Abstract
Objective
To test the hypothesis that routine intraperitoneal drainage is not required after pancreatic resection.
Summary Background Data
The use of surgically placed intraperitoneal drains has been considered routine after pancreatic resection. Recent studies have suggested that for other major upper abdominal resections, routine postoperative drainage is not required and may be associated with an increased complication rate.
Methods
After informed consent, eligible patients with peripancreatic tumors were randomized during surgery either to have no drains placed or to have closed suction drainage placed in a standardized fashion after pancreatic resection. Clinical, pathologic, and surgical details were recorded.
Results
One hundred seventy-nine patients were enrolled in the study, 90 women and 89 men. Mean age was 65.4 years (range 23–87). The pancreas was the tumor site in 142 (79%) patients, with the ampulla (n = 24), duodenum (n = 10), and distal common bile duct (n = 3) accounting for the remainder. A pancreaticoduodenectomy was performed in 139 patients and a distal pancreatectomy in 40 cases. Eighty-eight patients were randomized to have drains placed. Demographic, surgical, and pathologic details were similar between both groups. The overall 30-day death rate was 2% (n = 4). A postoperative complication occurred during the initial admission in 107 patients (59%). There was no significant difference in the number or type of complications between groups. In the drained group, 11 patients (12.5%) developed a pancreatic fistula. Patients with a drain were more likely to develop a significant intraabdominal abscess, collection, or fistula.
Conclusion
This randomized prospective clinical trial failed to show a reduction in the number of deaths or complications with the addition of surgical intraperitoneal closed suction drainage after pancreatic resection. The data suggest that the presence of drains failed to reduce either the need for interventional radiologic drainage or surgical exploration for intraabdominal sepsis. Based on these results, closed suction drainage should not be considered mandatory or standard after pancreatic resection.
Prophylactic drainage of the peritoneal cavity after gastrointestinal surgery has been widely practiced since the mid-1800s, with the dictum of Lawson Tait, the 19th-century British surgeon, “When in doubt, drain,” well known to all surgical trainees. However, data are lacking as to the efficacy of such an approach, and recent reports have suggested that many upper abdominal surgical procedures can be performed safely without drainage. In pancreatic surgery, the use of surgically placed drains is still considered routine after resection. Multiple suction catheters normally are placed in the right and left subhepatic space in relation to both the biliary and pancreatic anastomoses. The intent of these drains is to remove collected blood, bile, chyle, or pancreatic juice. They also may act as a warning of hemorrhage or anastomotic leakage. However, surgically placed drains are not without risk: they have been associated with increased rates of intraabdominal and wound infection, increased abdominal pain, decreased pulmonary function, and prolonged hospital stay. 1–4
Recent studies from both community and academic centers have shown a remarkable reduction in the death rate after pancreatic resection, with rates less than 5% reported. 5–9 Although the death rate has been reduced, a considerable rate of associated complications remains. In a recent retrospective report from our institution, the overall complication rate after pancreaticoduodenectomy was 48%. 5 Infectious complications occurred in 34%, with intraabdominal abscess occurring in 14% of patients. Although the majority of patients had a drain placed in the region of the biliary anastomosis or pancreaticojejunostomy, one cannot determine from this retrospective review the role, if any, that the drains played in the pathogenesis or prevention of these complications. However, a suggestion that surgically placed drainage may be unnecessary or in fact harmful to the patient comes from our observation in a previous retrospective review of a smaller cohort of patients that routine surgical placement of intraabdominal drains may not be necessary after pancreatic resection. 10 In that report, there was no difference in the rate of enteric fistula or abscess or the length of hospital stay whether a drain was placed or not.
As a result of that experience, we designed this prospective, randomized trial to test the hypothesis that intraabdominal drainage may be unnecessary after pancreatic resection.
PATIENTS AND METHODS
This study was performed under a human investigational protocol that was approved and monitored by the Institutional Review Board of Memorial Sloan-Kettering Cancer Center (IRB 97–057). Adult patients presenting to our institution with peripancreatic tumors who were being considered for surgical resection were eligible for inclusion in this study. Patients who had undergone a recent exploration before presentation or who had evidence of intraabdominal sepsis were excluded. After informed consent was obtained, eligible patients were randomized during surgery by the envelope method either to have no drains placed or to have closed suction intraabdominal drains placed on completion of their pancreatic resection.
Experienced pancreatic surgeons performed all of the surgical resections. In patients randomized to drainage, Jackson-Pratt closed suction drains (7 mm; Baxter Health Care Corp., Deerfield, IL) were placed through separate skin incisions in a standardized fashion into the right and left upper quadrants of the abdomen. The drains were placed in relation to the pancreatic and biliary anastomoses. Clinical, demographic, pathologic, and surgical details were recorded. Biochemical and hematologic data were also recorded. For patients randomized to drainage, drain output and amylase content were measured daily. In uncomplicated cases, if no increased amylase level was noted, drains were routinely removed at the discretion of the attending surgeon, regardless of drainage volume, between postoperative days 5 and 7. Complications were prospectively recorded by independent observers and follow-up was obtained. Routine abdominal imaging studies were not performed. A pancreatic fistula was defined when the drain output on postoperative day 5 or more exceeded 30 mL and the amylase level was greater than 150 IU/L and/or three times greater than the serum value. A biliary or enterocutaneous fistula was defined as the presence of bile or enteric drainage that persisted after postoperative day 5. An intraabdominal abscess was defined as a collection associated with fever and a positive culture requiring either surgical or radiologic drainage. Other infectious complications were classified as bacteremia, cholangitis, colitis, pneumonia, superficial or deep wound, or urinary tract infections. The need for either surgical or interventional radiologic procedures in the postoperative period was recorded. Follow-up was obtained by personal contact with the patient or responsible physician. Deaths occurring within 30 days of the procedure were considered surgical deaths. Complications associated with the index admission were tracked for 3 months.
