Abstract
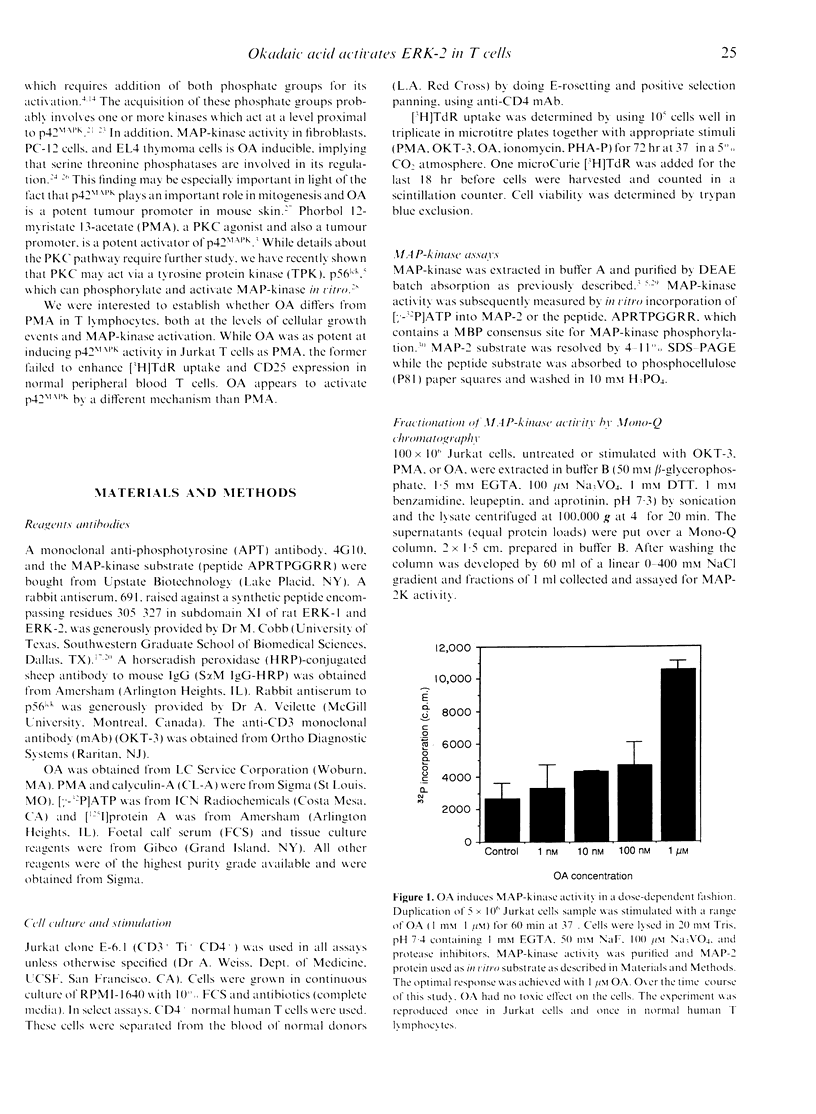
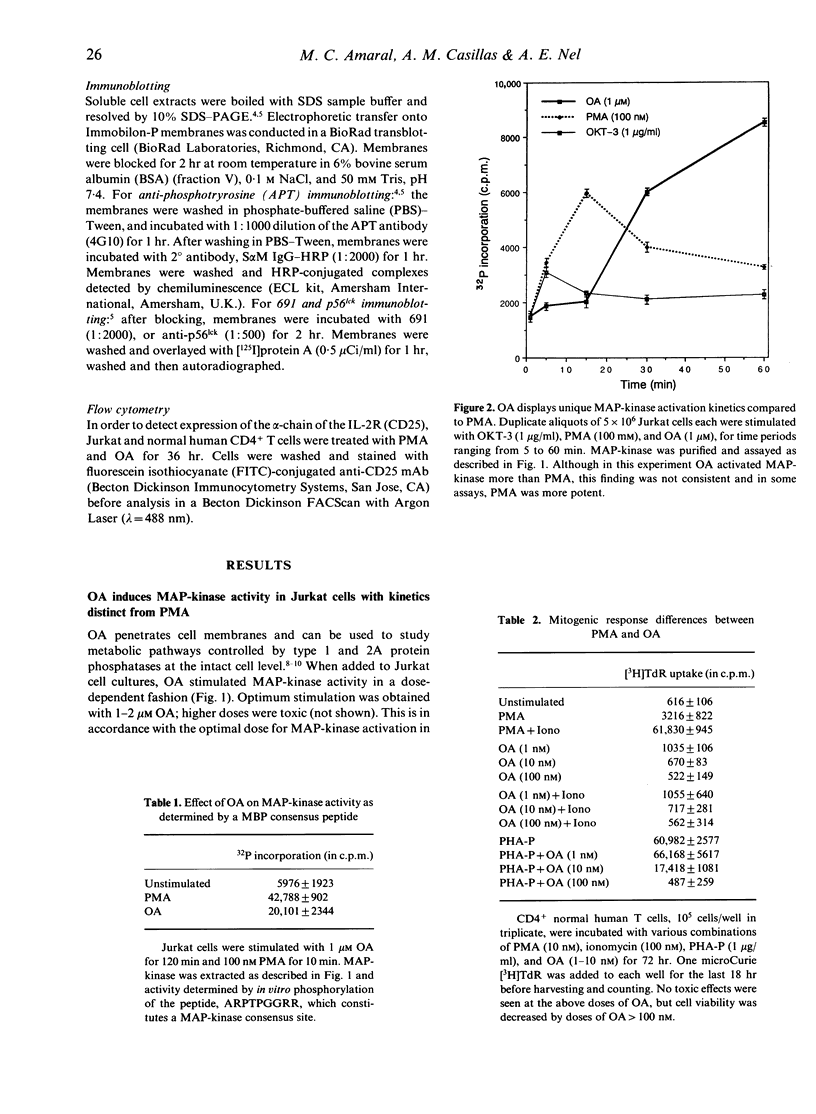
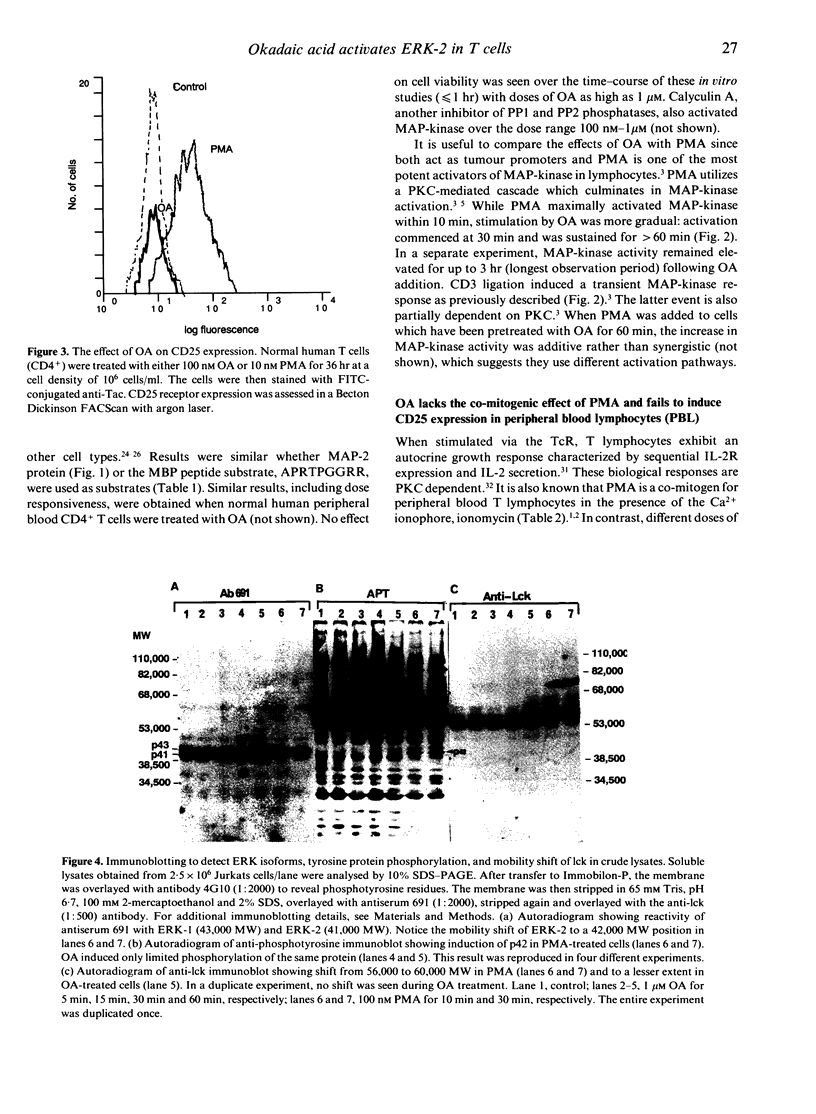
The induction of T-cell growth by the T-cell antigen receptor (TcR) is dependent on a co-ordinated process of phosphorylation and dephosphorylation of intracellular proteins. An intermediary in this signalling pathway is the serine kinase, p42 mitogen-activated protein kinase (p42MAPK), also known as microtubule-associated protein-2 kinase (MAP-2K). MAP-kinase is activated upon the acquisition of tyrosine as well as threonine phosphate groups and removal of either by specific tyrosine or serine/threonine