical studies. Controlled trials in well-defined patient groups and with discrete outcome measures will be essential to proper evaluation of the clinical value of current and evolving bioartificial devices.
Plasma exchange has been used sporadically in the management of acute liver failure during the past 30 years. Small series reported some benefit for patients with acute failure secondary to toxic ingestions. The rationale for treatment is to prevent life-threatening complications, because there is a likelihood for spontaneous recovery once the toxin is removed and liver regeneration is initiated. A similar argument has been used for the application of TPE in the treatment of patients with delayed liver allograft function, in whom, given the opportunity, many of these grafts will recover. A study by Mohandas et al 19 states that plasma exchange did not significantly affect graft survival in patients with primary graft dysfunction. However, Mandal et al 16 recently reported that TPE may have a beneficial effect on allograft recovery in patients with primary nonfunction. These results suggest that TPE may be indicated in settings where liver recovery is possible.
The present report represents the largest analysis of TPE in the management of acute liver failure by a single institution to date and is the first large series in children. The application of TPE in the management of 49 children allowed us to draw more meaningful conclusions regarding the potential of this technology to correct coagulopathy while maintaining euvolemia. The issue of fluid overload may be a severe problem in small children younger than 1 year of age.
This series shows the TPE can correct the bleeding diathesis associated with acute liver failure. Because TPE was extensively used in the management of liver failure at our institution during the entire period of study, no matched, untreated cohort is available for comparison. However, when compared with an historical cohort, the absence of any significant bleeding in our patients is notable. Interestingly, the presence or absence of the failing liver at the time of TPE did not affect the outcome, because it did not alter the efficacy of treatment. The correction of coagulopathy is a result of the replacement of clotting factors normally synthesized by the liver. Given its short half-life, the greatest benefit was likely a result of the restoration of factor VII levels. In theory, replacement of these factors by recombinant proteins may show the same benefit and should be considered once large commercial quantities are available.
The removal of accumulated anticoagulant toxins by TPE may be of clinical benefit, but from our data we cannot draw any conclusions regarding this possibility. We have noticed a more favorable outcome related to renal function. However, it is not clear whether the low incidence of renal failure in our study cohort is related to removal of specific nephrotoxic substances.
Despite a trend toward decreased ammonia levels, TPE did not provide durable improvement in hepatic encephalopathy in our patients. Although hyperammonemia may contribute to central neurologic dysfunction and may explain the transient improvement seen in some patients after their first exchanges, it is intracranial hypertension as well as the accumulation of many other neurotoxic substances over time that explains the persistent hepatic coma.
Liver function values were decreased with plasma exchange. Given that these values returned to pretreatment levels within 24 hours, this decrease can be interpreted as wholly an artifact of dilution. In case of spontaneous recovery, liver function test results remained at lower levels with each exchange, allowing extension of the interval between treatments.
In summary, TPE is a useful technique in the prevention of complications in the setting of acute liver failure. In children, correction of coagulopathy is possible without volume overload and with protection of renal function. This is not to say that TPE is a replacement for definitive therapy for the liver failure, which in most cases requires liver transplantation. In the subpopulation with toxic injury, TPE may allow sufficient time for hepatocyte recovery and regeneration.
DISCUSSION
Dr. Ronald W. Busuttil (Los Angeles, California): Dr. Singer, I enjoyed that paper. It was a very elegant presentation. Congratulations.
The use of plasma exchange has recently been used in a variety of clinical situations including trauma, septic shock, and liver failure to decrease cytokine response, to improve metabolic balance, to restore coagulation profiles, and, to extend this, to reduce the toxic milieu which promotes morbidity and mortality.
Unfortunately, there are no controlled trials showing efficacy from this treatment regarding increased patient survival. And in fact, this has now been extended to one of the bio-artificial liver devices which in uncontrolled small series appeared to have a survival advantage in acute liver failure. However, when it was subjected to a multi-center randomized trial, there really was no clear benefit that became apparent.