The primary endpoint of this study was the incidence of postoperative complications. The statistic of interest was the rate of postoperative complications in patients who received no drain versus drain at the time of surgery, measured by the odds ratio. This association was examined across a variety of stratifications to control for potentially confounding prognostic factors in addition to the presence of a drain. The hypothesis of a heterogenous odds ratio across strata was first tested. If this hypothesis was not rejected, estimates and 95% confidence intervals were provided for the common odds ratios under each choice of stratification (data not shown). In addition, several secondary postoperative categoric or continuous endpoints were examined between the drain and no-drain groups and analyzed using the Fisher exact test, the Mann-Whitney test, or the two-sided t test. Analyses were carried out using StatXact (Cytel Software Corp., Cambridge, MA) and sPlus (Mathsoft Inc., Seattle, WA). Significance was defined as P < .05.
RESULTS
During the study period, 379 patients underwent a pancreatic resection at our institution. Of these, 179 were enrolled in this study. There were 90 female and 89 male patients. Median age was 68 years (range 23–87).
The pancreas was the tumor site in 142 (79%) patient. Most of the lesions were present in the head of the gland (n = 102) compared with the pancreatic body or tail (n = 40). The ampulla of Vater (n = 24), duodenum (n = 10), and distal common bile duct (n = 3) accounted for the remainder of the lesions. The predominant histologic diagnosis was adenocarcinoma (n = 120, 67%).
A biliary endoprosthesis was present in 52 (29%) patients at the time of surgery. Ten patients had received radiation or chemotherapy before presentation. A comorbid medical condition was noted in 153 patients (drain group, n = 67; no-drain group, n = 86). The most common condition was hypertension (n = 66). Diabetes was present in 34 patients (drain group, n = 13; no-drain group, n = 21).
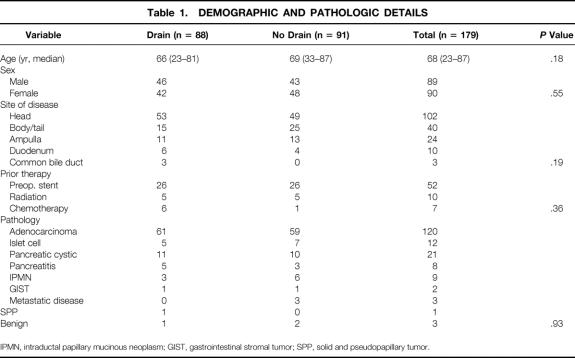
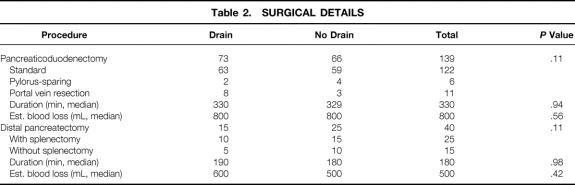
Preoperative antibiotics were given in 177 patients (99%). A second-generation cephalosporin was the most commonly administered antibiotic. A pancreaticoduodenectomy was performed in 139 patients and a distal pancreatectomy in 40 patients. Most patients underwent a pancreaticoduodenectomy that included a partial gastrectomy with a standard enteric loop reconstruction for the biliary and pancreatic anastomoses. Six patients had a pylorus-sparing procedure, and a portal vein resection was performed in 11 patients (8%). A spleen-preserving procedure was performed in 15 of the 40 patients (38%) who underwent a distal pancreatectomy. Laparoscopic staging was completed in 152 patients (85%) before their resection. Median surgical time and estimated blood loss for patients who underwent a pancreaticoduodenectomy were 330 minutes and 800 mL, compared with 180 minutes and 500 mL for those undergoing a distal pancreatectomy. Intraabdominal drains were placed in 88 patients, and 91 patients had no drains placed. Demographic, surgical, and pathologic details were similar between both groups (Tables 1 and 2). Seventy-eight percent of patients were treated by two surgeons. Median drain volume and amylase values are shown in Figure 1.
Table 1. DEMOGRAPHIC AND PATHOLOGIC DETAILS
IPMN, intraductal papillary mucinous neoplasm; GIST, gastrointestinal stromal tumor; SPP, solid and pseudopapillary tumor.