phosphatases abrogates kinase activity. Okadaic acid (OA), a tumour promoter and potent inhibitor of type 1 and 2A serine/threonine protein phosphatases (PP1 and PP2A), induced MAP-kinase activity in Jurkat T cells in a dose-dependent fashion with optimal effect at 1 microM. Compared to rapid activation (peak < 10 min) of MAP-kinase by another tumour promoter, the phorbol ester, PMA, the effect of OA was delayed (> 30 min) and more sustained. In spite of activating a growth-promoting kinase, OA differed from PMA by its lack of mitogenic activity and failure to induce CD25 [interleukin-2R alpha (IL-2R alpha)] expression in normal human T cells. This implies that PP1 and PP2A also act downstream of MAP-kinase to facilitate later cell cycle events. PMA induced a 42,000 MW tyrosine phosphoprotein which co-electrophoresed and co-chromatographed with ERK-2, a p42 MAP-kinase. Although OA induced an identical Mono-Q peak, there was less avid tyrosine phosphorylation of p42. OA also differed from PMA to the extent by which it induced mobility shift of the tyrosine protein kinase, p56lck, which has been implicated in p42MAPK activation in T cells. Taken together, these results indicate that OA and PMA exert both overlapping as well as divergent effects on lymphocyte growth pathways.
