Abstract
Objective
To document what can be accomplished with surgical resection done according to the classical principles of surgical oncology.
Methods
One hundred consecutive patients underwent en bloc esophagectomy for esophageal adenocarcinoma. No patient received pre- or postoperative chemotherapy or radiation therapy. Tumor depth and number and location of involved lymph nodes were recorded. A lymph node ratio was calculated by dividing the number of involved nodes by the total number removed. Follow-up was complete in all patients. The median follow-up of surviving patients was 40 months, with 23 patients surviving 5 years or more.
Results
The overall actuarial survival rate at 5 years was 52%. Survival rates by American Joint Commission on Cancer (AJCC) stage were stage 1 (n = 26), 94%; stage 2a (n = 11), 65%; stage 2b (n = 13), 65%; stage 3 (n = 32), 23%; and stage 4 (n = 18), 27%. Sixteen tumors were confined to the mucosa, 16 to the submucosa, and 13 to the muscularis propria, and 55 were transmural. Tumor depth and the number and ratio of involved nodes were predictors of survival. Metastases to celiac (n = 16) or other distant node sites (n = 26) were not associated with decreased survival. Local recurrence was seen in only one patient. Latent nodal recurrence outside the surgical field occurred in 9 patients and systemic metastases in 31. Tumor depth, the number of involved nodes, and the lymph node ratio were important predictors of systemic recurrence. The surgical death rate was 6%.
Conclusion
Long-term survival from adenocarcinoma of the esophagus can be achieved in more than half the patients who undergo en bloc resection. One third of patients with lymph node involvement survived 5 years. Local control is excellent after en bloc resection. The extent of disease associated with tumors confined to the mucosa and submucosa provides justification for more limited and less morbid resections.
During the past two decades, the management of esophageal cancer has changed from the treatment of patients with advanced-stage squamous cell cancer to those with early-stage adenocarcinoma occurring in the setting of Barrett’s esophagus. 1,2 Several new, unproven therapeutic approaches have been used in patients with early asymptomatic adenocarcinomas detected in the course of Barrett’s surveillance, such as mucosal ablation 3,4 or endoscopic mucosal resection. 5 For patients with more advanced tumors, combined modality therapy (neoadjuvant chemoradiotherapy) has been broadly applied, despite the lack of clear evidence regarding the superiority of this approach. 6–11 In some centers, the need for surgical resection has been questioned, advocating instead definitive chemoradiotherapy as primary treatment. When surgery is performed, controversy persists about the extent of resection necessary, 12–16 with much of the debate centered on the benefits of a systematic lymph node dissection. To clarify the clinical biology of esophageal adenocarcinoma, the outcome after complete surgical resection performed according to the classical principles of surgical oncology must be clearly defined.
This study reports 100 consecutive en bloc esophageal resections with systematic abdominal and mediastinal lymph node dissection for esophageal adenocarcinoma performed in patients who had only surgery as their primary mode of therapy. Patients who received preoperative neoadjuvant or postoperative adjuvant therapy were not included because these additional treatments can affect both accurate staging and outcome. This unique experience answers several important questions about the biology of esophageal adenocarcinoma. First, it defined the prevalence and extent of tumor spread at all stages. This is critical in determining the extent of treatment needed in various stages of the disease to achieve cure. Second, it showed the outcome to be expected after complete surgical resection. This is a benchmark for comparing the outcome of newly proposed treatment regimens and technologies. Finally, it identified predictors of systemic recurrence that can be used to predict patients in whom complete surgical resection alone is insufficient to cure the disease. These patients need additional therapy, and their identification would focus the expense and toxicity of chemotherapy on those who are most apt to benefit.