Thus, I am not completely convinced that this costly treatment does much to improve patient outcome except to temporarily improve some of the biochemical and coagulation profiles which you illustrated. And you have even acknowledged this in your manuscript by concluding that there is no impact on neurological recovery, regeneration, or patient survival.
Thus as I see it, the potential benefit of plasmapheresis might be extended as a viable bridge to the patient who has a failing allograft. Unfortunately, your study could not answer this, I looked at it, and you did not have a control group.
Now, whether plasmapheresis is able to salvage or bridge the failing allograft has received a fair amount of attention recently. It has been studied and reported by the John Hopkins group and also by the Mount Sinai group. We have had an interest in this, and we have had 30 patients with failed allografts in which we have done plasmapheresis as a bridge to retransplantation.
Interestingly enough, 75% of the grafts placed in patients with acute liver failure were salvaged by plasmapheresis. However, in the patients that were transplanted for chronic liver disease and had primary nonfunctioning, only 30% of those patients were salvaged — an interesting observation. And this, too, needs to be subjected to a more controlled trial.
I have three questions:
One, you reported 32 patients that were eventually transplanted, and 15 of these died. This seems like a pretty high number. Could you elaborate on this a bit?
Secondly, have you used plasmapheresis as a bridge for a failed graft? What is your experience with that?
Finally, a more practical question. What is the cost of plasmapheresis per exchange? Because with the managed care environment that we now exist in, we get a fixed reimbursement for the transplant, and this often extends to up to six months. And obviously, are we going to use this entire reimbursement by having these patients in plasmapheresis or significantly reduce it? I think that is a very important question that needs to be answered.
Presenter Dr. Andrew L. Singer (Philadelphia, Pennsylvania): Thank you very much, Dr. Busuttil, for your comments and questions.
With respect to the mortality rate in the transplanted population, it is important to realize that this series reflects the early transplant experience at the Children’s Hospital of Philadelphia. The sub-optimal outcomes were recognized by the institution and, in part, led to the recruitment of a more aggressive transplantation team. Over the past five years, greater than 85% of the patients in this population achieved long-term survival.
As to your second question, we routinely utilize plasmapheresis in the management of adult patients with primary non-function of their liver allografts and have seen similar results to those that you have described.
With respect to your final question, the cost of plasmapheresis, I acknowledge that the treatment is expensive but I cannot comment on the specifics of the cost.
Dr. Charles M. Miller (New York, New York): Dr. Singer, congratulations to your new team at Penn for this very interesting piece of work. A special congratulations go because you were able to institute this rather logistically complex procedure in very small children, which is something we actually haven’t been able to do. Hepatic failure in very critically ill kids presents very complex diagnostic and treatment challenges, and, as you pointed out, survival without transplantation is truly dismal.
Plasmapheresis is an excellent way to replenish clotting factors in a euvolemic fashion, thereby not exacerbating the cerebral pressure that will lead to premature brain death. I have always thought that it would be beneficial as a bridge in primary non-function or failing grafts as a means of diluting out toxins or cytokines that could actually exacerbate the graft failure. But we were actually unable to show that and our plasmapheresis team has unfortunately lost enthusiasm.
We have been able to maintain their enthusiasm for one aspect of it, and that is in adults with fulminant hepatic failure. Plasmapheresis is performed to replenish the clotting factors with AB plasma and to dilute out all the anti-A and anti-B isohemoglutinins in anticipation of possibly having to perform an ABO mismatched graft. When we have done that, we have had a relatively low incidence of graft threatening rejection. My first question is, do you think plasmapheresis in the pre-transplant period helps prevent rejection?
With respect to renal function, you point out that plasmapheresis maintains the renal function, which ultimately correlates with good survival. Have you seen that impact on your post-transplant survival?
Finally, I wanted to focus not on the post-transplant survival but on the 14 patients who never made it to the transplant. Do you think you overstretched the indications of the plasmapheresis hoping it would actually allow for spontaneous recovery and didn’t move to aggressive and timely transplantation? Has the incidence of waiting list death fallen with the aggressive approach that started in 1995?