Table 2. SURGICAL DETAILS
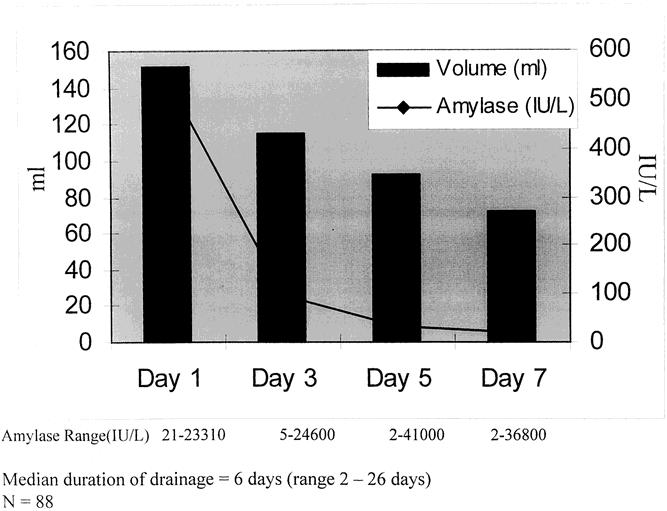
Figure 1. Median drain volume and drain amylase level by day (day 1 vs. days 3, 5, and 7, P < .05).
Median hospital stay for all patients was 9 days. The overall 30-day death rate was 2% (n = 4). Complications occurred in 123 patients (68%). In 107 patients (59%), this complication was recognized during the initial hospital stay (Table 3). A total of 258 complications were recorded in these patients (Table 4). Infectious complications accounted for 24% of the total complications (n = 61). In the drained group, 11 patients (12.5%) developed a pancreatic fistula. There was no significant difference in the complication rate or the number or type of complications between the drained and no-drain groups. However, if we examine the incidence of intraabdominal abscesses/collections and fistulas, a significant increase was noted in the drained group (Table 5) (P < .02). Variables such as hyperbilirubinemia, the presence of a preoperative biliary stent, or perioperative blood transfusion were not significant with regard to overall postoperative complications in either group. For patients without complications, the median length of hospital stay was 8 days (range 5–11); for those with a complication, it was 11 days (range 3–34;P < .001). Patients in the drain group with a pancreatic fistula had a median length of stay of 14 days (range 6–34).
Table 3. IN-HOSPITAL DEATHS AND COMPLICATIONS
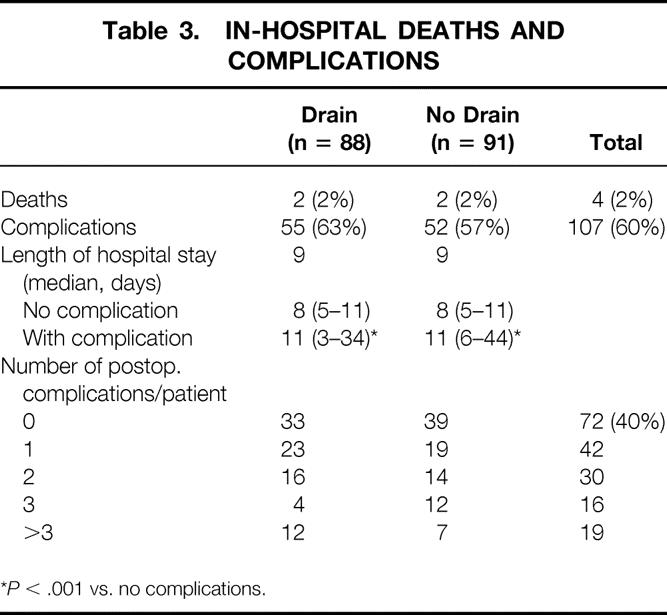
*P < .001 vs. no complications.
Table 4. IN-HOSPITAL COMPLICATIONS (INITIAL STAY)
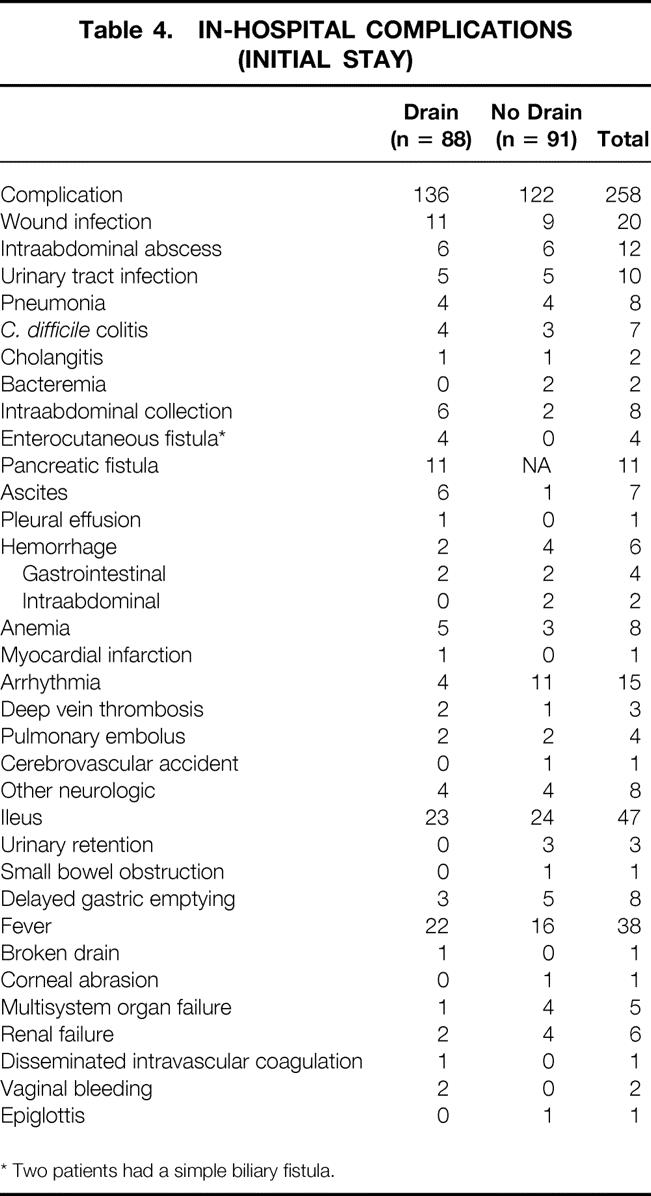
* Two patients had a simple biliary fistula.