PATIENTS AND METHODS
The study population consisted of 100 consecutive patients who underwent an en bloc resection with a systematic abdominal and mediastinal lymph node dissection as primary therapy for their cancer. They were accrued between January 1982 and November 2000, at a rate that reflected the increasing incidence of esophageal adenocarcinoma from its historical baseline in the late 1970s. None of the patients received neoadjuvant radiation or chemotherapy, and none had previous esophageal or gastric resections. There were 89 male and 11 female patients, with a median age at surgery of 57 years (interquartile range [IQR] 52–65.5). The age of the patients at presentation did not differ significantly over the time period studied. Patient demographic information and tumor characteristics are summarized in Table 1. The most common symptom leading to the detection of the cancer was dysphagia (present in 55 patients). Nineteen patients were asymptomatic, with the cancer detected during screening endoscopy or during surveillance programs for Barrett’s esophagus. A prior history of gastroesophageal reflux was present in 69 patients. Twenty-eight were known to have Barrett’s esophagus before the diagnosis of carcinoma.
Table 1. DEMOGRAPHIC INFORMATION AND TUMOR CHARACTERISTICS
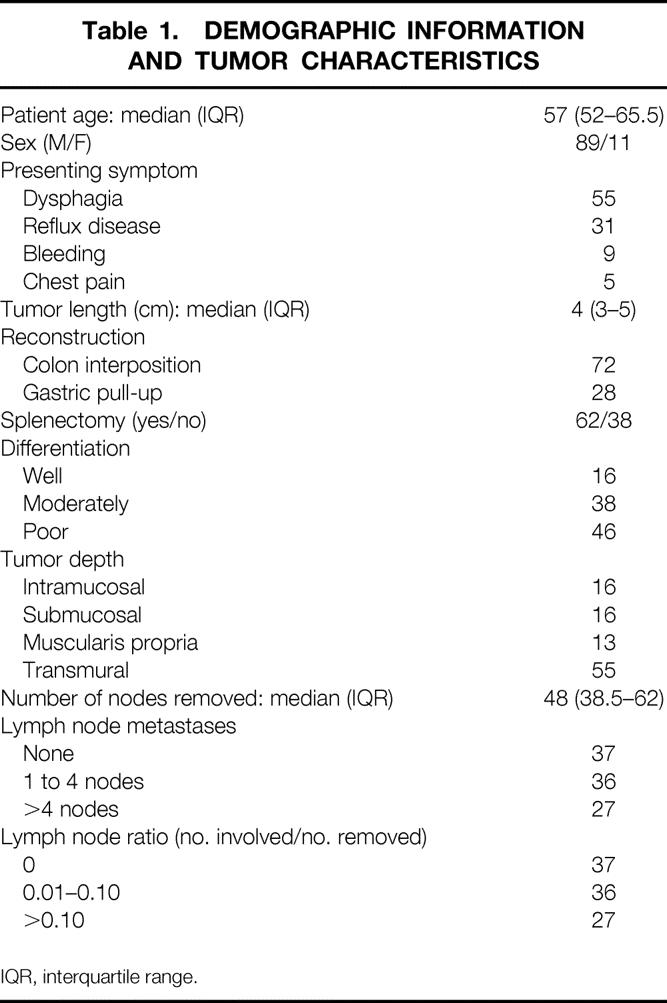
IQR, interquartile range.
Preoperative Assessment
All patients underwent an endoscopic evaluation to assess the primary tumor. Endoscopic ultrasound performed at 7.5 and 12 Hz was used selectively and provided additional information regarding the depth of tumor penetration and the presence of lymph node metastases. A computed tomography scan of the chest and abdomen was performed in all patients to exclude the presence of metastatic disease. An assessment of cardiopulmonary reserve was done by a noninvasive cardiac evaluation (dobutamine stress echo or dipyridamole thallium study) and pulmonary function tests. Patients younger than 75 who had adequate cardiopulmonary reserve and appeared to have locoregional disease based on the imaging studies described above were considered candidates for the en bloc resection. Patients found to have disease beyond the borders of an en bloc resection at surgery (i.e., inability to perform an R0 resection) received a palliative resection and were excluded.
