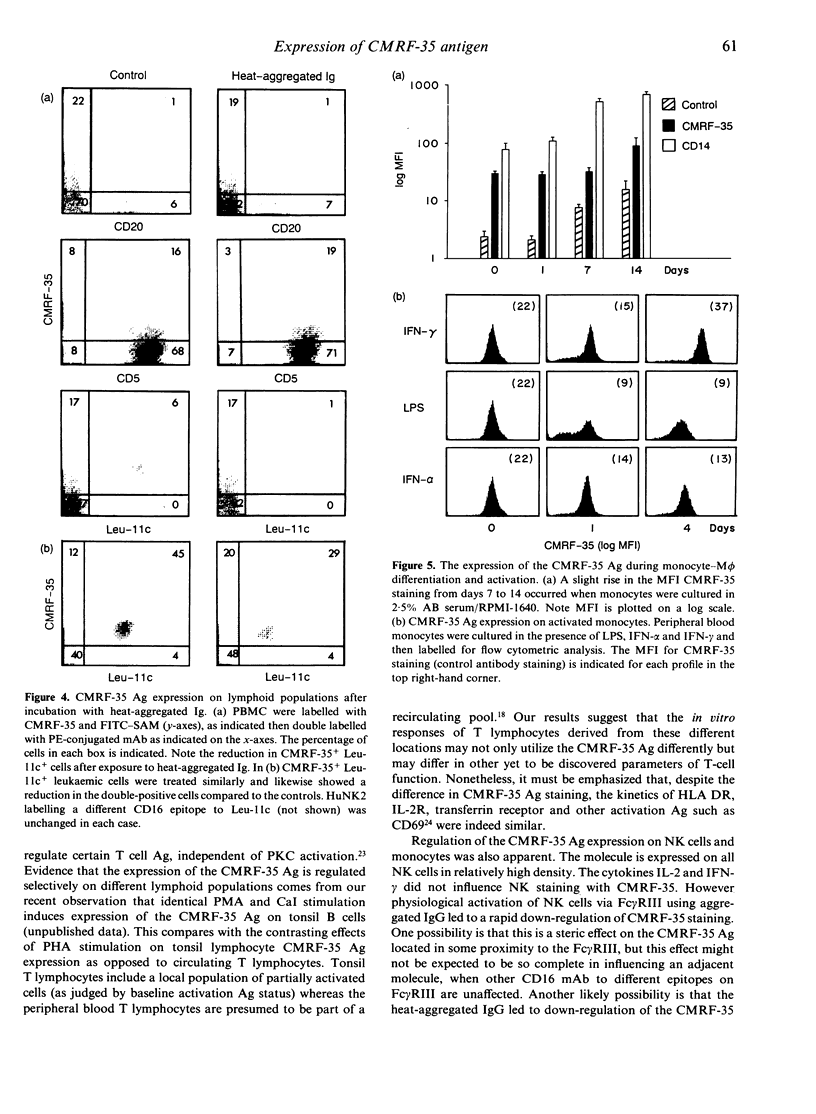
Abstract
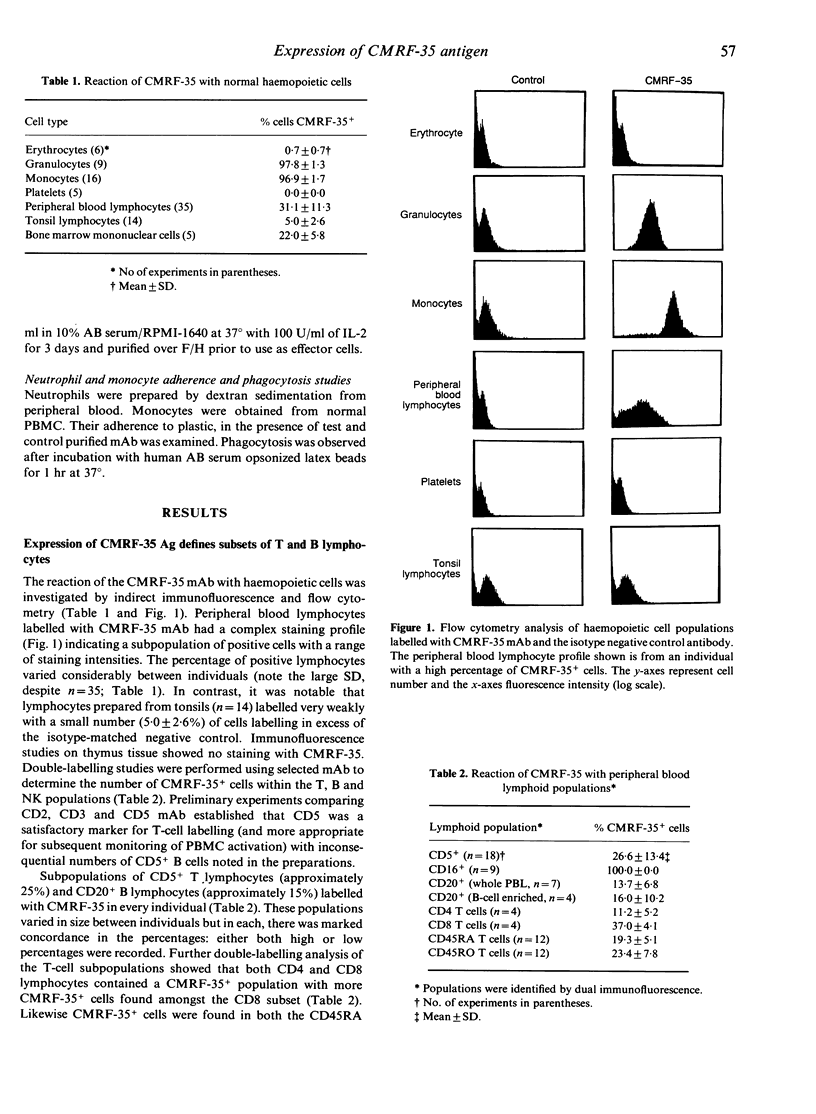
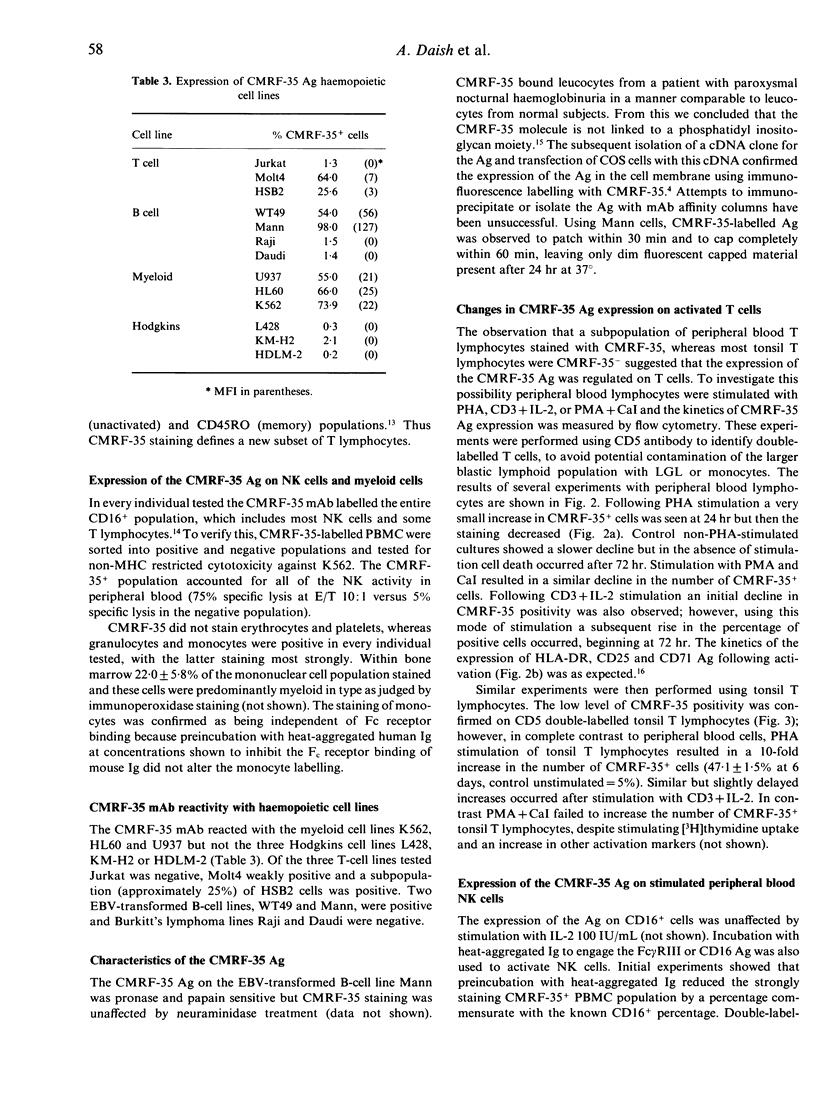
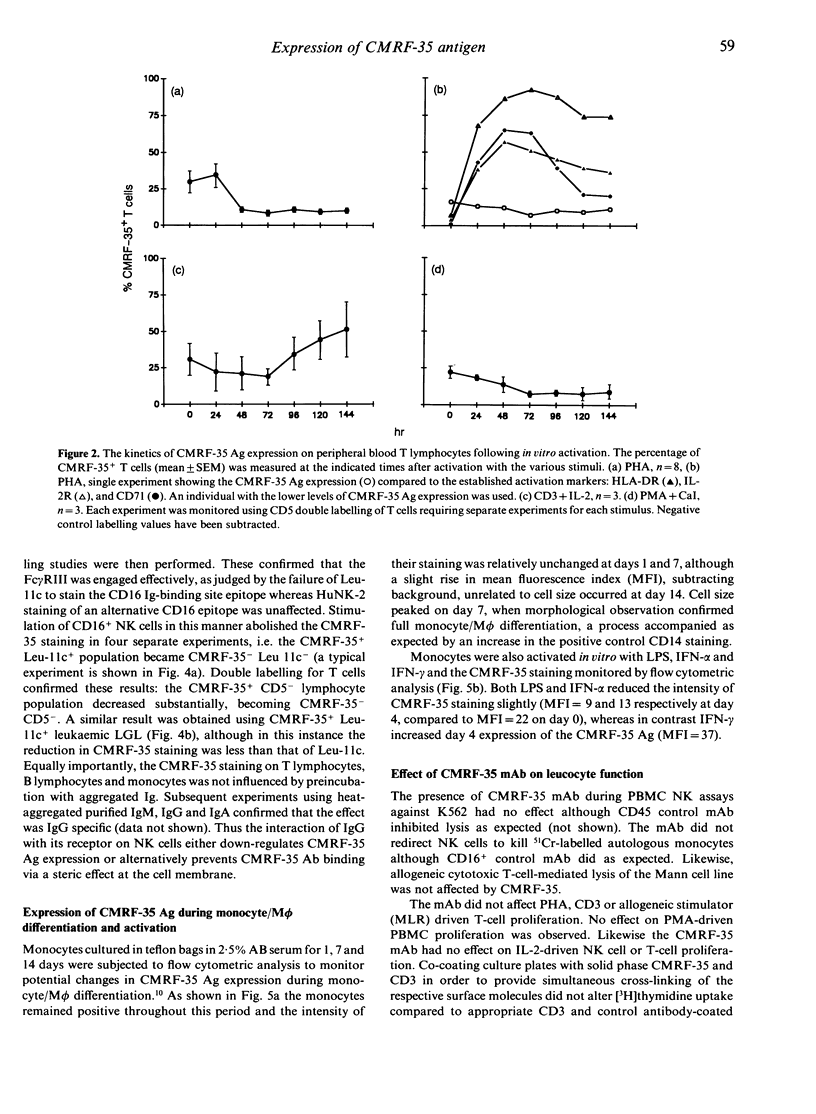
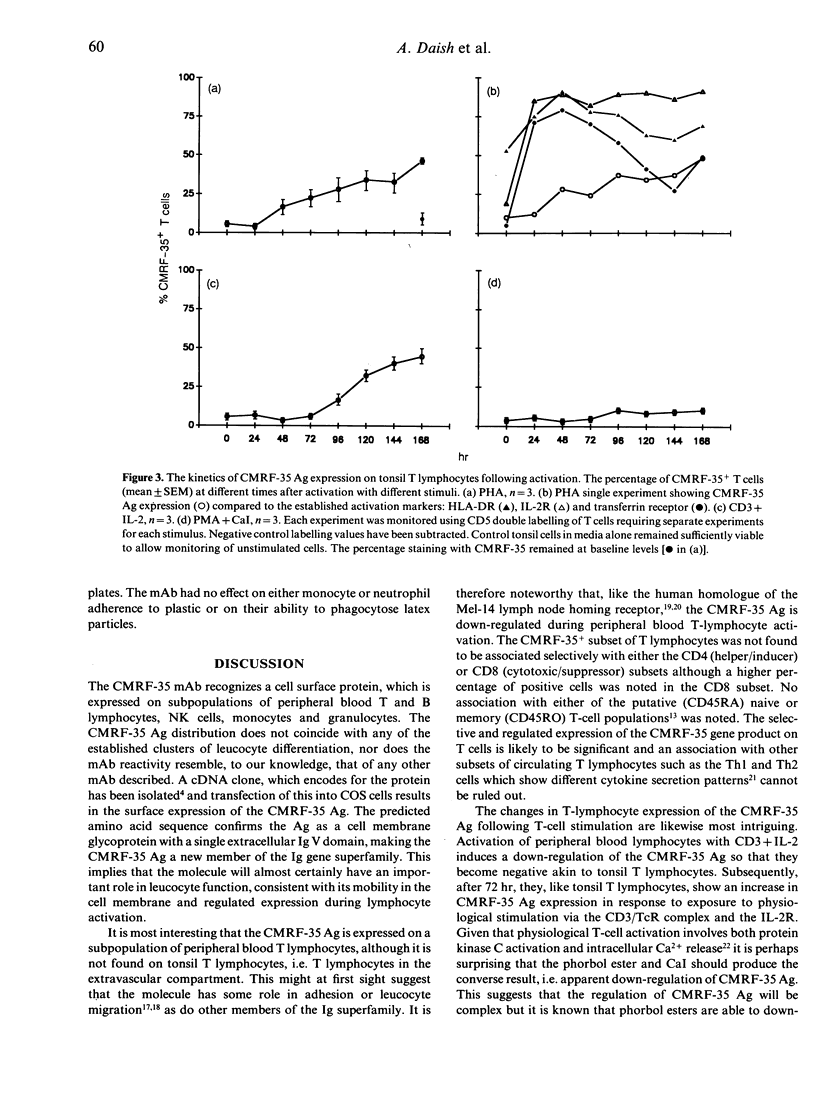
A new monoclonal antibody, CMRF-35, has been generated that recognized a 224 amino acid cell surface protein which is a novel member of the immunoglobulin gene superfamily. The antibody, raised against large granular lymphocytes (LGL), stains LGL, monocytes, macrophages and granulocytes but not platelets or erythrocytes. In addition, a subset of peripheral blood T lymphocytes (26.6 +/- 13.4% CD5+ cells) and B lymphocytes (13.7 +/- 6.8% CD20+ cells) stained with CMRF-35 but tonsil T and B cells were essentially negative. Expression of the CMRF-35 antigen (Ag) on different leucocyte populations was markedly influenced by stimulation of the cells with mitogens and cytokines. Activation of peripheral blood T cells with phytohaemagglutinin (PHA), or phorbol myristate acetate (PMA) and calcium ionophore (CaI) led to a decrease in the proportion of CMRF-35+ T lymphocytes. In contrast, PHA activation of tonsil T lymphocytes resulted in an increase in CMRF-35 Ag expression (47.1 +/- 1.5% CD5 cells at 6 days). An increase in CMRF-35 Ag was also seen on phorbol ester and CaI-activated tonsil B cells. No change in CMRF-35 expression on natural killer (NK) cells occurred following activation with interleukin-2 (IL-2) but the CMRF-35 Ag was