Table 5. INCIDENCE OF INTRAABDOMINAL COLLECTIONS AND FISTULAS
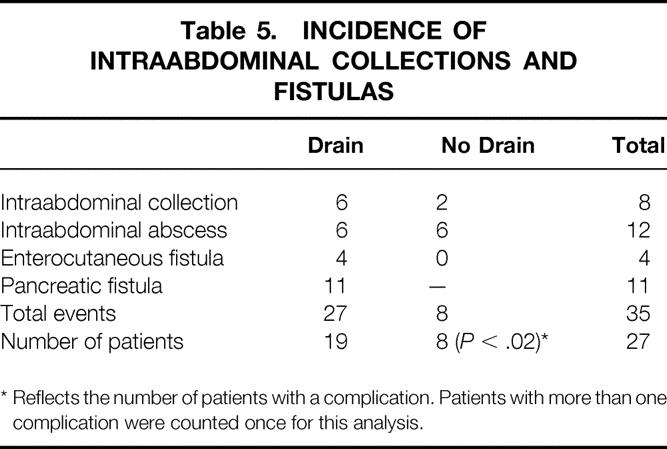
* Reflects the number of patients with a complication. Patients with more than one complication were counted once for this analysis.
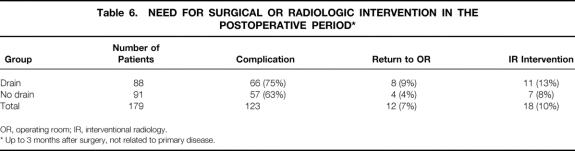
Reoperation occurred in 12 patients (7%) (Table 6). Indications for this surgical procedure are listed in Table 7. Interventional radiologic intervention was required in 18 (10%) patients. Drainage of an intraabdominal collection was the main indication for intervention. Of the 13 patients who required computed tomography-guided catheter drainage, 8 (62%) were in the drain group.
Table 6. NEED FOR SURGICAL OR RADIOLOGIC INTERVENTION IN THE POSTOPERATIVE PERIOD*
OR, operating room; IR, interventional radiology.
* Up to 3 months after surgery, not related to primary disease.
Table 7. INDICATION FOR INTERVENTIONAL RADIOLOGIC INTERVENTION OR REEXPLORATION
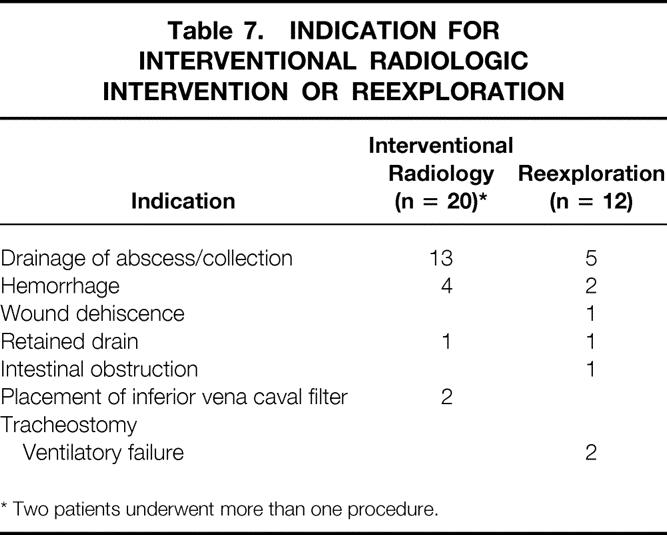
* Two patients underwent more than one procedure.
Sixteen patients (9%) had their first complication recorded after discharge from the hospital. Twenty-nine patients were readmitted to the hospital within 3 months after discharge for reasons other than progression of their disease. Most of these admissions occurred in the drained patients (n = 19 vs. 10, P = .07). The median duration of this readmission was also longer for the drained patients (10 vs. 5 days, P < .05).
DISCUSSION
Since the initial description of a pancreatic resection by Kausch in 1912 and its subsequent modification by Whipple in 1935, surgical placement of drains has been a standard component of the procedure. Multiple drains are often placed on the basis that leakage of blood, bile, chyle, or pancreatic juice may occur and that the potential adverse consequences of such leakage can be lessened by drainage. Unfortunately, the principle of drainage is not based on any scientific data, and in general the prophylactic value of drains in abdominal surgery remains controversial.