Surgical Approach
The en bloc procedure was performed through an initial right thoracotomy followed by a midline laparotomy. 17 In all patients the proximal anastomosis was performed through an incision in the left neck. The thoracic dissection included removal of the azygos vein with its associated nodes, the thoracic duct, and the low paratracheal, subcarinal, paraesophageal, and parahiatal nodes in continuity with the resected esophagus. The block of tissue removed was bounded laterally on each side by the excised mediastinal pleura, anteriorly by the pericardium and membranous trachea, and posteriorly by the aorta and vertebral bodies.
The abdominal dissection included removal of the lymph nodes along the hepatic artery and portal vein from the porta hepatis to the celiac trunk, around the celiac trunk, and along the left gastric artery and lesser curvature of the stomach. In addition, all the retroperitoneal tissue cephalad to the hepatic artery was removed, including the tissue that lies over the inferior vena cava and the right crus of the diaphragm. On the left side, the tissues and lymph nodes surrounding the splenic artery and the tissue overlying the adrenal gland and left crus of the diaphragm were removed. In 62 patients, the spleen and the lymph nodes along the splenic artery and in the splenic hilum were also removed. In 72 patients, gastrointestinal continuity was reestablished by the use of an isoperistaltic colon interposition based on the left colic artery. In these patients, the abdominal dissection also included the removal of the proximal two thirds of the stomach, the omentum, and the lymph nodes along the proximal two thirds of the greater curvature of the stomach. In 28 patients, the esophageal reconstruction was done by creating a gastric tube after a wide resection of the gastric cardia down to the fourth vein on the lesser curvature of the stomach. The use of the stomach for reconstruction was based on the size of the primary tumor, the degree to which it involved the proximal stomach, and the presence of intrinsic colonic disease (e.g., polyps, diverticula) or variations in vascular supply that precluded the use of the colon.
Analysis of the Resected Specimens
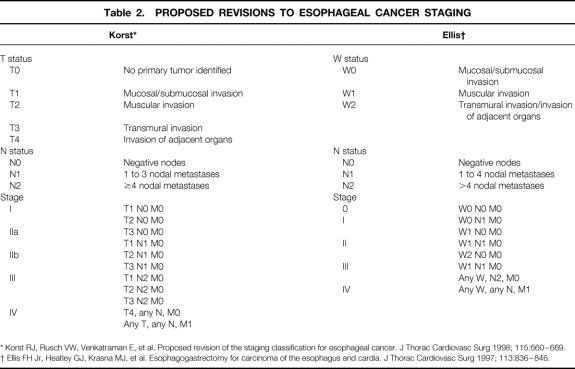
Two experienced gastrointestinal pathologists evaluated the resected specimens to identify the depth of invasion of the primary tumor and the number and location of involved and uninvolved lymph nodes. In 16 patients, the tumors invaded the lamina propria but did not invade beyond the muscularis mucosae; these were defined as intramucosal cancers. Sixteen tumors invaded beyond the muscularis mucosae but not into the muscularis propria; these were classified as intramural/submucosal tumors. In 13 patients, the tumor invaded into but not through the muscularis propria; these were classified as intramural/muscularis propria tumors. Fifty-five patients had penetration of the tumor beyond the muscularis propria into the periesophageal adventitia; these were classified as transmural tumors. The pathology report included information regarding the presence or absence of Barrett’s epithelium in 76 patients, with specialized intestinal metaplasia identified in 60 (79%). The median number of nodes removed per patient was 48 (IQR 38.5–62). The locations of the lymph nodes were categorized according to a group of standard lymph node stations. 18 The specimens were fixed in formalin, cut into 5-μm sections, stained with hematoxylin and eosin, and examined by light microscopy. Based on the microscopic findings, the tumors were categorized according to the most recent staging classification proposed by the American Joint Commission on Cancer. 19 Because of recognized inadequacies in this staging system, the tumors were also categorized according to two recent modifications of an alternative staging classification system 20,21 proposed by Skinner et al 22 (Table 2). A lymph node ratio was calculated by dividing the number of lymph nodes containing metastatic tumor by the total number of lymph nodes.