down-regulated following Fc receptor stimulation. A moderate increase in CMRF-35 expression occurred during monocyte-macrophage differentiation and the expression of the Ag on monocytes was differentially regulated by interferon-gamma (IFN-gamma). This regulation of the CMRF-35 Ag on the leucocyte surface suggests that the molecule has an important function common to diverse leucocyte types.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Anderson S. E., McKenzie J. L., McLoughlin K., Beard M. E., Hart D. N. The inheritance of abnormal sialoglycoproteins found in a Gerbich negative individual. Pathology. 1986 Oct;18(4):407–412. doi: 10.3109/00313028609087560. [DOI] [PubMed] [Google Scholar]
- Andreesen R., Brugger W., Scheibenbogen C., Kreutz M., Leser H. G., Rehm A., Löhr G. W. Surface phenotype analysis of human monocyte to macrophage maturation. J Leukoc Biol. 1990 Jun;47(6):490–497. doi: 10.1002/jlb.47.6.490. [DOI] [PubMed] [Google Scholar]
- Brière F., Paliard X., De Vries J. E. Induction of the receptor for the Fc portion of IgA by secretory IgA on human T cell lines and T cell clones. Eur J Immunol. 1988 Mar;18(3):445–450. doi: 10.1002/eji.1830180319. [DOI] [PubMed] [Google Scholar]
- Camerini D., James S. P., Stamenkovic I., Seed B. Leu-8/TQ1 is the human equivalent of the Mel-14 lymph node homing receptor. Nature. 1989 Nov 2;342(6245):78–82. doi: 10.1038/342078a0. [DOI] [PubMed] [Google Scholar]