Dr. Andrew L. Singer: Thank you very much, Dr. Miller.
With respect to your first question as to whether we see increased graft survival after pre-treatment with plasmapheresis in the setting of transplantation, this series did not allow for such an analysis. However, plasma exchange should theoretically reduce circulating alloantibodies and in turn may reduce rejection after transplantation. In fact, many years ago Dr. Terasaki reported the use of plasmapheresis prior to renal transplantation. He noted increased graft survival in patients that had undergone plasma exchange, presumably a result of the removal of alloreactive antibodies.
As to your second question, renal function as a predictor of outcome, we too have seen poor survival associated with pre-operative renal failure. We would predict that the maintenance of normal renal function should result in improved patient survival after liver transplantation. Despite the fact that good kidney function was maintained in 46 out of 49 patients in this series, the high mortality associated with non-renal factors in these transplanted patients likely precludes us from seeing the predicted benefit.
With respect to your question regarding the patients who never made it to transplantation, the aggressive strategies now employed at Children’s Hospital, specifically segmental transplantation from either an in situ cadaveric split or from a living donor, have led to reduced waiting times such that the development of sepsis or progressive neurological dysfunction which would preclude transplantation has decreased and as a result more children make it to successful transplantation.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association, April 26–28, 2001, the Broadmoor Hotel, Colorado Springs, Colorado.
Correspondence: Abraham Shaked, MD, PhD, Liver Transplant Program, University of Pennsylvania School of Medicine, 34th and Spruce Streets, First Floor Gates Pavilion, Philadelphia, PA 19104.
E-mail: shaked@mail.med.upenn.edu
Accepted for publication April 26, 2001.
References
- 1.Ascher NL, Lake JR, Emond JC, et al. Liver transplantation for fulminant hepatic failure. Arch Surg 1993; 128: 677–682. [DOI] [PubMed] [Google Scholar]
- 2.Manns MP. New therapeutic aspects in fulminant hepatic failure. Chest 1991; 100 (3 Suppl): 193S–196S. [DOI] [PubMed] [Google Scholar]
- 3.Esquivel CO, Marino IR, Fioravanti V, et al. Liver transplantation for metabolic disease of the liver. Gastroenterol Clin North Am 1988; 17: 167–175. [PubMed] [Google Scholar]
- 4.Spannagl M, Schramm W. Replacement of coagulation factors in liver or multiple organ dysfunction. Thromb Res 1999; 95 (4 Suppl 1): S51–56. [DOI] [PubMed] [Google Scholar]
- 5.Jones EA. Pathogenesis of hepatic encephalopathy. Clin Liver Dis 2000; 4: 467–485. [DOI] [PubMed] [Google Scholar]
- 6.Planas R, Bataller R, Rodes J. Hepatorenal syndrome. Curr Treat Options Gastroenterol 2000; 3: 445–450. [DOI] [PubMed] [Google Scholar]
- 7.Larsen FS, Hansen BA, Blei AT. Intensive care management of patients with acute liver failure with emphasis on systemic hemodynamic instability and cerebral edema: a critical appraisal of pathophysiology. Can J Gastroenterol 2000; 14 (Suppl D): 105D–111D. [DOI] [PubMed] [Google Scholar]
- 8.Goss JA, Shackleton CR, McDiarmid SV, et al. Long-term results of pediatric liver transplantation: an analysis of 569 transplants. Ann Surg 1998; 228: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davenport A. Artificial hepatic support. Where are we now? Blood Purif 2001; 19: 1–3. [DOI] [PubMed] [Google Scholar]
- 10.Kaptanoglu L, Blei AT. Current status of liver support systems. Clin Liver Dis 2000; 4: 711–729. [DOI] [PubMed] [Google Scholar]