Table 2. PROPOSED REVISIONS TO ESOPHAGEAL CANCER STAGING
* Korst RJ, Rusch VW, Venkatraman E, et al. Proposed revision of the staging classification for esophageal cancer. J Thorac Cardiovasc Surg 1998; 115:660–669.
† Ellis FH Jr, Heatley GJ, Krasna MJ, et al. Esophagogastrectomy for carcinoma of the esophagus and cardia. J Thorac Cardiovasc Surg 1997; 113:836–846.
Patients with lymph node metastases were separated into two groups: those with only local lymph node involvement, and those with distant lymph node involvement. Distant nodes in the thorax were defined as those not normally removed by a transhiatal approach. This included paratracheal, subcarinal, and retrocrural nodes and the nodes around the azygos vein. In the abdomen, distant nodes included the porta hepatis nodes and nodes around the hepatic, celiac, and splenic arteries.
Follow-up
The operating surgeon followed up patients at 3-month intervals for the first 3 years, and every 4 to 6 months thereafter. The median length of follow-up in the surviving patients was 40 months (IQR 15.5–63.5), with 23 patients alive 5 years or more. Follow-up evaluation included a history, physical examination, a complete blood count, and serum liver panel. Computed tomography scans of the chest and abdomen were obtained at each visit. Recurrent disease was suggested by enlarging nodes or by localized abnormalities in the densities of solid organs. Suspected recurrent disease was confirmed by biopsy or imaging studies.
Statistical Analysis
Comparisons of proportions were performed using the chi-square or Fisher exact test. Continuous variables were compared using either the Mann-Whitney test or the Kruskal-Wallis test. Survival estimates were calculated according to the method of Kaplan and Meier, 23 with comparison between survival curves performed using the log-rank method. P < .05 was considered significant.
RESULTS
Deaths and Complications
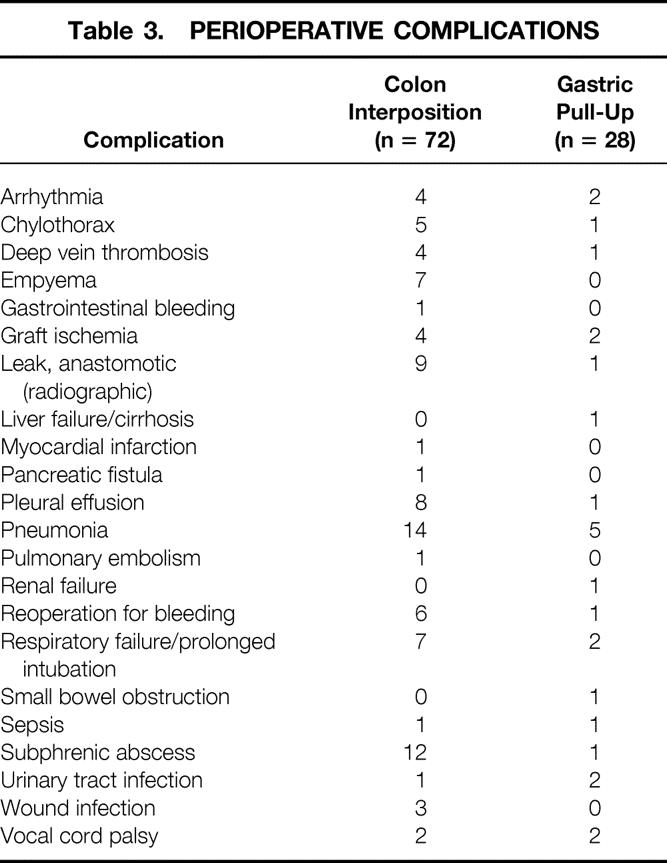
The perioperative death rate, including all deaths that occurred in the hospital or within 30 days of surgery, was 6%. There was only one postoperative death in the 40 patients who underwent surgery in the last 4 years of the study period. Complications occurred in 71 patients (Table 3). Respiratory complications were most common, occurring in 30 patients, with complications related to the reconstruction (graft necrosis, bleeding, and anastomotic leak on Gastrografin swallow) in 22 patients. There was no difference in complication rates (54/72 vs. 17/28) between patients who underwent a colon interposition versus those who had a gastric pull-up (P = .219, Fisher exact test). Likewise, the death rate was similar in patients reconstructed with colon (4/72) or stomach (2/28) (P = .6714, Fisher exact test).