Several well-constructed, prospective studies have examined this question and failed to show any benefit from surgically placed closed suction drainage. After a variety of intraabdominal procedures such as colorectal resection, 4,11,12 closure of perforated duodenal ulceration, 3 open or laparoscopic cholecystectomy, 1,13 radical hysterectomy and pelvic lymphadenectomy, 14 or retroperitoneal lymphadenectomy, 15 there appears to be no statistical difference in the rate of complications between patients who are drained and those who are not, suggesting at best that routine placement of intraperitoneal drains is unnecessary. In fact, many of the studies imply that peritoneal drainage may be associated with adverse effects.
Critics have argued that these studies cannot be applied to hepatobiliary and pancreatic surgery because the potential ramification of an undrained collection could be devastating to the patient. Even in this area, however, existing data appear to be consistent with the experience cited for other abdominal procedures. Franco et al, 16 in a prospective nonrandom fashion, examined the hospital course of 61 patients who underwent a liver resection without drainage. They concluded that an 8% complication rate suggested that the routine use of drains was unnecessary. Similar results were reported by Belghiti et al, 17 who prospectively randomized 81 patients either to have surgically placed closed suction drainage on completion of a hepatic resection or to have no drains placed. The overall complication rates appeared similar between groups. However, postoperative ultrasonography showed in patients who had a minor hepatic resection that there was a significantly greater rate of subphrenic collections in the drain versus the no-drain group.
Our experience at Memorial Hospital mirrors this experience. Between 1992 and 1994, 120 patients were randomized after hepatic resection to receive closed suction drainage or not. 18 There was no significant difference between groups with regard to the rates of death and complications, the length of hospital stay, or the need for subsequent percutaneous drainage. The three patients who developed an infected subhepatic collection were all in the drain group. Although the study was too small to determine whether drainage was truly responsible for these infected collections, it is consistent with an earlier prospective study of intraperitoneal drainage after open cholecystectomy reported by Monson et al. 19
Before the current study, there were no randomized studies examining the role of intraperitoneal drainage after pancreatectomy. Although the death rate related to pancreatic resection has diminished in recent years, there remain considerable associated complications. Geer and Brennan 20 reviewed the Memorial Hospital experience with pancreatic resection for adenocarcinoma. During the time period of their study, 146 patients underwent pancreatic resection. Major complications (abscess, hemorrhage, fistulas) were noted in 27% of patients, with minor complications such as wound infection or ileus occurring in a further 10%. Intraabdominal abscess was the most common complication, occurring in 12%. Similar results have been reported by others. More recently, Povoski et al 5 updated our institutional experience and noted a 48% complication rate in a retrospective review of 240 patients undergoing pancreaticoduodenectomy. Infectious complications were noted in 34% of patients. A drain in relation to the pancreaticojejunostomy was placed during surgery in 178 (74%) patients. Drainage did not appear to be a significant variable with regard to either the overall or infectious complication rate.
In an earlier retrospective report, Jeekel 21 described 22 patients who underwent a pancreaticoduodenectomy without drainage. After surgery, an intraabdominal abscess was diagnosed in three patients, all of whom were treated without surgery, leading to the suggestion that drainage could be omitted without producing clinically significant complications. Heslin et al, 10 from our institution, reviewed a cohort of patients entered in a prospective trial to assess the value of early enteral feeding. In this retrospective analysis they identified 89 patients who underwent a pancreaticoduodenectomy, 51 of whom had drains placed. Complications occurred in 38 patients (43%). No statistical difference was seen in the rate or type of complication between patients who had a drain placed and those who did not.
Our current study was designed to test the hypothesis that the presence or absence of a surgically placed drain has no impact on the rate of either death or complications after resection. The overall 30-day death rate was 2%, with no difference between the groups. This death rate is similar to results recently reported from other specialized units. 9,22 Our data show that the majority of patients had at least one complication recorded during their initial hospital stay. Most of these complications were minor (see Table 4). An infectious complication accounted for 24% of the total. Preoperative variables such as the presence of a biliary stent or jaundice did not appear to be significant determinants with regard to complications. This differs somewhat from an earlier retrospective analysis from our unit, which noted that preoperative biliary stenting was associated with an increased rate of intraabdominal infection, but probably reflects the decreased prevalence of preoperative stenting in the present series of 37% (52/139 patients) versus 53% (126/240 patients) and a change to more appropriate antibiotic prophylaxis. In the current study, all but two patients received preoperative broad-spectrum antibiotic coverage for biliary and enteric organisms, in contrast to our practice in the past, in which antibiotic coverage was variable.
As one would expect, a complication resulted in a significant increase in the median hospital stay (8 vs. 11 days, P < .001). Although there was no significant difference in the overall rate or type of complication whether intraperitoneal drains were present or not, there was a significant increase in the number of patients in the drained group who developed an intraabdominal abscess, collection, or fistula versus those who were not drained (P < .02). This supports the suggestion from other prospective trials that drains may in fact be harmful.