- 11.Hirasawa H, Sugai T, Oda S, et al. Efficacy and limitation of apheresis therapy in critical care. Ther Apheresis 1997; 1: 228–232. [DOI] [PubMed] [Google Scholar]
- 12.Buckner CD, Clift RA, Volwiler W, et al. Plasma exchange in patients with fulminant hepatic failure. Arch Intern Med 1973; 132: 487–492. [PubMed] [Google Scholar]
- 13.Freeman JG, Matthewson K, Record CO. Plasmapheresis in acute liver failure. Int J Artif Organs 1986; 9: 433–438. [PubMed] [Google Scholar]
- 14.Kondrup J, Almdal T, Vilstrup H, et al. High-volume plasma exchange in fulminant hepatic failure. Int J Artif Organs 1992; 15: 669–676. [PubMed] [Google Scholar]
- 15.Lepore MJ, Stutman LJ, Bonanno CA, et al. Plasmapheresis with plasma exchange in hepatic coma. II. Fulminant viral hepatitis as a systemic disease. Arch Intern Med 1972; 129: 900–907. [PubMed] [Google Scholar]
- 16.Mandal AK, King KE, Humphreys SL, et al. Plasmapheresis: an effective therapy for primary allograft nonfunction after liver transplantation. Transplantation 2000; 70: 216–220. [PubMed] [Google Scholar]
- 17.Redeker AG, Yamahiro HS. Controlled trial of exchange-transfusion therapy in fulminant hepatitis. Lancet 1973; 1: 3–6. [DOI] [PubMed] [Google Scholar]
- 18.Riviello JJ Jr, Halligan GE, Dunn SP, et al. Value of plasmapheresis in hepatic encephalopathy. Pediatr Neurol 1990; 6: 388–390. [DOI] [PubMed] [Google Scholar]
- 19.Skerrett D, Mor E, Curtiss S, et al. Plasmapheresis in primary dysfunction of hepatic transplants. J Clin Apheresis 1996; 11: 10–3. [DOI] [PubMed] [Google Scholar]
- 20.Leevy CM. Clinical diagnosis, evaluation and treatment of liver disease in alcoholics. Fed Proc 1967; 26: 1474–1481. [PubMed] [Google Scholar]
- 21.Karrer FM, Lilly JR, Stewart BA, et al. Biliary atresia registry, 1976 to 1989. J Pediatr Surg 1990; 25: 1076–1081. [DOI] [PubMed] [Google Scholar]
- 22.Gibbons RD, Meltzer D, Duan N. Waiting for organ transplantation. Institute of Medicine Committee on Organ Transplantation. Science 2000; 287: 237–238. [DOI] [PubMed] [Google Scholar]
- 23.Millis JM, Cronin DC, Brady LM, et al. Primary living-donor liver transplantation at the University of Chicago: technical aspects of the first 104 recipients. Ann Surg 2000; 232: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcos A, Fisher RA, Ham JM, et al. Right lobe living donor liver transplantation. Transplantation 1999; 68: 798–803. [DOI] [PubMed] [Google Scholar]
- 25.Gerlach JC, Schnoy N, Encke J, et al. Improved hepatocyte in vitro maintenance in a culture model with woven multicompartment capillary systems: electron microscopy studies. Hepatology 1995; 22: 546–552. [PubMed] [Google Scholar]
- 26.Nagamori S, Hasumura S, Matsuura T, et al. Developments in bioartificial liver research: concepts, performance, and applications. J Gastroenterol 2000; 35: 493–503. [DOI] [PubMed] [Google Scholar]
- 27.Riordan SM, Williams R. Acute liver failure: targeted artificial and hepatocyte-based support of liver regeneration and reversal of multiorgan failure. J Hepatol 2000; 32: 63–76. [DOI] [PubMed] [Google Scholar]
- 28.Rozga J, Holzman MD, Ro MS, et al. Development of a hybrid bioartificial liver. Ann Surg 1993; 217: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozga J, Podesta L, LePage E, et al. A bioartificial liver to treat severe acute liver failure. Ann Surg 1994; 219: 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe FD, Mullon CJ, Hewitt WR, et al. Clinical experience with a bioartificial liver in the treatment of severe liver failure. A phase I clinical trial. Ann Surg 1997; 225: 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]