Abstract
Objective
To analyze resectability and survival in patients with hilar cholangiocarcinoma according to a proposed preoperative staging scheme that fully integrates local, tumor-related factors.
Summary Background Data
In patients with hilar cholangiocarcinoma, long-term survival depends critically on complete tumor resection. The current staging systems ignore factors related to local tumor extent, preclude accurate preoperative disease assessment, and correlate poorly with resectability and survival.
Methods
Demographics, results of imaging studies, surgical findings, pathology, and survival were analyzed prospectively in consecutive patients. Using data from imaging studies, all patients were placed into one of three stages based on the extent of ductal involvement by tumor, the presence or absence of portal vein compromise, and the presence or absence of hepatic lobar atrophy.
Results
From March 1991 through December 2000, 225 patients were evaluated, 77% of whom were seen and treated within the last 6 years. Sixty-five patients had unresectable disease; 160 patients underwent exploration with curative intent. Eighty patients underwent resection: 62 (78%) had a concomitant hepatic resection and 62 (78%) had an R0 resection (negative histologic margins). Negative histologic margins, concomitant partial hepatectomy, and well-differentiated tumor histology were associated with improved outcome after all resections. However, in patients who underwent an R0 resection, concomitant partial hepatectomy was the only independent predictor of long-term survival. Of the 9 actual 5-year survivors (of 30 at risk), all had a concomitant hepatic resection and none had tumor-involved margins; 3 of these 9 patients remained free of disease at a median follow-up of 88 months. The rates of complications and death after resection were 64% and 10%, respectively. In the 219 patients whose disease could be staged, the proposed system predicted resectability and the likelihood of an R0 resection and correlated with metastatic disease and survival.
Conclusion
By taking full account of local tumor extent, the proposed staging system for hilar cholangiocarcinoma accurately predicts resectability, the likelihood of metastatic disease, and survival. Complete resection remains the only therapy that offers the possibility of long-term survival, and hepatic resection is a critical component of the surgical approach.
Cholangiocarcinoma is a rare disease, accounting for less than 2% of all human malignancies. 1 Although the entire biliary tree is potentially at risk, tumors involving the biliary confluence or the right or left hepatic ducts (hilar cholangiocarcinoma) are most common and account for 40% to 60% of all cases. 2–6 Meaningful clinical experience in managing hilar cholangiocarcinomas has been limited to a few referral centers because of the infrequency with which they are encountered.
Although resection has long been recognized as the most effective therapy for hilar cholangiocarcinoma, 7 the importance of partial hepatectomy and the willingness of surgeons to use it routinely are relatively recent developments. 2,8–10 Many clinical series extend over a prolonged period, often greater than 20 years. 4,5,9,11,12 As a result, these reports lack a uniform approach to diagnosis, assessment of disease extent, and resection, and the results are therefore difficult to interpret. Further, most studies originate from surgical departments and tend to focus on surgical findings and results and often do not provide a full accounting of all patients seen.
Long-term survival in patients with hilar cholangiocarcinoma depends critically on complete tumor resection. 2,10 In the absence of widespread disease, the likelihood of achieving a complete resection requires examination of all factors related to local tumor extent, which increasingly has become possible with noninvasive imaging studies. 13,14 Tumor location and extent within the biliary tree, as provided by the Bismuth-Corlette classification system, 15,16 is only one component. Additional factors that must be addressed relate to radial tumor growth and its impact on adjacent structures, specifically portal venous involvement and consequent hepatic lobar atrophy. Both the modified Bismuth-Corlette and the American Joint Committee on Cancer 17 staging systems fail to account for all of these local, tumor-related factors, which frequently influence therapy. We have shown previously that a preoperative staging system that accounts fully for local tumor-related factors accurately predicts resectability and correlates with survival. 2
The present study represents an analysis of all patients with hilar cholangiocarcinoma seen and treated at a single institution during a 9-year period. The organizational structure of the hepatobiliary program at Memorial Sloan-Kettering Cancer Center (MSKCC) allows a multidisciplinary review of all patients, regardless of disease stage. Thus, a full accounting of all patients, including those with unresectable tumors, is possible. The relatively short time interval ensures homogeneity with respect to disease assessment and surgical approach. This study also proposes a preoperative staging system, based on imaging data and modified from a previous report, 2 that stratifies patients into treatment groups with predictable resectability rates and survival.
METHODS
All patients with a diagnosis of hilar cholangiocarcinoma evaluated and treated at MSKCC since 1991 were identified from a database maintained by the Hepatobiliary Service of the Department of Surgery. Clinical, radiologic, histopathologic, and survival data were entered prospectively and analyzed retrospectively. Only patients with biliary adenocarcinoma arising from the biliary confluence or the right or left main hepatic ducts were included. Patients with tumors originating in the proximal common hepatic duct were included if the tumor extended to the biliary confluence. Patients with diffuse or multifocal cholangiocarcinoma were included, provided that the right or left hepatic ducts or the biliary confluence was involved. Patients with papillary cholangiocarcinoma were included if the tumor arose from a base within the proximal bile ducts, as defined above.
Patient Evaluation
Initial patient assessment included a complete history and physical examination, assessment of general health, and review of the available imaging studies and histopathology. Most patients were referred after at least a partial radiographic evaluation had been completed, usually consisting of a computed tomographic (CT) scan and some form of direct cholangiography (endoscopic retrograde cholangiopancreatography or percutaneous translepatic cholangiogram. After referral, further evaluation of tumor extent within the biliary tree and assessment of possible vascular involvement or metastatic disease were performed with magnetic resonance cholangiopancreatography (MRCP) and duplex ultrasonography, which are currently the preferred studies. More recently, CT angiography has been used in addition to MRCP and ultrasound. Since 1997, staging laparoscopy has been used with greater frequency and was used in 55 patients in this series. 18 All cases were reviewed at a multidisciplinary hepatobiliary disease management conference.