Computed tomography-guided drainage of an intraabdominal collection was required in 13 patients, 8 of whom had been randomized to peritoneal drainage. This at first appears counterintuitive because one of the reasons given for drainage is to prevent the accumulation of “fluid” in the surgical bed. However, it is consistent with the data reported by Monson et al, 19 who by using ultrasonography showed a statistically significant increase in subhepatic collections in the patients drained after cholecystectomy versus those in whom no drainage catheter was placed (18% vs. 1.8%, P < .01).
A pancreatic fistula was noted in 12.5% of patients randomized to the drain group. Yeo et al 23 from Johns Hopkins recently reported a 10% rate in patients enrolled in a prospective trial examining the efficacy of perioperative octreotide in reducing complications after pancreaticoduodenectomy. In this well-controlled study, prophylactic octreotide had no impact on the rate of complications. In our study, although no patient in the drained group with a fistula died, a fistula was associated with an increased rate of complications, need for computed tomographic or surgical intervention, and increased median hospital stay (14 days vs. 8 days for patients with an uncomplicated course, P < .001). In addition, 6 of 11 patients with a fistula required readmission to the hospital for treatment of associated complications. Overall, 13% of all complications in this study were first noted after discharge from the index admission. Twenty-nine patients required readmission to the hospital within 3 months, 19 of them in the drained group.
In summary, the results of this prospective randomized trial failed to show that surgically placed intraperitoneal drains reduce the rate of either death or complications associated with pancreatic resection. These results are consistent with those of other prospective trials that have examined the role of drainage after a variety of other abdominal procedures, suggesting that the routine placement of drains is unnecessary and may indeed be harmful to the patient.
Acknowledgments
The authors thank the staff of the Gastric and Mixed Tumor and Hepatobiliary Services, who facilitated this study, and Diane Bassman for her help in preparing the manuscript.
Discussion
Dr. Keith D. Lillemoe (Baltimore, Maryland): I would like to congratulate Drs. Conlon, Brennan, and colleagues for their efforts in conducting this important prospective randomized trial and compliment Dr. Conlon for his excellent presentation and well written manuscript.
The authors have demonstrated that the use of peritoneal drains does not lessen perioperative morbidity and mortality following pancreatic resection and they suggest in their secondary end-point analysis that certain intra-abdominal complications may actually be increased with the use of drains. I would like to ask Dr. Brennan a few questions.
First a technical question. What was the size, number, and specific location of the drains with respect to the pancreatic and biliary anastomoses? Your incidence of patients developing postoperative collections in the drain group makes me question whether these drains were optimally utilized. We tend to use two large quarter-inch suction drains immediately adjacent to both of the key anastomoses, and although our fistula rates are similar, we do not seem to see the same incidence of collections.
Second, although your fair randomization makes these points unlikely to affect your conclusion, what was the incidence of fistula or collection related to the texture of the gland? Our recent octreotide study from Hopkins demonstrated the incidence of pancreatic anastomotic complications in hard texture glands was almost zero while in soft glands it approached 20%. Do soft glands have a higher rate of anastomotic complications? If so, what is your current practice with respect to drains in such patients?
Similarly, was there a difference in these complications in patients who underwent the Whipple versus the distal pancreatectomy, noting that 60% of your distal resections were in the no-drain group.
By your own definition, there were no pancreatic fistulas in the no-drain group. But when these patients did require drainage of collections, was there evidence of infection or pancreatic leak based on the aspiration in the no-drain group?
How were your postoperative pancreatic fistulas managed? This is important because it appears that a number of these patients required a late hospital readmission and probably a procedure for drainage of a collection or an abscess. If they did, were they considered to have had two complications when you did your analysis of specific intra-abdominal complications? In other words, did you ‘double-dip‘ in this analysis?
Finally, what was the nature of the four enterocutaneous fistulas? Were these at an anastomotic site or elsewhere and how exactly were the drains considered to have contributed to the development of these complications?
Presenter Dr. Murray F. Brennan (New York, New York): Dr. Lillemoe, thank you. I appreciate your comments but you are one of the big guns in this area!
For the technical questions, we used Jackson-Pratt drains placed at the site of both the choledochojejunal and the pancreaticojejunal anastomosis. As many of you appreciate in the audience, the pressure generated by these closed suction drains can be very significant – create in excess of 100 millimeters of mercury, and I do believe that it is possible for a suction of that degree to create fistula formation.
Yes, we agree with your very nice analysis, as I think most people do who practice this, the risk complication from a soft gland is much more significant than a firm and hard gland. However, this is a prospective randomized trial and we could not show any difference between the distribution between both soft and hard glands in the trial. As we believe the data in the prospective randomized trial, we have not changed our approach to the placement of drains in the soft glands.
You are correct that 139 of these patients had a pancreaticoduodenectomy. Again, it was a randomized trial, there was equal distribution between the two, by procedure.
You are absolutely correct that a collection that has to be subsequently drained either operatively or by interventional radiology is almost always associated with at least some degree of minor leak, or certainly associated with infection, often, as previously published, with identical organisms to that contained in the bile culture sample at the time of resection.
The fistula question I think I answered. I believe that drains can contribute to fistula formation, and this will support but not prove that.
Dr. Andrew L. Warshaw (Boston, Massachusetts): I would like to commend the Memorial group on this randomized trial, not only for the quality with which it was carried out but also for your courage in actually undertaking the trial.