Table 3. PERIOPERATIVE COMPLICATIONS
Prevalence of Nodal Disease
A total of 5,026 lymph nodes were examined. The median number of nodes removed per patient was 48 (IQR 38.5–62). Lymph node metastases were identified in 63 patients, with limited node disease (one to four involved nodes) in 36 patients and extensive node disease (more than four involved nodes) in 27. Distant lymph node metastases were identified in 27 patients, with celiac node metastases in 18. Five patients with celiac node metastases had limited node disease and 13 had extensive node disease.
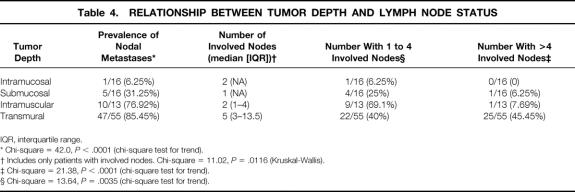
Relationship Between Tumor Depth and Lymph Node Metastases
The depth of tumor invasion was intramucosal in 16 patients, intramural/submucosal in 16, intramural/muscularis propria in 13, and transmural in 55. The prevalence of involved nodes and the number of lymph node metastases per patient increased with increasing tumor depth (Table 4). As the depth of the tumor increased, the percentage of patients with more than four lymph node metastases increased, and the proportion of patients with four or fewer nodes involved decreased. The likelihood of distant node metastases, specifically celiac node metastases, also increased with increasing tumor depth (Table 5). Only one patient with tumor invasion limited to the submucosa had more than four nodes involved. He was also the only patient with a tumor of this depth who had a distant node involved. Nodal disease in patients whose tumors invaded the muscularis propria was similar to those with transmural tumors with regard to prevalence, distant location, and celiac node involvement. The only difference between these latter two levels of invasion was that patients with tumors limited to the muscularis propria were less likely to have extensive nodal disease (more than four nodes involved).
Table 4. RELATIONSHIP BETWEEN TUMOR DEPTH AND LYMPH NODE STATUS
IQR, interquartile range.
* Chi-square = 42.0, P < .0001 (chi-square test for trend).
† Includes only patients with involved nodes. Chi-square = 11.02, P = .0116 (Kruskal-Wallis).
‡ Chi-square = 21.38, P < .0001 (chi-square test for trend).
§ Chi-square = 13.64, P = .0035 (chi-square test for trend).
Table 5. RELATIONSHIP BETWEEN TUMOR DEPTH AND NODE LOCATION
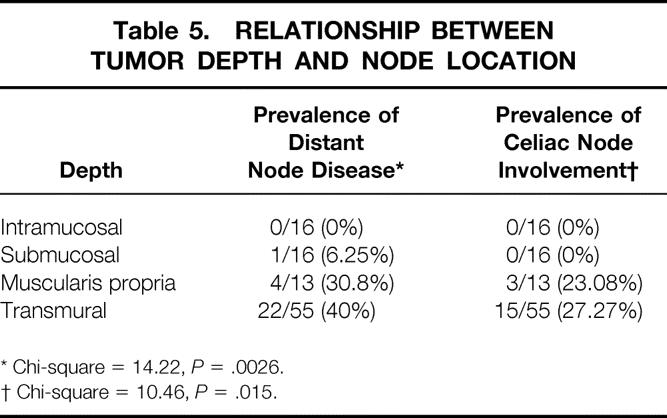
* Chi-square = 14.22, P = .0026.