Full text
PDF
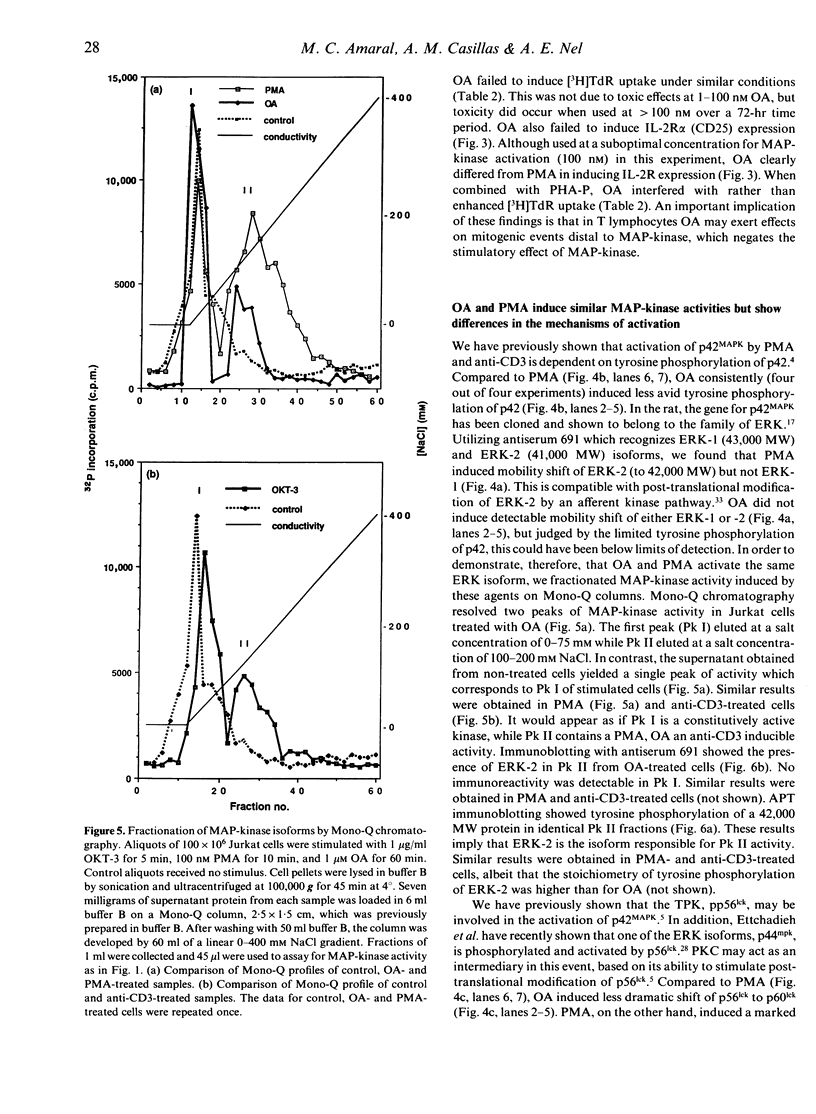
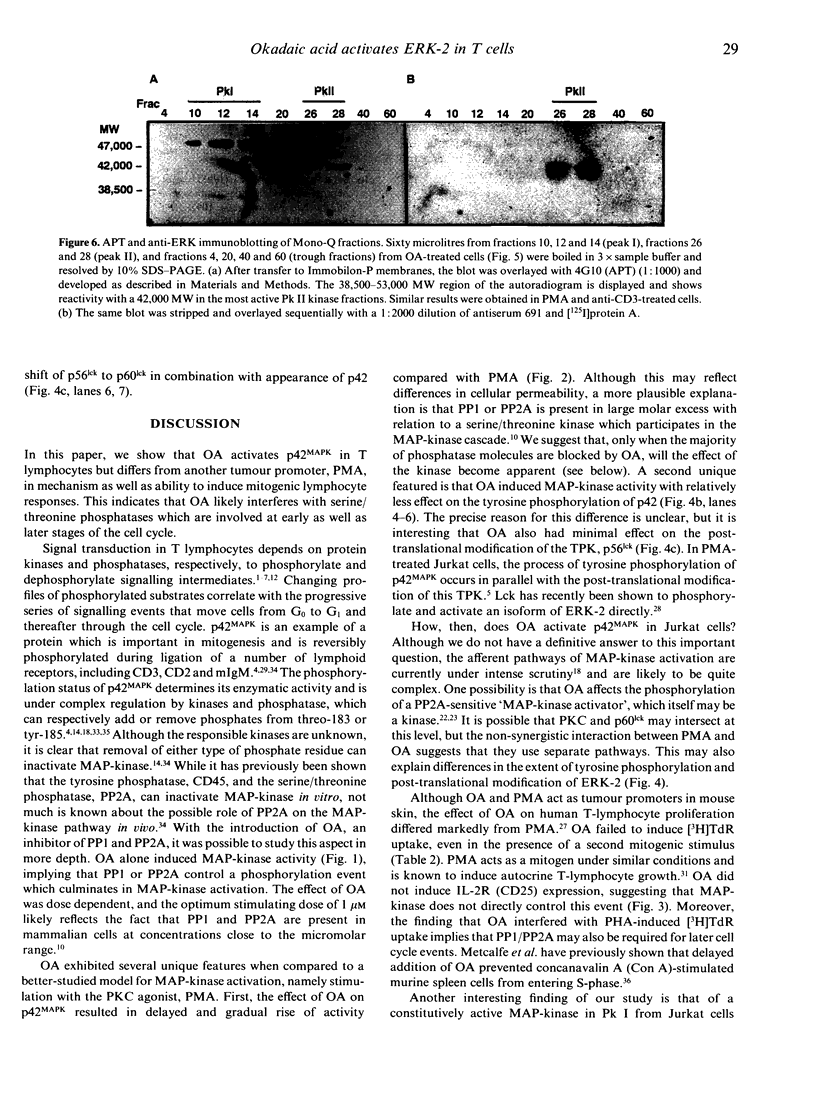


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Reinherz E. L. The human T-cell receptor. Structure and function. N Engl J Med. 1985 Apr 25;312(17):1100–1111. doi: 10.1056/NEJM198504253121706. [DOI] [PubMed] [Google Scholar]
- Ahn N. G., Seger R., Bratlien R. L., Diltz C. D., Tonks N. K., Krebs E. G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991 Mar 5;266(7):4220–4227. [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Casillas A., Hanekom C., Williams K., Katz R., Nel A. E. Stimulation of B-cells via the membrane immunoglobulin receptor or with phorbol myristate 13-acetate induces tyrosine phosphorylation and activation of a 42-kDa microtubule-associated protein-2 kinase. J Biol Chem. 1991 Oct 5;266(28):19088–19094. [PubMed] [Google Scholar]
- Clark-Lewis I., Sanghera J. S., Pelech S. L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem. 1991 Aug 15;266(23):15180–15184. [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Ettehadieh E., Sanghera J. S., Pelech S. L., Hess-Bienz D., Watts J., Shastri N., Aebersold R. Tyrosyl phosphorylation and activation of MAP kinases by p56lck. Science. 1992 Feb 14;255(5046):853–855. doi: 10.1126/science.1311128. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Sakai H. Okadaic acid activates microtubule-associated protein kinase in quiescent fibroblastic cells. Eur J Biochem. 1990 Nov 13;193(3):671–674. doi: 10.1111/j.1432-1033.1990.tb19385.x. [DOI] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Hanekom C., Nel A., Gittinger C., Rheeder A., Landreth G. Complexing of the CD-3 subunit by a monoclonal antibody activates a microtubule-associated protein 2 (MAP-2) serine kinase in Jurkat cells. Biochem J. 1989 Sep 1;262(2):449–456. doi: 10.1042/bj2620449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabak M. J., Hui S. W. The mitogenic activities of phosphatidate are acyl-chain-length dependent and calcium independent in C3H/10T1/2 cells. Cell Regul. 1991 Jan;2(1):57–64. doi: 10.1091/mbc.2.