Patients considered to have potentially resectable tumors (Table 1) underwent further evaluation with a screening chest radiograph, routine laboratory studies, and assessment by an anesthesiologist. A formal cardiopulmonary evaluation was obtained in all patients older than age 65 and in any patient with comorbid medical conditions suggesting an increased surgical risk. Complications related to biliary tract obstruction or previous biliary intervention (i.e., cholangitis, pancreatitis, biliary injury) were treated accordingly before surgery. In some patients, this required replacement of existing biliary drainage catheters, placement of new catheters, percutaneous drainage of fluid collections, or a prolonged course of antibiotics. Because of the association between postoperative infective complications and biliary prostheses, 19,20 jaundiced patients not previously stented and with no evidence of cholangitis did not undergo routine biliary intubation, provided that surgery was anticipated within approximately 1 week.
Table 1. CRITERIA OF UNRESECTABILITY

* Metastatic disease to peripancreatic, periduodenal, celiac, superior mesenteric, or posterior pancreaticoduodenal lymph nodes was considered to represent disease not amenable to a potentially curative resection. By contrast, metastatic disease to cystic duct, pericholedochal, hilar, or portal lymph nodes (i.e., within the hepatoduodenal ligament) did not necessarily constitute unresectability.
All pathologic material from the referring institution was reexamined by pathologists at MSKCC. In patients with obviously advanced disease or in those unfit for surgery, biopsy confirmation of the diagnosis was performed, if not done previously, and plastic biliary drainage catheters were converted to Wallstents. However, in patients being considered for resection and in whom the diagnosis of hilar cholangiocarcinoma was consistent with the radiographic findings, histologic confirmation of malignancy was not pursued before surgery.
Preoperative Tumor Staging
Using imaging data, all patients were staged according to a proposed clinical T-staging system (Table 2). This staging system, a modification of a previously reported scheme, 2 classifies tumors according to three factors related to local tumor extent: the location and extent of bile duct involvement (according to the Bismuth-Corlette system), 15,16 the presence or absence of portal venous invasion, and the presence or absence of hepatic lobar atrophy. The first 90 patients in the series had been staged according to the original system 2 and were retrospectively converted to the current system, which comprises three stage groupings rather than four and represents a simple combining of two stages from the earlier format. The remaining patients were all staged prospectively after thorough review of all imaging studies.
Table 2. PROPOSED T-STAGE CRITERIA FOR HILAR CHOLANGIOCARCINOMA
The extent of biliary and portal venous involvement by tumor was assessed mainly with duplex ultrasound and MRCP. Direct cholangiography was also used, mainly in the first few years of the study; it is not performed solely for this purpose at present. Portal vein involvement was considered to be present if the tumor contacted and either distorted or narrowed the vein or if the vein was encased or occluded. Hepatic lobar atrophy was considered to be present if cross-sectional imaging (CT or MRCP) showed a small, often hypoperfused lobe with crowding of dilated intrahepatic bile ducts 2,21 (Fig. 1).

Figure 1. Axial magnetic resonance cholangiopancreatography images of a patient with hilar cholangiocarcinoma. The bile ducts appear white. The left liver is shrunken; its medial extent is indicated by the white arrows. The bile ducts in the left liver are dilated and crowded (white arrowheads), with little interposed liver tissue. The tumor is indicated by the black arrow (C).
Surgical Technique
In all patients who underwent a potentially curative resection, a standardized surgical approach was used that has been described previously. 2,22 Full exploration was performed to exclude disseminated disease. Exposure of the biliary confluence and assessment for vascular involvement were accomplished by early transection of the common bile duct at the level of the duodenum, with reflection superiorly. Intraoperative bile cultures were sent routinely. The entire extrahepatic biliary apparatus (supraduodenal bile duct and gallbladder), usually with en bloc partial hepatectomy, was resected, along with a subhilar lymphadenectomy (clearance of all lymph nodes within the hepatoduodenal ligament to the level of the common hepatic artery). Caudate lobectomy was performed routinely for all tumors involving the left hepatic duct and in any case when considered necessary to achieve complete tumor clearance. Histologic assessment of resection margins was performed during surgery. Additional tissue was resected, if feasible, when residual microscopic disease was suspected based on frozen-section histology. Roux-en-Y biliary-enteric reconstruction was performed to a segment of jejunum approximately 70 cm long.
Some patients with unresectable tumors were treated with systemic chemotherapy or chemoradiation therapy, as appropriate based on disease extent. However, adjuvant therapy was not used in patients who underwent a complete resection.
Data Analysis
Patient demographics, findings of radiographic investigations, and final patient disposition were recorded. In patients who underwent surgery, the surgical findings, operation performed, estimated blood loss (as recorded by the anesthesiologist), hospital stay (from the time of surgery to discharge), and postoperative complications were recorded. Surgical death was defined as any death resulting from a complication of surgery, whenever it occurred. Transfusion of any blood products (packed red cells, whole blood, fresh-frozen plasma, or platelets) during surgery or at any time during the hospital stay after surgery was also recorded. Data regarding the resected specimen, including tumor size and differentiation, status of the resection margin, metastatic disease to resected lymph nodes, and final tumor stage according to the American Joint Commission on Cancer (AJCC), 17 were analyzed. Tumor differentiation (well, moderate, poor) was determined on histologic review of the resected specimen. In this study, tumors classified as well to moderately differentiated or well differentiated with areas of moderate differentiation were not counted as well-differentiated lesions.
Statistical calculations were performed using SPSS, version 9.0 (Statistical Package for the Social Sciences, Chicago, IL). Continuous variables were compared using the Student t test (two-tailed) and categorical variables with a chi-square test. Survival probabilities were estimated using the Kaplan-Meier method 23 and compared by the log-rank test. Survival (in months) was measured from the date initially seen at MSKCC to the date of death or date of last contact. Cox regression 24 was used to determine independent predictors of outcome, using survival as the dependent variable and factors significant (P < .05) on univariate analysis as covariates. The predictive value of the proposed T-staging system and of the AJCC staging system was assessed with logistic regression analysis, using T stage or AJCC stage as the covariate and resectability, the presence of metastatic disease, and the likelihood of a complete resection (R0, histologically negative margins) as dependent variables. P < .05 was considered significant. Numeric data are presented as median values and/or mean values ± standard deviation.