- Chevailler A., Monteiro R. C., Kubagawa H., Cooper M. D. Immunofluorescence analysis of IgA binding by human mononuclear cells in blood and lymphoid tissue. J Immunol. 1989 Apr 1;142(7):2244–2249. [PubMed] [Google Scholar]
- Davidson S. E., McKenzie J. L., Beard M. E., Hart D. N. The tissue distribution of the 3 alpha-fucosyl-N-acetyl lactosamine determinant recognized by the CD15 monoclonal antibodies CMRF-7 and 27. Pathology. 1988 Jan;20(1):24–31. doi: 10.3109/00313028809085192. [DOI] [PubMed] [Google Scholar]
- Driscoll P. C., Cyster J. G., Campbell I. D., Williams A. F. Structure of domain 1 of rat T lymphocyte CD2 antigen. Nature. 1991 Oct 24;353(6346):762–765. doi: 10.1038/353762a0. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A., Hamann A. Mechanisms and regulation of lymphocyte migration. Immunol Today. 1989 Jan;10(1):23–28. doi: 10.1016/0167-5699(89)90061-3. [DOI] [PubMed] [Google Scholar]
- Eiffert H., Quentin E., Decker J., Hillemeir S., Hufschmidt M., Klingmüller D., Weber M. H., Hilschmann N. Die Primärstruktur der menschlichen freien Sekretkomponente und die Anordnung der Disulfidbrücken. Hoppe Seylers Z Physiol Chem. 1984 Dec;365(12):1489–1495. [PubMed] [Google Scholar]
- Jackson D. G., Hart D. N., Starling G., Bell J. I. Molecular cloning of a novel member of the immunoglobulin gene superfamily homologous to the polymeric immunoglobulin receptor. Eur J Immunol. 1992 May;22(5):1157–1163. doi: 10.1002/eji.1830220508. [DOI] [PubMed] [Google Scholar]