Your findings are really quite disturbing to me, and the request to comment on them is a challenge – mostly because the study challenges my closely held beliefs that the pancreatic anastomosis or closure is at much greater risk of breakdown and leak than liver resections, bowel anastomoses, or retroperitoneal node dissections.
You have concluded that drains didn’t help, and, God forbid, maybe they were even harmful. 9% of the drained patients returned to the operating room versus 4% of those who were not drained. 13% required percutaneous intervention in the drain group and 8% not. Those data are terribly bothersome, even though the complication rate does seem a little high. Although not statistically significant, pancreatic fistulas, intra-abdominal abscesses, and other fluid collections were equal or higher in the drained group.
I would like to know more about the non-abscess collections that you referred to. Were these simple fluid collections that could have been ignored and therefore been discarded from your database? You mentioned earlier that the drains produce a high negative pressure (much higher than I had believed). Do you think the suction caused injury? Is there introduction of infection along the two-way street of a drain? Or does the presence of the drain prevent apposition of tissues which might seal the anastomosis?
When did the fistulas appear? In our experience with pancreatic anastomotic breakdown, the pancreaticojejunocutaneous fistulas tend to occur after day five. Perhaps mistakenly we have left drains in at least that long in order that they be there when needed. Maybe we are exactly wrong. Maybe we should take the drains out at one or two days, once we see no evidence of bile or enteric leak but before the drains can cause trouble.
What was the cause of the readmissions? You combined abscesses and ‘collections.‘ Among those 16 patients who had new complications after discharge, how many were uninfected collections? How many were amylase-rich fluid collections (pseudocysts) versus harmless fluid collections that might have been spontaneously resorbed? Was the pattern of complications different in the drained versus undrained groups?
This study has made me rethink our practices. We will have to consider a follow-up prospective trial of our own to test some of the questions you have provoked.
Dr. Murray F. Brennan: Thank you, Dr. Warshaw. Dr. Lillemoe would want me to concede that you are certainly Big Gun Number 2.
It is important to emphasize in this trial that all the complications are evaluated by an independent observer. So the surgeon has no role in defining those. I think that is important because it gives you the most accurate data of the true complication rate. Again, I also think it is important that complications include the patients that are re-admitted. The evaluation is all done by an independent observer.
You asked the question of what is a collection and what is an abscess. That is difficult. It is obviously easier from a CT scan. If there is gas in a collection, that is an abscess. However, it is not easy to define a collection that is symptomatic, pain, delayed gastric emptying, that may be a contained pancreatic leak that is not contaminated. I know of no way to be sure of that other than to either re-explore the patient or place a needle into the collection. Obviously, for a late presentation, we would aspirate, then drain, and if it was infected we would leave the drain in; if it was not infected and the amylase level was low, then, we would expeditiously remove the drain.
I do not know for certain that drains do cause problems. Our data suggests they contribute to problems. Yes, I do think that pressure is an issue. And yes, I do think that it prevents tissue apposition. Whether or not it is a two-way street, as has been found in essentially all other drains, including closed suction drains, I just do not know.
Obviously in a cancer hospital we have significant experience with intraperitoneal treatment with chemotherapy and have certainly been surprised at how difficult that is to do within a week of the operation.
It is important to emphasize that 44 of the patients in the drain group still had the drain in place at seven days. So I do not think it was premature removal of the drain that could have contributed. Yes, the readmissions are for delayed gastric emptying. Those that have fever and a collection that contains gas are abscesses.
Dr. Johannus Jeekel (Rotterdam, Netherlands): I think it is an excellent initiative to study certain things that we do for decades or centuries and consider to be normal. Since the time that pancreatic resection started in 1898 – I think most surgeons will drain. In this study the question of drainage was investigated in a prospective randomized manner.
My question, though, is can you really conclude from this study that a drain should not be used? Your hypothesis was and the conclusion is that there is no reduction in morbidity with a drain. How many patients did you need for this hypothesis? Is this conclusion based upon statistical significant data? Did you have enough patients included to show statistical evidence?
It may be your leakage rate is lower than the average surgeon will have. What was exactly your incidence of pancreaticojejunostomy breakdown? It may be that surgeons who have a higher incidence of leakage rate would need the drain.
Dr. Murray F. Brennan: Thank you, Dr. Jeekel. We have now completed prospective randomized trials that relate to the management of pancreatic malignancy, one on total parental nutrition, one on enteral nutrition, and now one on drainage. In all of those, there was a null hypothesis, i.e. the routine use of either intravenous, enteral nutrition or drainage would not improve outcome. And I believe that null hypothesis has been proven.
You asked about the assumptions. We assumed we would need 140 patients randomized. We were concerned at the interim analysis that there was a minimal maldistribution of distal pancreas without analyzing the data. So we took it to 179, which would comfortably exclude – missing a 10% benefit to drainage. As the results were all in the opposite direction, the likelihood that this is a statistical aberration is extremely small, and would approximate zero.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association, April 26–28, 2001, the Broadmoor Hotel, Colorado Springs, Colorado
Funded in part by the Milton and Bernice Stern Fund for Pancreatic Research.
Correspondence: Murray F. Brennan, MD, FACS, Department of Surgery, 1275 York Ave., New York, NY 10021.