RESULTS
Demographics
From March 1, 1991, through December 31, 2000, 225 patients with hilar cholangiocarcinoma were evaluated and treated at MSKCC, the first 90 of whom have been analyzed previously. 2 There were 124 men (55%) and 101 women (45%). The median age was 68 years (range 35–87; mean 66 ± 11). One hundred seventy-three patients (77%) were seen during the last 6 years of the study (1995–2000).
Results of Initial Evaluation
Sixty-five patients (29%) either had unresectable disease or were unfit for surgery at initial presentation (Fig. 2). Twenty-six patients had locally advanced lesions with extensive biliary involvement (n = 8), vascular invasion (n = 14), or both (n = 4). Twenty-six patients had metastatic disease precluding resection; five of them also had unresectable, locally advanced primary tumors. Twenty-three of these 26 patients had distant metastases (liver, peritoneal cavity, lung, or bone); 3 had proven disease in retroperitoneal lymph nodes. Eleven patients were unfit for surgery, principally because of comorbid conditions that prevented a major operation; however, three patients died of septic complications during evaluation and two patients declined to undergo surgery.

Figure 2. Flow diagram showing the results of the initial investigation and surgical findings of all patients in the series. *Seven patients were judged unfit for extended hepatic resection, two because of unexpected cirrhosis and five because of significant underlying coronary artery disease. **Resection of supraduodenal bile duct, cholecystectomy, subhilar lymphadenectomy. +Twenty-three patients had distant metastases (liver, peritoneal cavity, lung, or bone), whereas three had disease in retroperitoneal lymph nodes. ++Twenty-five patients had metastases to N2-level lymph nodes and 22 had distant disease (9 to the liver, 9 to the peritoneum, and 4 to liver and peritoneum).
Surgical Procedures and Findings
After complete evaluation, 160 patients (71%) were considered to have potentially resectable tumors. At exploration, 80 patients had findings that precluded resection. Forty-seven patients had metastatic disease: 25 to N2-level lymph nodes, 9 to the liver, 9 to the peritoneum, and 4 to the liver and peritoneum. Twenty-six patients had locally advanced tumors, 14 with extensive biliary involvement by tumor and 9 with technically insurmountable vascular invasion. In 3 of these 26 patients, a combination of local tumor extent and adhesions from prior operations, radiation therapy, or both precluded resection. Seven patients were judged unfit for extended hepatic resection, two because of unsuspected cirrhosis and five because of significant underlying coronary artery disease (see Fig. 2).
Eighty patients (36% of the entire group or 50% of those considered to have resectable disease before surgery) underwent a potentially curative resection. Eighteen patients underwent resection of the extrahepatic biliary apparatus only (resection of supraduodenal bile duct, cholecystectomy, subhilar lymphadenectomy), and 62 patients, or 78% of those undergoing resection, underwent partial hepatectomy in addition to this. The 62 hepatic resections consisted of a right trisegmentectomy (n = 22), right lobectomy (n = 16), left trisegmentectomy (n = 3), left lobectomy (n = 17), and central hepatectomy (n = 4). En bloc caudate lobe resection was performed in 22 of these patients, and 9 underwent portal vein resection and reconstruction. Two patients with tumor extension into the distal bile duct underwent concomitant pancreaticoduodenectomy to achieve clear margins.
Histopathology
Of the 80 patients who underwent resection, 62 had negative margins histologically (R0 resection); in 18 patients, the margins were microscopically involved with tumor (R1 resection). An R0 resection was more likely to be achieved in patients who had a concomitant hepatic resection than in those who underwent bile duct resection alone (84% vs. 56%;P < .01) (Table 3). The average tumor size was 2.2 ± 1.4 cm; 38% were well differentiated and 18% were papillary tumors. There was a 24% overall incidence of metastatic disease to hepatoduodenal lymph nodes resected with the primary tumor. In the hepatic resection group, the tumors were larger than those removed without hepatic resection (2.8 ± 1.5 vs. 2 ± 0.8 cm;P < .05) and there was a lower incidence of papillary tumors (13% vs. 33%;P < .05). Tumors in the hepatic resection group were less often well differentiated (32% vs. 56%;P < .09) and more often associated with lymph node metastases (26% vs. 17%;P < .5), but the differences in these variables were not statistically significant.
Table 3. HISTOPATHOLOGIC FEATURES OF RESECTED TUMORS, PERIOPERATIVE RESULTS, AND SURVIVAL
Survival calculations include the perioperative deaths.
*P < .01.
†P < .05.
‡P < .04.
Perioperative Results and Complications
The median blood loss for all resections was 800 mL (range 130–7,000). Forty-six percent of patients required transfusion of blood products during or after surgery, and the median number of transfused units was 1 (range 0–71). Although the blood loss and use of blood products was greater in the hepatic resection group than in the patients who did not undergo hepatic resection, these differences were not statistically significant. The median hospital stay was 13 days (range 6–49) overall and likewise was not significantly different between the two groups.
A total of 62 complications occurred in 51 patients (64%). Forty infective complications were observed in 39 patients, accounting for 66% of the total number of complications seen. These included intraabdominal abscess (n = 24), wound infection (n = 9), infected ascites (n = 2), pneumonia (n = 2), Clostridiumdifficile colitis (n = 2), and Candida esophagitis (n = 1). Twelve patients incurred a total of 22 noninfective complications, accounting for 34% of the total: bile leak or sterile bile collection (n = 9), upper gastrointestinal hemorrhage (n = 3), small bowel obstruction (n = 2), hepatic failure (n = 2), supraventricular tachycardia (n = 2), portal vein thrombosis (n = 1), renal failure (n = 1), pancreatitis (n = 1), and urinary retention (n = 1). Positive intraoperative bile cultures more than doubled the incidence of postoperative infective complications (79% vs. 33%;P < .04), and positive cultures correlated strongly with the presence of preoperatively placed biliary stents (84% with stents vs. 12% without stents;P < .00001).