- Jung L. K., Bjorndahl J. M., Fu S. M. Dissociation of TPA-induced down-regulation of T cell antigens from protein kinase C activation. Cell Immunol. 1988 Dec;117(2):352–359. doi: 10.1016/0008-8749(88)90124-4. [DOI] [PubMed] [Google Scholar]
- Kanof M. E., James S. P. Leu-8 antigen expression is diminished during cell activation but does not correlate with effector function of activated T lymphocytes. J Immunol. 1988 Jun 1;140(11):3701–3706. [PubMed] [Google Scholar]
- Kay J. E. Mechanisms of T lymphocyte activation. Immunol Lett. 1991 Jul;29(1-2):51–54. doi: 10.1016/0165-2478(91)90198-j. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Buck D. W., Rhodes L., Ding A., Evans E., Barney C., Phillips J. H. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J Exp Med. 1988 May 1;167(5):1572–1585. doi: 10.1084/jem.167.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Maliszewski C. R., March C. J., Schoenborn M. A., Gimpel S., Shen L. Expression cloning of a human Fc receptor for IgA. J Exp Med. 1990 Dec 1;172(6):1665–1672. doi: 10.1084/jem.172.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet I., Panaye G., Revillard J. P. Expression of receptors for IgA on mitogen-stimulated human T lymphocytes. Eur J Immunol. 1988 Apr;18(4):621–626. doi: 10.1002/eji.1830180420. [DOI] [PubMed] [Google Scholar]