E-mail: brennanm@mskcc.org
Accepted for publication April 26, 2001.
References
- 1.Monson JR, Guillou PJ, Keane FB, et al. Cholecystectomy is safer without drainage: the results of a prospective, randomized clinical trial. Surgery 1991; 109: 740–746. [PubMed] [Google Scholar]
- 2.Benedetti-Panici P, Maneschi F, Cutillo G, et al. A randomized study comparing retroperitoneal drainage with no drainage after lymphadenectomy in gynecologic malignancies. Gynecol Oncol 1997; 65: 478–482. [DOI] [PubMed] [Google Scholar]
- 3.Pai D, Sharma A, Kanungo R, et al. Role of abdominal drains in perforated duodenal ulcer patients: a prospective controlled study. Aust NZ J Surg 1999; 69: 210–213. [DOI] [PubMed] [Google Scholar]
- 4.Merad F, Yahchouchi E, Hay JM, et al. Prophylactic abdominal drainage after elective colonic resection and suprapromontory anastomosis: a multicenter study controlled by randomization. French Associations for Surgical Research. Arch Surg 1998; 133: 309–314. [DOI] [PubMed] [Google Scholar]
- 5.Povoski SP, Karpeh MS Jr, Conlon KC, et al. Association of preoperative biliary drainage with postoperative outcome following pancreaticoduodenectomy. Ann Surg 1999; 230: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohn TA, Yeo CJ, Cameron JL, Pitt HA, Lillemoe KD. Do preoperative biliary stents increase postpancreaticoduodenectomy complications? J Gastrointest Surg 2000; 4: 258–267. [DOI] [PubMed] [Google Scholar]
- 7.Akhtar K, Perricone V, Chang D, Watson RJ. Experience of pancreaticoduodenectomy in a district general hospital. Br J Surg 2000; 87: 362–373. [DOI] [PubMed] [Google Scholar]
- 8.Grobmyer SR, Rivadeneira DE, Goodman CA, et al. Pancreatic anastomotic failure after pancreaticoduodenectomy. Am J Surg 2000; 180: 117–120. [DOI] [PubMed] [Google Scholar]
- 9.Rosemurgy AS, Bloomston M, Serafini FM, et al. Frequency with which surgeons undertake pancreaticoduodenectomy determines length of stay, hospital charges, and in-hospital mortality. J Gastrointest Surg 2001; 5: 21–26. [DOI] [PubMed] [Google Scholar]
- 10.Heslin MJ, Harrison LE, Brooks AD, et al. Is intra-abdominal drainage necessary after pancreaticoduodenectomy? J Gastrointest Surg 1998; 2: 373–378. [DOI] [PubMed] [Google Scholar]
- 11.Sagar PM, Couse N, Kerin M, et al. Randomized trial of drainage of colorectal anastomosis. Br J Surg 1993; 80: 769–771. [DOI] [PubMed] [Google Scholar]
- 12.Merad F, Hay JM, Fingerhut A, et al. Is prophylactic pelvic drainage useful after elective rectal or anal anastomosis? A multicenter controlled randomized trial. French Association for Surgical Research. Surgery 1999; 125: 529–535. [PubMed] [Google Scholar]
- 13.Hawasli A. Laparoscopic cholecysto-jejunostomy for obstructing pancreatic cancer: technique and report of two cases. J Laparoendosc Surg 1992; 2: 351–355. [DOI] [PubMed] [Google Scholar]
- 14.Patsner B. Closed-suction drainage versus no drainage following radical abdominal hysterectomy with pelvic lymphadenectomy for stage IB cervical cancer. Gynecol Oncol 1995; 57: 232–234. [DOI] [PubMed] [Google Scholar]
- 15.Benedetti-Panici P, Maneschi F, Cutillo G, et al. A randomized study comparing retroperitoneal drainage with no drainage after lymphadenectomy in gynecologic malignancies. Gynecol Oncol 1997; 65: 478–482. [DOI] [PubMed] [Google Scholar]
- 16.Franco D, Karaa A, Meakins JL, et al. Hepatectomy without abdominal drainage. Results of a prospective study in 61 patients. Ann Surg 1989; 210: 748–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belghiti J, Kabbej M, Sauvanet A, et al. Drainage after elective hepatic resection. A randomized trial. Ann Surg 1993; 218: 748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fong Y, Brennan MF, Brown K, et al. Drainage is unnecessary after elective liver resection. Am J Surg 1996; 171: 158–162. [DOI] [PubMed] [Google Scholar]
- 19.Monson JR, MacFie J, Irving H, et al. Influence of intraperitoneal drains on subhepatic collections following cholecystectomy: a prospective clinical trial. Br J Surg 1986; 73: 993–994. [DOI] [PubMed] [Google Scholar]
- 20.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993; 165: 68–72. [DOI] [PubMed] [Google Scholar]
- 21.Jeekel J. No abdominal drainage after Whipple’s procedure. Br J Surg 1992; 79: 182. [DOI] [PubMed] [Google Scholar]
- 22.Yeo CJ, Cameron JL, Sohn TA, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg 1999; 229: 613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeo CJ, Cameron JL, Lillemoe KD, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg 2000; 232: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]