Eight patients (10%) died of postoperative complications at a median time of 1.1 months (range 0.6–4) from the time of surgery. Infective complications were the underlying cause of death in six patients and included intraabdominal abscess (n = 4), infected ascites/pneumonia (n = 1), and pneumonia/gastrointestinal hemorrhage (n = 1). The two remaining patients died of hepatic failure and gastrointestinal hemorrhage, respectively. All six patients who died of infective complications had positive intraoperative bile cultures but the other two did not (P < .005), and five of the six had preoperatively placed biliary stents (vs. 0/2 noninfective deaths;P < .035). The perioperative rates of complications and death were greater in patients who underwent hepatic resection, but the differences were not statistically significant.
Survival
Median survival for all patients was 16 months at a median follow-up time of 11 months. As expected, patients who underwent resection (median follow-up 20 months), including the perioperative deaths, had a significantly longer survival than those who did not undergo resection (35 vs. 10 months;P < .00001). Likewise, survival after an R0 resection was significantly longer than after an R1 resection (42 vs. 21 months;P < .0075, including perioperative deaths) (Fig. 3). In addition, the median survival of patients with positive resection margins was similar to that of patients who underwent exploration but were found to have unresectable, locally advanced tumors (21 vs. 16 months;P < .35).

Figure 3. Survival after resection stratified by margin status. Resection with a negative histologic margin is indicated by the solid line (n = 62; median survival 42 months), and resection with a positive margin is indicated by the dashed line (n = 18; median survival 21 months). P < .0075 by log-rank test.
Median survival in the hepatic resection group was greater than in the group that did not undergo hepatic resection (46 vs. 28 months;P < .04, including perioperative deaths). The actuarial 5-year survival rate was 37% in patients who underwent hepatic resection versus 0% in those who underwent bile duct excision alone. Further, of the 30 patients who underwent resection 5 or more years ago, actual 5-year survival was observed only in those who underwent a partial hepatectomy (9/23 [39%] vs. 0/7). In addition, none of these nine patients had tumor-involved margins.
At the time of this analysis, 32 of the 80 patients who underwent resection were alive at a median follow-up of 21 months; 27 patients remained free of disease and 5 were alive with disease recurrence. Thirty-nine patients had died of disease at a median of 28 months. Nine patients died of other causes: eight of perioperative complications, and one patient died 6 months after surgery of an unrelated illness. Of the nine actual 5-year survivors, three were alive without disease (median follow-up 88 months) and six had died of disease recurrence and progression (median follow-up 77 months).
The clinical and tumor-related factors associated with improved survival are detailed in Table 4. Patients who died perioperatively were not included in this assessment. On univariate analysis of all resections, negative resection margin, concomitant partial hepatectomy, well-differentiated histopathology, and portal vein involvement by tumor were associated with significantly improved survival. Multivariate analysis using Cox regression identified negative resection margin, concomitant partial hepatectomy, and well-differentiated histopathology as independent predictors of outcome. Metastatic disease in resected hepatoduodenal lymph nodes was not a significant factor. Because of the importance of resection margin status in dictating outcome, this analysis was repeated for patients who underwent R0 resections to determine whether additional significant variables might emerge. On univariate analysis, concomitant partial hepatectomy, well-differentiated tumor histology, portal vein involvement by tumor, and lobar atrophy were associated with improved survival. On multivariate analysis, however, only concomitant hepatic resection remained a significant predictor of outcome; well-differentiated histology approached significance. Once again, lymph node involvement had no impact on survival.
Table 4. FACTORS ASSOCIATED WITH IMPROVED SURVIVAL
Analysis of factors associated with favorable long-term survival after any resection and after R0 resection (negative margins). This analysis excluded 8 patients who died in the perioperative period and 1 patient who died 6 months after surgery of an unrelated illness. Cox regression analysis was performed using only those variables that were significant on univariate analysis.
* Two patients had incomplete data.
† Six patients in the overall resection group and three patients in the R0 group had incomplete data.
Proposed T-Staging system
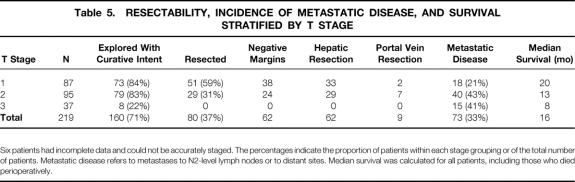
Two hundred nineteen patients were staged according to the proposed preoperative clinical system, as described above (Table 5). Six patients had incomplete data. Eighty-seven patients had tumor involvement of the biliary confluence (with or without unilateral extension to second-order biliary radicles), no portal vein involvement, and no lobar atrophy and were therefore classified as having T1 tumors. Ninety-five patients had T2 lesions because of ipsilateral portal vein involvement (n = 82) or ipsilateral lobar atrophy (n = 81); 69 patients had both findings. Thirty-seven patients had T3 tumors: 8 because of biliary extent alone, 17 because of main portal vein involvement, and 12 because of a combination of vascular involvement, biliary extent, and/or lobar atrophy. A similar proportion of patients with T1 (84%) and T2 (83%) tumors underwent exploration with curative intent. Eight patients with T3 tumors (22%) were also considered to have potentially Fig. 4 resectable disease; these patients all had main portal vein involvement that was possibly amenable to resection with portal vein reconstruction.
Table 5. RESECTABILITY, INCIDENCE OF METASTATIC DISEASE, AND SURVIVAL STRATIFIED BY T STAGE
Six patients had incomplete data and could not be accurately staged. The percentages indicate the proportion of patients within each stage grouping or of the total number of patients. Metastatic disease refers to metastases to N2-level lymph nodes or to distant sites. Median survival was calculated for all patients, including those who died perioperatively.

Figure 4. Survival of all patients stratified by T stage. Solid line indicates T1 tumors (n = 87; median survival 20 months). Dashed line indicates T2 tumors (n = 95; median survival 13 months). Dotted line indicates T3 tumors (n = 37; median survival 8 months). P < .0092 by Cox regression (likelihood ratio test for overall significance with 2 df).