- Monteiro R. C., Kubagawa H., Cooper M. D. Cellular distribution, regulation, and biochemical nature of an Fc alpha receptor in humans. J Exp Med. 1990 Mar 1;171(3):597–613. doi: 10.1084/jem.171.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., Friedlander M., Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature. 1984 Mar 1;308(5954):37–43. doi: 10.1038/308037a0. [DOI] [PubMed] [Google Scholar]
- Peach S. F., Davidson S. E., McKenzie J. L., Nimmo J. C., Hart D. N. An activation antigen on a subpopulation of B lymphocytes identified by the monoclonal antibody CMRF-17. Immunology. 1989 Jul;67(3):351–358. [PMC free article] [PubMed] [Google Scholar]
- Prickett T. C., Hart D. N. Anti-leucocyte common (CD45) antibodies inhibit dendritic cell stimulation of CD4 and CD8 T-lymphocyte proliferation. Immunology. 1990 Feb;69(2):250–256. [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991 Aug;12(8):256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- Sandor M., Gajewski T., Thorson J., Kemp J. D., Fitch F. W., Lynch R. G. CD4+ murine T cell clones that express high levels of immunoglobulin binding belong to the interleukin 4-producing T helper cell type 2 subset. J Exp Med. 1990 Jun 1;171(6):2171–2176. doi: 10.1084/jem.171.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert J., Alvarado M., Uciechowski P., Zielinska-Skowronek M., Freund M., Vogt H., Schmidt R. E. Diagnosis of paroxysmal nocturnal haemoglobinuria using immunophenotyping of peripheral blood cells. Br J Haematol. 1991 Nov;79(3):487–492. doi: 10.1111/j.1365-2141.1991.tb08060.x. [DOI] [PubMed] [Google Scholar]