† Chi-square = 10.46, P = .015.
Survival Analysis
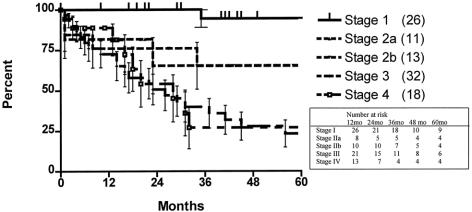
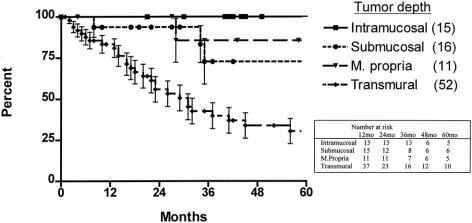
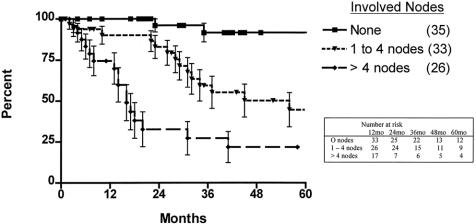
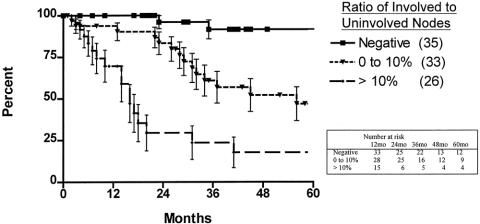
The 5-year survival rate in the 100 patients, including surgical deaths, was 52.2% (Fig. 1). Twenty-three patients were alive 5 years or more after surgery. Survival according to the current AJCC classification system is shown in Figure 2. To investigate the relationship between the various tumor characteristics and survival, the survival estimates shown in Table 6 were calculated excluding surgical deaths. Tumor depth significantly predicted survival (Fig. 3). As expected, the presence of nodal metastases also predicted survival. As the number of nodal metastases per patient increased (Fig. 4) or the lymph node ratio increased (Fig. 5), survival decreased. When lymph nodes were involved, the location of the nodes did not have an impact on survival; that is, patients with involvement of celiac nodes or other distant nodes had the same survival as patients with only local nodes involved.
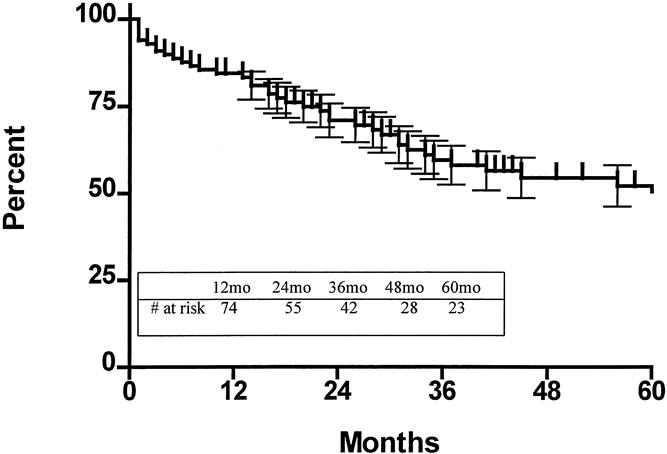
Figure 1. Actuarial survival, including surgical deaths, for the entire series.
Figure 2. Actuarial survival, including surgical deaths, according to the current American Joint Commission on Cancer staging classification system.