Resectability and the likelihood of achieving an R0 resection both decreased progressively with increasing T stage. On logistic regression analysis, increasing T stage significantly reduced the resectability rate (P < .00001, odds ratio = 0.21 [0.13–0.35]) and the likelihood of an R0 resection (P < .00001, odds ratio = 0.3 [0.18–0.5]). Of the 51 patients with T1 tumors who underwent resection, 33 (65%) had a concomitant partial hepatectomy and 2 (4%) required portal vein resection because of tumor adherence. This is in contrast to patients with T2 lesions, all of whom required a liver resection and 7 (24%) of whom required a portal vein resection and reconstruction. In addition, metastatic disease to N2-level lymph nodes or to distant sites (i.e., metastatic disease that contraindicated resection) correlated with increasing T stage (P < .007, odds ratio = 0.6 [0.39–0.86]).
The proposed T-staging system also correlated with survival (P < .0092, likelihood ratio test for overall significance with 2 df). Median survival (including perioperative deaths) was 20 months for patients with T1 tumors, 13 months for patients with T2 tumors, and 8 months for patients with T1 lesions (see Table 5, Fig. 3). Using Cox regression with T stage as a categorical covariate and T1 as the reference stage, median survival was reduced significantly in moving from stage T1 to T2 (P < .002, odds ratio = 0.47 [0.29–0.76]) and from stage T2 to T3 (P < .02, odds ratio = 0.57 [0.36–0.93], 95% CI).
One hundred eighty-seven patients were staged according to the AJCC system. Stage was determined from analysis of the resected specimen in 80 patients and from the imaging studies or the surgical findings for the remaining 101 patients. Thirty-eight patients could not be adequately staged with the available information. On logistic regression, AJCC tumor stage, unlike the proposed T-staging system, neither correlated with resectability (P < .9) nor predicted the likelihood of an R0 resection (P < .4). Likewise, using Cox regression, AJCC tumor stage did not correlate with survival (P < .23, likelihood ratio test for overall significance with 3 df). In addition, 46 of 80 patients who underwent resection (58%) and 7 of 9 actual 5-year survivors (78%) had AJCC stage 4 tumors.
DISCUSSION
Hilar cholangiocarcinoma remains among the most difficult management problems faced by surgeons. In the past, although partial hepatectomy was recognized as a possible means of achieving complete tumor extirpation, such resections stretched the limits of surgical expertise and hepatic resection was not performed routinely. 7,25 Many surgeons accepted positive resection margins as adequate rather than risk a more extensive resection. More recently, however, improvements in surgical technique have been accompanied by better results, leading many to pursue a more aggressive resectional approach. The accumulated results from many centers show convincingly that only a resection with negative histologic margins can be considered potentially curative and that hepatic resection is often required to achieve this objective. 2,10,11
The use of hepatic resection not only increases the number of patients potentially eligible for a complete resection but also demands a reevaluation of the current approach to patient assessment and tumor staging. Tumors at the biliary confluence frequently involve the portal vein and often result in hepatic lobar atrophy. These additional factors, which are related to local tumor extent, do not necessarily preclude resection but must be evaluated in relation to the extent of ductal cancer spread before resectability can be determined. For example, unilateral tumor extension to second-order biliary radicles with ipsilateral portal vein involvement or ipsilateral lobar atrophy may well be amenable to resection with a concomitant partial hepatectomy, but contralateral involvement is not. Thus, the willingness to perform a partial hepatectomy has redefined irresectability in patients with hilar cholangiocarcinoma.
Currently, no clinical staging system embraces all of the relevant local, tumor-related variables and stratifies patients before surgery into subgroups based on potential for resection. The modified Bismuth-Corlette system 15,16 classifies patients based on the extent of biliary ductal involvement by tumor; although useful to some extent, it does not correlate with resectability or survival. The current AJCC 17 system is based largely on histopathologic criteria, and although provision is made for tumor invasion into the liver and for distant disease, there is little applicability for preoperative staging of potentially resectable lesions.
The present study analyzes 225 patients seen and treated at a single center during a 9-year period, with most of this experience concentrated in the past 6 years. This relatively short time interval ensures homogeneity with respect to patient evaluation and treatment. Further, this represents a consecutive series of all patients seen during this time, allowing a thorough analysis of resectability and construction of a preoperative staging system based on local factors that determine resectability and, therefore, outcome: biliary tumor extent, vascular involvement, and lobar atrophy.
The clinical staging system proposed in this report, derived from a thorough analysis of patients with resectable and unresectable tumors, is an approach aimed at full radiologic diagnosis using all the available preoperative data. By taking full account of local factors that influence resectability, the proposed scheme, in contrast to the AJCC staging system, 26 reliably stratified patients into treatment groups, predicted the need for hepatic resection to clear all tumor, and correlated with overall resectability and survival. Of the patients who underwent exploration with curative intent, the proposed classification accurately staged the local extent of disease in 86%. This system, in its current form, does not consider the presence of nodal or distant metastases, but the incidence of these findings increased with increasing T stage. The proposed staging classification thus fills a gap by allowing a more rational assessment of resectability and prediction of outcome. In addition, the correlation between more locally advanced tumors (i.e., higher T stage) and metastatic disease may be useful in identifying patients who would benefit from more intensive radiologic investigation or from staging laparoscopy, thereby sparing them an unnecessary laparotomy.
Most patients in this series had disease that was not amenable to resection. Only 50% of those who underwent exploration with curative intent (36% of the entire group) underwent a resection of all gross tumor, whereas 39% of those explored (28% of the entire group) underwent an R0 resection. However, nearly 80% of resections were performed with negative margins, which correlated strongly with concomitant hepatic resection, including en bloc caudate lobectomy when appropriate. Thus, the data confirm the importance of hepatic resection to achieve a complete resection, the significance of which is clear in analyzing long-term outcome. Survival was markedly reduced in patients with tumor-involved margins, and none of these patients were among the group that survived 5 or more years. In fact, survival after an R1 resection was no better than that of patients with locally advanced tumors who underwent an exploration with no resection.
At first glance, the close association between hepatic resection and negative margins would appear to explain the improved survival observed in patients who underwent concomitant partial hepatectomy. Because of the powerful influence of margin status on outcome, multivariate analysis was repeated for patients who had an R0 resection to determine whether additional significant variables might emerge. This analysis identified concomitant partial hepatectomy as the only independent predictor of survival after resection with negative margins. To date, 5-year survival has been seen only in patients who underwent hepatic resection. These results suggests that bile duct excision alone is less effective in clearing all disease, despite the interpretation of negative resection margins, and further suggests that hepatic resection should be considered for all patients. However, this observation must be confirmed in a larger series of patients with more mature follow-up before a firm recommendation can be made in this regard.
