Abstract
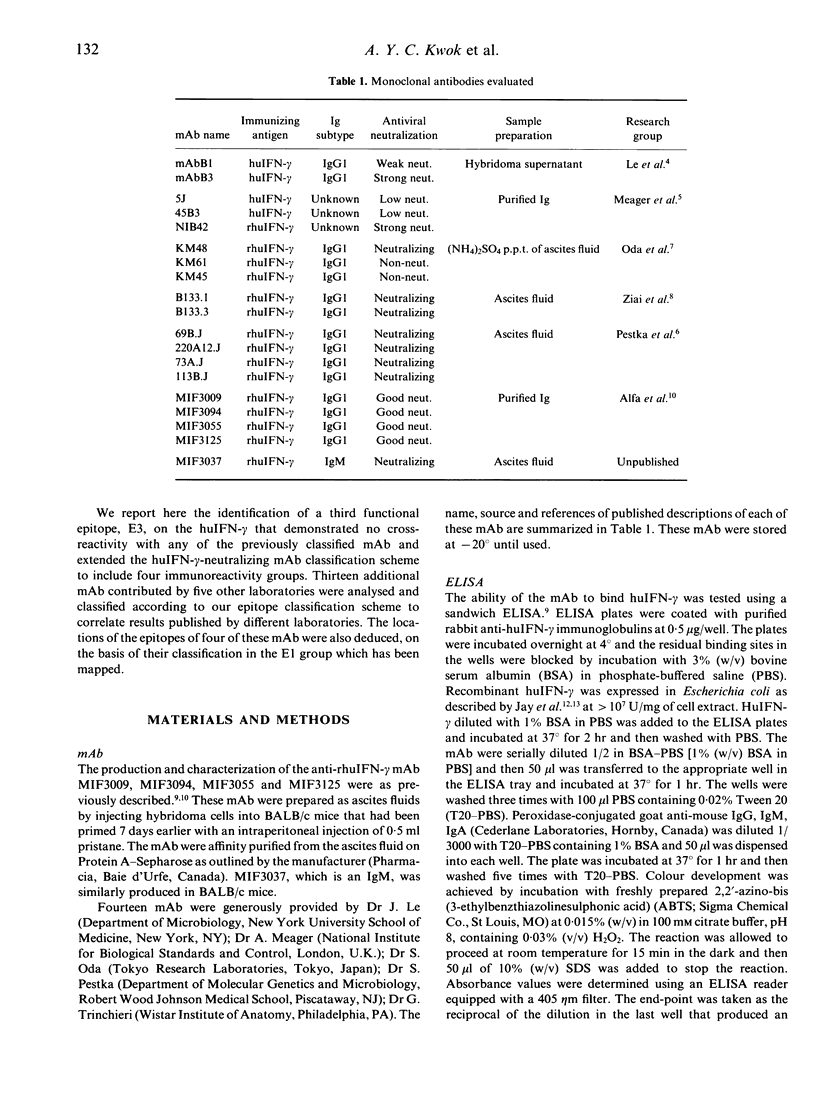
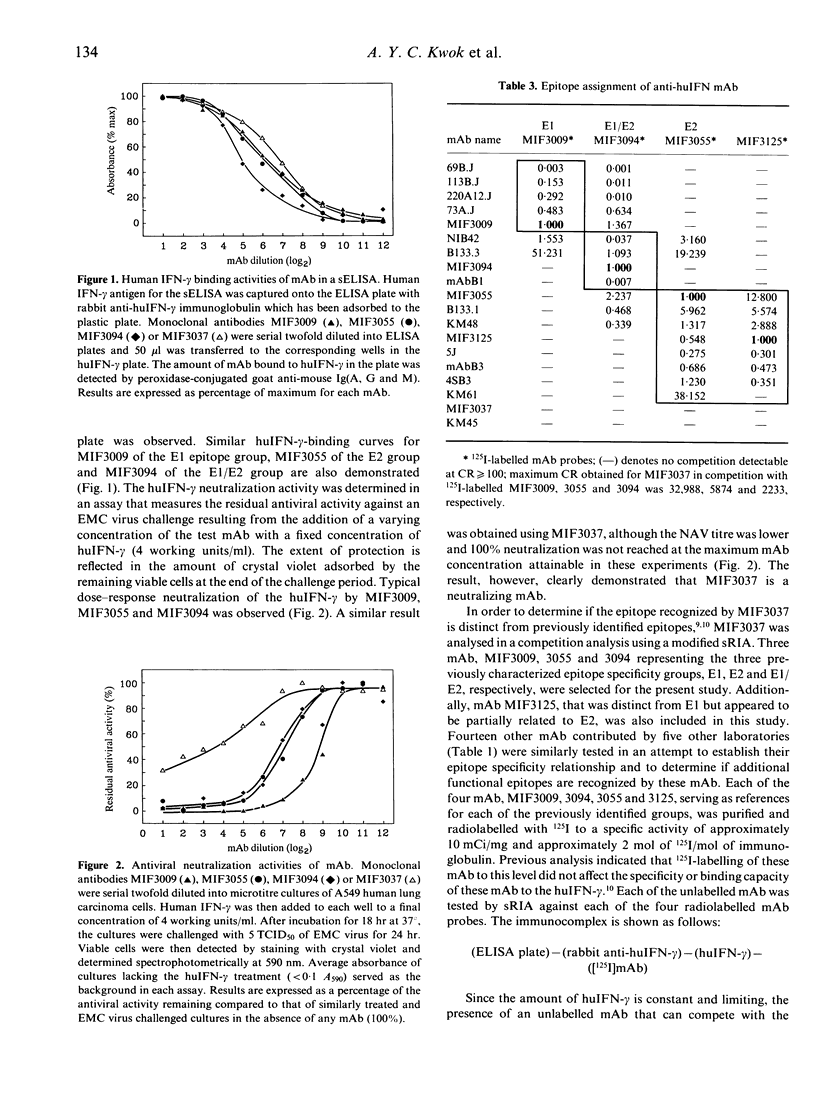
An antiviral activity-neutralizing monoclonal antibody (mAb), MIF3037, was developed by the immunization of BALB/c mice with recombinant human interferon-gamma (rhuIFN-gamma). Its neutralizing activity suggests that its epitope may be at or adjacent to a functional domain on the huIFN-gamma. MIF3037 was compared with representative mAb of previously identified epitope-specific groups in a competitive binding assay. In an attempt to determine if there are other functional epitopes recognized by mAb developed with different preparations of huIFN-gamma or different hybridoma screening methods, 14 additional mAb contributed by five other laboratories were similarly analysed. Based on their ability to bind to huIFN-gamma, all the neutralizing mAb except MIF3037 may be classified into three previously defined groups: E1, E2 and E1/E2. Monoclonal antibodies of the E1 group do not compete with those of the E2 group for huIFN-gamma binding, indicating that the E1 and E2 epitopes are distinct domains on the huIFN-gamma important for the antiviral function. Monoclonal antibodies of the E1/E2 group compete with some of the mAb of E1 and/or E2 groups and may bind to regions of the huIFN-gamma that partially overlap the E1 and E2 epitopes. MIF3037 demonstrated no competitive binding inhibition with mAb of the previously identified epitope specificity groups and, therefore, must represent a distinct functional epitope, E3. The huIFN-gamma, therefore, must have at least three distinct functional domains; none of these appeared to be responsible for cell surface receptor binding. Based on this finding, the epitope typing scheme must be extended to include the E3 epitope. The epitope specificity relationships of 13 neutralizing mAb developed by five other laboratories were established which allows correlation of results obtained with these mAb by different laboratories. The location of the epitopes of four widely studied mAb, 69B, 73A, 113B and 220A12, have been deduced based on their competition with the E1 mAb which have recently been mapped.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfa M. J., Dembinski J. J., Jay F. T. Production of monoclonal antibodies against recombinant human interferon-gamma: screening of hybridomas without purified antigen. Hybridoma. 1987 Oct;6(5):509–520. doi: 10.1089/hyb.1987.6.509. [DOI] [PubMed] [Google Scholar]