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S., Kosako H., Takenaka K., Moriyama K., Sakai H., Akiyama T., Gotoh Y., Nishida E. Xenopus MAP kinase activator: identification and function as a key intermediate in the phosphorylation cascade. EMBO J. 1992 Mar;11(3):973–982. doi: 10.1002/j.1460-2075.1992.tb05136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier K. E., Licciardi K. A., Haystead T. A., Krebs E. G. Activation of messenger-independent protein kinases in wild-type and phorbol ester-resistant EL4 thymoma cells. J Biol Chem. 1991 Jan 25;266(3):1914–1920. [PubMed] [Google Scholar]
- Metcalfe S., Milner J. Phosphatases PP1 and PP2A act after the G0/G1 interface in lymphocyte activation. Immunol Lett. 1990 Nov;26(2):177–182. doi: 10.1016/0165-2478(90)90142-d. [DOI] [PubMed] [Google Scholar]
- Miyasaka T., Miyasaka J., Saltiel A. R. Okadaic acid stimulates the activity of microtubule associated protein kinase in PC-12 pheochromocytoma cells. Biochem Biophys Res Commun. 1990 May 16;168(3):1237–1243. doi: 10.1016/0006-291x(90)91161-k. [DOI] [PubMed] [Google Scholar]
- Nel A. E., Bouic P., Lattanze G. R., Stevenson H. C., Miller P., Dirienzo W., Stefanini G. F., Galbraith R. M. Reaction of T lymphocytes with anti-T3 induces translocation of C-kinase activity to the membrane and specific substrate phosphorylation. J Immunol. 1987 May 15;138(10):3519–3524. [PubMed] [Google Scholar]
- Nel A. E., Hanekom C., Hultin L. Protein kinase C plays a role in the induction of tyrosine phosphorylation of lymphoid microtubule-associated protein-2 kinase. Evidence for a CD3-associated cascade that includes pp56lck and that is defective in HPB-ALL. J Immunol. 1991 Sep 15;147(6):1933–1939. [PubMed] [Google Scholar]
- Nel A. E., Hanekom C., Rheeder A., Williams K., Pollack S., Katz R., Landreth G. E. Stimulation of MAP-2 kinase activity in T lymphocytes by anti-CD3 or anti-Ti monoclonal antibody is partially dependent on protein kinase C. J Immunol. 1990 Apr 1;144(7):2683–2689. [PubMed] [Google Scholar]
- Nel A. E., Ledbetter J. A., Williams K., Ho P., Akerley B., Franklin K., Katz R. Activation of MAP-2 kinase activity by the CD2 receptor in Jurkat T cells can be reversed by CD45 phosphatase. Immunology. 1991 Jun;73(2):129–133. [PMC free article] [PubMed] [Google Scholar]