Patients with well-differentiated tumors appeared to have a more favorable outcome after resection, an observation that has been made previously. 26,27 Indeed, in analyzing all resections, well-differentiated tumor histology, along with a negative resection margin and concomitant hepatic resection, was an independent predictor of long-term survival. However, after an R0 resection, tumor differentiation did not appear to confer the same benefit, underscoring the importance of a complete resection in dictating outcome. Tumor involvement of resected lymph nodes within the hepatoduodenal ligament did not adversely affect survival, which is contrary to prior reports 9,11 and may merely reflect the relatively small number of patients. Portal vein involvement and lobar atrophy paradoxically seemed to suggest a more favorable outcome, but the close association between these variables and hepatic resection accounts for this, because neither was significant on multivariate analysis.
The traditional view of hilar cholangiocarcinoma is that of a slow-growing, locally invasive cancer that infrequently gives rise to metastases. The results of the current study do not support this view. Seventy-three patients (32%) had metastatic disease that precluded resection, and another 18 had metastatic disease in resected lymph nodes in the hepatoduodenal ligament. Unsuspected metastatic disease was seen in 29% of patients who underwent surgery for a potentially curative resection. Thus, in all patients in the series, the overall incidence of metastases of any type was 40%, and 20% of patients had metastases to distant sites, which is supported by previous reports. 28,29
The death and complication rates, although greater than those from this unit after resection of other tumor types, 30,31 are consistent with reports from other centers of 5% to 17%. 6,9,11 Infective complications dominated the postoperative course for many patients and were responsible for two thirds of all complications seen and six of the eight surgical deaths. In only one patient was hepatic failure the primary cause of death. Bacterial colonization of bile at the time of resection correlated strongly with preoperatively placed biliary stents and significantly increased the risk of death and complications. Another problem in this regard concerns patients with complications related to the initial biliary intervention, which delayed resection and probably influenced the surgical complication rate. The relationship between biliary stents, colonized bile, and postoperative infections is not a novel finding: we have previously reported this in patients with proximal biliary obstruction undergoing resection or bypass. 19 Further, in patients with distal bile duct obstruction, there is evidence that preoperative stents increase not only the incidence of postoperative infections but also the surgical death rate. 20 Clearly, efforts to reduce the rate of surgical complications in patients with hilar cholangiocarcinoma are warranted. It is hoped that the increasing availability of high-quality, noninvasive imaging will reduce the use of biliary stents before referral for resection.
In summary, the current study shows the value of a preoperative staging system that accounts fully for factors related to local tumor extent and reliably stratifies patients into treatment groups. This system predicts resectability and the likelihood of metastatic disease and correlates with survival. As suggested in earlier work, 7,25 the current study shows that an aggressive surgical approach to hilar cholangiocarcinoma is warranted and can result in long-term survival in one third of patients, provided that a complete resection is performed. The results show the importance of partial hepatectomy in achieving a complete resection and further suggest that only a concomitant hepatic resection effectively clears all disease and offers the possibility of prolonged survival.
Discussion
Dr. Christoph Broelsch (Essen, Germany): Dr. Jarnagin and his colleagues certainly need to be congratulated for tackling a very complex problem affecting very few patients but requiring utmost expertise. They present a unique collection of data of over more than a decade, allowing for an intriguing analysis. For comparison of data, a systematic approach for staging is required.
In past years, however, the new invasive imaging studies like MRCP were instrumental in assessing a more accurate staging by endoscopic ultrasound and refined ERC techniques. Together, in the clinical assessment, it seems we have a full armamentarium to select patients for surgery or drainage or even predict outcome.
However, what counts is not the staging but the operability. And that, I believe, is determined by the competence of the surgeon. With increasing expertise he has shown in the past few years to get many more referrals to the institution, which is over 60% in the last four years.
Now, with the staging imaging completed, 75% of your patients were considered to have resectable tumors. While in exploration, 80 patients, about 50%, already had findings precluding resection, mostly because of metastatic disease. But 26 had locally advanced disease even with biliary involvement or vascular invasion.
My first question, therefore, is whether this was not detectable by preoperative evaluation including your laparoscopic approach or were those patients you considered for your famous segment 3 bypass operation?
Secondly, I would like to challenge your proposed T-staging criteria on the basis of your own resectability data as compared to those who did not use it as comprehensive as you did in the past. For example, our own group in 1983 reported an RO rate of resection, being 11 out of 23 patients. It was extended later in 1996 with a 73% resectability, including 125 patients with hilar resections and 95 lobectomies. That was published in the Annals in 1996. Neuhaus recently published an excellent series of 80 resections, including 14 hilar resections and 66 hepatectomies. The five-year survival rates now range around 35%, which is as good as we get in this field.
We all agree with you that RO resection should actually be attempted, even at the price of higher morbidity, including partial lymphadenectomy or vascular reconstructions. Now achieving RO resections, in your paper, lymph node involvement did not significantly change prognosis except for some distant metastatic disease.
Thirdly, would you consider local irradiation to be added to your armamentarium in borderline cases such as T-3 or T-2 staging? Our approach in Essen is much more simple. We now divide our series into two branches. One is clear RO resection and no metastatic diseases outside the liver. All the others with positive lymph nodes and vascular involvement, and irrespective of RI resection receive intraoperative radiation by 20 gray and subsequently 45 gray plus 5. Do you think this is an additional tool for your series as well?
Thank you for the opportunity of reviewing your manuscript. The issue remains challenging and burning because it decides the fate of very desperate patients.
Presenter Dr. Leslie H. Blumgart (New York, New York): Thank you very much indeed for your comments. I am going to take your last point first because I think it is quite important. That is question of adjuvant irradiation. Perhaps we should be doing that although we have elected not to do so during the period of this study. Irradiation is a matter that we have given consideration to but up till now have used it, usually as brachytherapy combined with external beam irradiation, in patients who are irresectable.