- Shen L., Lasser R., Fanger M. W. My 43, a monoclonal antibody that reacts with human myeloid cells inhibits monocyte IgA binding and triggers function. J Immunol. 1989 Dec 15;143(12):4117–4122. [PubMed] [Google Scholar]
- Starling G. C., Davidson S. E., McKenzie J. L., Hart D. N. Inhibition of natural killer-cell mediated cytolysis with monoclonal antibodies to restricted and non-restricted epitopes of the leucocyte common antigen. Immunology. 1987 Jul;61(3):351–356. [PMC free article] [PubMed] [Google Scholar]
- Starling G. C., Davidson S. E., Nimmo J. C., Beard M. E., Hart D. N. Inhibition of non-MHC-restricted cytotoxicity by CD45 but not CD3 monoclonal antibodies in patients with large granular lymphoproliferative disease. Clin Exp Immunol. 1989 Dec;78(3):396–401. [PMC free article] [PubMed] [Google Scholar]
- Taggart R. T., Samloff I. M. Stable antibody-producing murine hybridomas. Science. 1983 Mar 11;219(4589):1228–1230. doi: 10.1126/science.6402815. [DOI] [PubMed] [Google Scholar]
- Wallace D. L., Beverley P. C. Phenotypic changes associated with activation of CD45RA+ and CD45RO+ T cells. Immunology. 1990 Mar;69(3):460–467. [PMC free article] [PubMed] [Google Scholar]
- Wang J. H., Yan Y. W., Garrett T. P., Liu J. H., Rodgers D. W., Garlick R. L., Tarr G. E., Husain Y., Reinherz E. L., Harrison S. C. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990 Nov 29;348(6300):411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]