- Alfa M. J., Jay F. T. Distinct domains of recombinant human IFN-gamma responsible for anti-viral effector function. J Immunol. 1988 Oct 1;141(7):2474–2479. [PubMed] [Google Scholar]
- Devos R., Cheroutre H., Taya Y., Degrave W., Van Heuverswyn H., Fiers W. Molecular cloning of human immune interferon cDNA and its expression in eukaryotic cells. Nucleic Acids Res. 1982 Apr 24;10(8):2487–2501. doi: 10.1093/nar/10.8.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Jay E., Rommens J., Pomeroy-Cloney L., MacKnight D., Lutze-Wallace C., Wishart P., Harrison D., Lui W. Y., Asundi V., Dawood M. High-level expression of a chemically synthesized gene for human interferon-gamma using a prokaryotic expression vector. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2290–2294. doi: 10.1073/pnas.81.8.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Kelder B., Rashidbaigi A., Pestka S. A sandwich radioimmunoassay for human IFN-gamma. Methods Enzymol. 1986;119:582–587. doi: 10.1016/0076-6879(86)19079-3. [DOI] [PubMed] [Google Scholar]
- Meager A., Parti S., Barwick S., Spragg J., O'Hagan K. Detection of hybridomas secreting monoclonal antibodies to human gamma interferon using a rapid screening technique and specificity of certain monoclonal antibodies to gamma interferon. J Interferon Res. 1984 Fall;4(4):619–625. doi: 10.1089/jir.1984.4.619. [DOI] [PubMed] [Google Scholar]
- Oda S., Akinaga S., Kumagai A., Inoue A., Nakamizo N., Yoshida H. Generation of a new monoclonal antibody and its application for determination and purification of biologically active human gamma-interferon. Hybridoma. 1986 Winter;5(4):329–338. doi: 10.1089/hyb.1986.5.329. [DOI] [PubMed] [Google Scholar]
- Rinderknecht E., O'Connor B. H., Rodriguez H. Natural human interferon-gamma. Complete amino acid sequence and determination of sites of glycosylation. J Biol Chem. 1984 Jun 10;259(11):6790–6797. [PubMed] [Google Scholar]
- Ziai M. R., Imberti L., Kobayashi M., Perussia B., Trinchieri G., Ferrone S. Distinct functional domains on the recombinant human immune interferon molecule. Cancer Res. 1986 Dec;46(12 Pt 1):6187–6190. [PubMed] [Google Scholar]
- Zu X. W., Jay F. T. The E1 functional epitope of the human interferon gamma is a nuclear targeting signal-like element. Mapping of the E1 epitope. J Biol Chem. 1991 Apr 5;266(10):6023–6026. [PubMed] [Google Scholar]