- Nel A. E., Pollack S., Landreth G., Ledbetter J. A., Hultin L., Williams K., Katz R., Akerley B. CD-3-mediated activation of MAP-2 kinase can be modified by ligation of the CD4 receptor. Evidence for tyrosine phosphorylation during activation of this kinase. J Immunol. 1990 Aug 1;145(3):971–979. [PubMed] [Google Scholar]
- Nel A. E., Schabort I., Rheeder A., Bouic P., Wooten M. W. Inhibition of antibodies to CD3 surface antigen and phytohemagglutinin-mediated T cellular responses by inhibiting Ca2+/phospholipid-dependent protein kinase activity with the aid of 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine dihydrochloride. J Immunol. 1987 Oct 1;139(7):2230–2236. [PubMed] [Google Scholar]
- Posada J., Cooper J. A. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science. 1992 Jan 10;255(5041):212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. N., Klausner R. D., Rapp U. R., Samelson L. E. T cell antigen receptor engagement stimulates c-raf phosphorylation and induces c-raf-associated kinase activity via a protein kinase C-dependent pathway. J Biol Chem. 1990 Oct 25;265(30):18472–18480. [PubMed] [Google Scholar]
- Sturgill T. W., Wu J. Recent progress in characterization of protein kinase cascades for phosphorylation of ribosomal protein S6. Biochim Biophys Acta. 1991 May 17;1092(3):350–357. doi: 10.1016/s0167-4889(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Suganuma M., Fujiki H., Suguri H., Yoshizawa S., Hirota M., Nakayasu M., Ojika M., Wakamatsu K., Yamada K., Sugimura T. Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1768–1771. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffs R. E., Redegeld F. A., Sitkovsky M. V. Modulation of cytolytic T lymphocyte functions by an inhibitor of serine/threonine phosphatase, okadaic acid. Enhancement of cytolytic T lymphocyte-mediated cytotoxicity. J Immunol. 1991 Jul 15;147(2):722–728. [PubMed] [Google Scholar]
- Thomas G. MAP kinase by any other name smells just as sweet. Cell. 1992 Jan 10;68(1):3–6. doi: 10.1016/0092-8674(92)90199-m. [DOI] [PubMed] [Google Scholar]
- Weiss A., Wiskocil R. L., Stobo J. D. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J Immunol. 1984 Jul;133(1):123–128. [PubMed] [Google Scholar]