Of course, I agree with you that the competence of the surgical group is important. As recent studies have shown, surgeons doing a high turn over of a particular complex operation and working in high turn over hospitals produce the best results. This is particularly true in specialized fields.
As regards the resectability rate – I think we can debate forever the question as to whether the resectability rate is higher in one institution than in another. Firstly, we are all prisoners of our own referral pattern and perhaps this is what resectability rates relate to. It should be remembered that the series we have just presented is a series of all comers referred to the institution both to the gastroenterologists and to ourselves in the surgical group and the resectability rate is likely to reflect this rather than a group of patients selected and sent to a surgical specialist.
However, it is interesting that the overall resectability rate is about 30%. I have been involved in the treatment of this disease for some 28 years or so and whether it was in London or Switzerland or now in New York, my resectability rate has changed little.
Of course, definition of resectability rate is also important and RO resections (that is resection with clear margins) are very important and we have seen a considerable improvement in this group probably as a result of selecting patients according to our staging system and the willingness to carry out hepatic resection.
Dr. Lawrence W. Way (San Francisco, California): In this interesting work, Dr. Blumgart and his colleagues report the findings and results of treatment of a large series of patients with cholangiocarcinoma of the bile duct. Many questions could be asked, but I would like to concentrate on a couple of areas in an attempt to draw out even more useful information.
The first concerns the role of local resection without hepatectomy. No patient was cured by this procedure, whether or not the margins were clear of disease. In my opinion, the shortcomings of local resection have been generally known for some time. Can you clarify, therefore, the circumstances where you believe local resection is still justified? The data suggest that it could rarely be more than palliative. But was palliation actually achieved in these cases? In retrospect, do you think that some of your patients who had local resections and negative margins were undertreated? If so, what should have been done instead? A large proportion of the papillary tumors had local resections, which suggests that your surgeons may have thought that these lesions were more easily cured than the sclerosing tumors. If so, are you revising this concept?
Secondly, I wonder whether you could expand on the importance of the caudate lobe and the indications for its removal. The bifurcation of the common hepatic duct normally rests in direct contact with the caudate lobe. Although the two can often be separated by blunt dissection, very small ducts usually join them. It may be tempting to believe that in the absence of gross invasion across this boundary, clear lateral margins can be obtained by lifting a bifurcation tumor off the caudate lobe. But on the other hand, that may be wishful thinking. The caudate lobe did not receive much analysis in the manuscript. What was the relationship between caudate lobe excision and outcome? What are your current thoughts on these questions?
Finally, there was no mention of adjuvant therapy. Did any of these patients receive radiotherapy? If so, this would be important to know in interpreting the outcomes, which in the current manuscript appear solely attributable to surgical management. The implication at present is that you do not use postoperative radiotherapy.
And before concluding, this presentation demonstrates to my satisfaction that patients with this disease are best cared for in centers where surgeons and ancillary services have the special expertise to perform the full array of procedures and to understand the nuances of treatment planning. It is highly specialized. The number of centers prepared to deliver this type of quaternary care is few.
Dr. Leslie H. Blumgart: Dr. Way, thank you very much. You raised some very important points, some of which, of, course, are still under debate.
Firstly, the question of local resection – I was delighted to hear you say that the shortcomings of this procedure have been generally known for some time. I must say I had some difficulty, until fairly recently, convincing people, some of whom are in this audience, that this was the case. Naturally, I think we should proceed to liver resection if there is any doubt as to extension of the tumor into right or left biliary system. However, there are some difficult problems when the tumor is very localized at the confluence. In this situation, it becomes very difficult to proceed to liver resection since it may not be quite clear whether one should resect the right or the left side. Some of the difficulty may be related to shortcomings in pathological interpretation and it may be that we are not detecting spread of disease accurately. Nevertheless, your point is well taken and I think we will see fewer and fewer series with local resections in the future.
Your point about palliation – you asked was palliation actually achieved? Well, it depends what you mean by palliation. If you mean relief of jaundice and itching, yes, it was achieved in exactly the same way as it would have been with a biliary enteric bypass to the segment III duct. If you mean, by palliation, that it is clear that they didn’t survive any better than patients not operated upon, then there was no palliation. So I think the real answer to the question is that palliation in terms of relief of jaundice and itching and perhaps of cholangitis was achieved but at the expense of a big operation.
Your question about the caudate lobe – this is an area of debate and it is again difficult. You will notice we had 22 caudate resections most of them carried out in association with left lobar or extended left hepatic resections. Indeed it is difficult to remove these lesions on the left side with complete clearance without including the caudate lobe. On the other hand if the lesion is well localized on the right side then a caudate resection is often not necessary. I find it difficult to advocate caudate resection for all lesions and indeed it has been uncommon for us to carry out such resections for patients with tumors on the right. Does it influence outcome? Well, I don’t think have an answer to that yet but probably so because it does help achieve RO resections.
Chris Broelsch has already raised the question of radiotherapy. I have said that I think this is a valid point and should be considered in the future although we have been somewhat unwilling to irradiate these often small biliary anastomoses. Indeed the question presupposes that recurrence is always local and this is not the case.
Dr. Robert M. Beazley (Boston, Massachusetts): I did not have the benefit of the manuscript, so I did the next best thing and went back looking at some work that Dr. Blumgart and I did some 20 years ago on the first 16 cases that he resected. There are a lot of similarities, I must say.
The deaths that we had in the first series were mostly from infection, you didn’t mention anything in this presentation about the operative deaths and whether they were related to infection or liver failure?
My second point is, there were no long-term survivors in the initial group. The longest survivor was 57 months. The initial series was criticized to some extent in that it was a ‘palliative‘ exercise. What your comments today, 20 years later, looking back on that critique?
Dr. Leslie H. Blumgart: You raised a very important point. I think this was alluded to also by Dr. Broelsch. We had eight deaths, and six of these eight postoperative deaths were caused by infection. Indeed all of these patients had positive bile cultures and five of the six had had preoperative stents. Positive intraoperative bile cultures increased the infective complication rate (79% versus 33%) and correlated strongly with the presence of preoperative stents (84% versus 12%). All patients who died had had invasive radiological procedures beforehand. It is quite clear that the ‘captain of the men death‘ is infection and that this is related to direct cholangiograplhy and biliary stenting.
Yes, Dr. Beazley, we were heavily criticized when this subject was presented to the Southern Surgical Society in 1984. This early work was published in the Annals of Surgery (1984;199:623-636) and if anyone is interested they can read the discussion. A somewhat subjective criticism stated at that time ‘when Les Blumgart does this operation, he feels good. But it makes me (the discussant) feel bad.’ Things have changed somewhat. Results are now increasingly good and after some years of continuous effort in the field I simply feel tired.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association, April 26–28, 2001, the Broadmoor Hotel, Colorado Springs, Colorado
Correspondence: William R. Jarnagin, MD, Department of Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Ave., New York, NY 10021.
Dr. Burke is currently at the Department of Surgery, Kaiser Permanente, Honolulu, Hawaii.
E-mail: jarnagiw@mskcc.org
Accepted for publication April 26, 2001.
References
- 1.Parker SL. Cancer statistics. CA Cancer J Clin 1996; 46: 5–27. [DOI] [PubMed] [Google Scholar]
- 2.Burke EC, Jarnagin WR, Hochwald SN, et al. Hilar cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg 1998; 228: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagorney DM, Donohue JH, Farnell MB, et al. Outcomes after curative resections of cholangiocarcinoma. Arch Surg 1993; 128: 871–879. [DOI] [PubMed] [Google Scholar]
- 4.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996; 224: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tompkins RK, Thomas D, Wile A, Longmire WP. Prognostic factors in bile duct carcinoma. Analysis of 96 cases. Ann Surg 1981; 194: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Launois B, Reding R, Lebeau G, et al. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepat Bil Panc Surg 2000; 7: 128–134. [DOI] [PubMed] [Google Scholar]
- 7.Launois B, Campion JP, Brissot P, Gosselin M. Carcinoma of the hepatic hilus. Surgical management and the case for resection. Ann Surg 1979; 190: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadjis NS, Blenkharn JI, Alexander N, et al. Outcome of radical surgery in hilar cholangiocarcinoma. Surgery 1990; 107: 597–604. [PubMed] [Google Scholar]
- 9.Klempnauer J, Ridder GJ, von Wasielewski R, et al. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol 1997; 15: 947–954. [DOI] [PubMed] [Google Scholar]
- 10.Pichlmayr R, Weimann A, Klempnauer J, et al. Surgical treatment in proximal bile duct cancer. A single-center experience. Ann Surg 1996; 224: 628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nimura Y, Kamiya J, Kondo S, et al. Aggressive preoperative management and extended surgery for hilar cholangiocar cinoma: Nagoya experience. J Hep Bil Panc Surg 2000; 7: 155–162. [DOI] [PubMed] [Google Scholar]
- 12.Launois B, Terblanche J, Lakehal M, et al. Proximal bile duct cancer: high resectability rate and 5-year survival. Ann Surg 1999; 230: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hann LE, Greatrex KV, Bach AM, et al. Cholangiocarcinoma at the hepatic hilus: sonographic findings. AJR Am J Roentgenol 1997; 168: 985–989. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz LH, Coakley FV, Sun Y, et al. Neoplastic pancreaticobiliary duct obstruction: evaluation with breath-hold MR cholangiopancreatography. AJR Am J Roentgenol 1998; 170: 1491–1495. [DOI] [PubMed] [Google Scholar]
- 15.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 1975; 140: 170–176. [PubMed] [Google Scholar]
- 16.Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg 1988; 12: 39–47. [DOI] [PubMed] [Google Scholar]
- 17.American Joint Committee on Cancer. Extrahepatic bile ducts. In: Fleming ID, Cooper JS, et al, eds. AJCC cancer staging manual. Philadelphia: Lippencott-Raven; 1997: 109–111.
- 18.Jarnagin WR, Bodniewicz J, Dougherty E, et al. A prospective analysis of staging laparoscopy in patients with primary and secondary hepatobiliary malignancies. J Gastrointest Surg 2000; 4: 34–43. [DOI] [PubMed] [Google Scholar]
- 19.Hochwald SN, Burke EC, Jarnagin WR, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg 1999; 134: 261–266. [DOI] [PubMed] [Google Scholar]
- 20.Povoski SP, Karpeh MS Jr, Conlon KC, et al. Preoperative biliary drainage: impact on intraoperative bile cultures and infectious morbidity and mortality after pancreaticoduodenectomy. J Gastrointest Surg 1999; 3: 496–505. [DOI] [PubMed] [Google Scholar]
- 21.Czerniak A, Soreide O, Gibson RN, et al. Liver atrophy complicating benign bile duct strictures. Surgical and interventional radiologic approaches. Am J Surg 1986; 152: 294–300. [DOI] [PubMed] [Google Scholar]
- 22.Blumgart LH, Benjamin IS. Liver resection for bile duct cancer. Surg Clin North Am 1989; 69: 323–337. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 24.Cox DR. Regression models and life tables (with discussion). JR Stat Soc B 1972; 187–220.
- 25.Beazley RM, Hadjis N, Benjamin IS, Blumgart LH. Clinicopathological aspects of high bile duct cancer. Experience with resection and bypass surgical treatment. Ann Surg 1984; 199: 623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg 1996; 223: 384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strong RW, Lynch SV. Surgical resection for hilar cholangiocarcinoma. J Hepat Bil Pancr Surg 1995; 2: 233–238. [Google Scholar]
- 28.Cameron JL, Pitt HA, Zinner MJ, et al. Management of proximal cholangiocarcinomas by surgical resection and radiotherapy. Am J Surg 1990; 159: 91–97. [DOI] [PubMed] [Google Scholar]
- 29.Lai CS, Tompkins RK, Mann L, Roslyn JJ. Proximal bile duct cancer: quality of survival. Ann Surg 1987; 205: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fong Y, Fortner JG, Sun R, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1,001 consecutive cases. Ann Surg 1999; 230: 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg 1999; 229: 790–979. [DOI] [PMC free article] [PubMed] [Google Scholar]