Table 6. PREDICTORS OF SURVIVAL
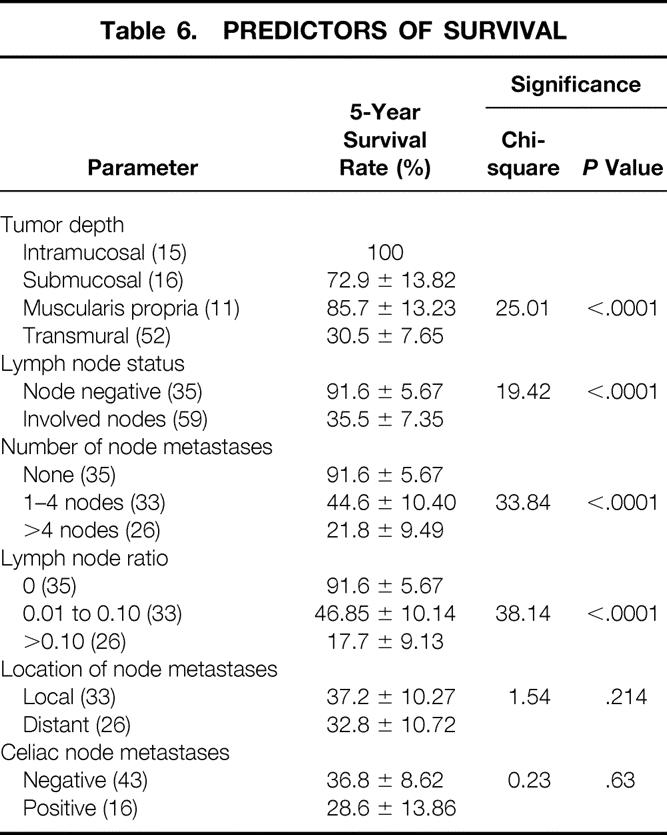
Figure 3. Actuarial survival, excluding surgical deaths, by tumor depth.
Figure 4. Actuarial survival, excluding surgical deaths, for patients with uninvolved nodes, one to four involved nodes, and more than four involved nodes.
Figure 5. Actuarial survival, excluding surgical deaths, according to lymph node ratio.
Three important observations are of note. First, of the 55 patients with transmural tumors, 8 (14.5%) had no lymph node metastases and enjoyed an excellent survival. Only one of these patients has developed recurrent disease. Second, the nine patients with tumors that invaded the muscularis propria or beyond who had lymph node metastases had a 83% 5-year survival rate after en bloc resection. Third, the 45 patients with transmural tumors who had lymph node metastases had a 25% 5-year survival after enbloc resection.
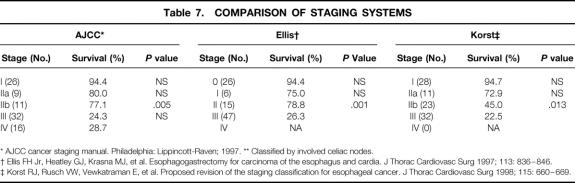
The proposed modifications of Skinner et al’s staging system 22 suggested by Ellis et al 21 and Korst et al 20 are compared with the current AJCC classification system in Table 7. Again, the calculations in this table exclude surgical deaths to evaluate which better predicted disease-related survival. The modifications of the Skinner staging system proposed by Korst allowed better stratification of patients in terms of disease-related survival.
Table 7. COMPARISON OF STAGING SYSTEMS
* AJCC cancer staging manual. Philadelphia: Lippincott-Raven; 1997. ** Classified by involved celiac nodes.
† Ellis FH Jr, Heatley GJ, Krasna MJ, et al. Esophagogastrectomy for carcinoma of the esophagus and cardia. J Thorac Cardiovasc Surg 1997; 113: 836–846.
‡ Korst RJ, Rusch VW, Vewkatraman E, et al. Proposed revision of the staging classification for esophageal cancer. J Thorac Cardiovasc Surg 1998; 115: 660–669.
Risk of Local Recurrence
Local recurrence was defined as either the development of nodal disease within the surgical field, or an anastomotic recurrence. After en bloc resection, no nodal recurrences were detected within the surgical field, and anastomotic recurrence was rare. Only one patient developed an anastomotic recurrence despite the use of colon for reconstruction and the performance of the anastomosis well above the tumor in the neck.
Risk of Systemic Recurrence
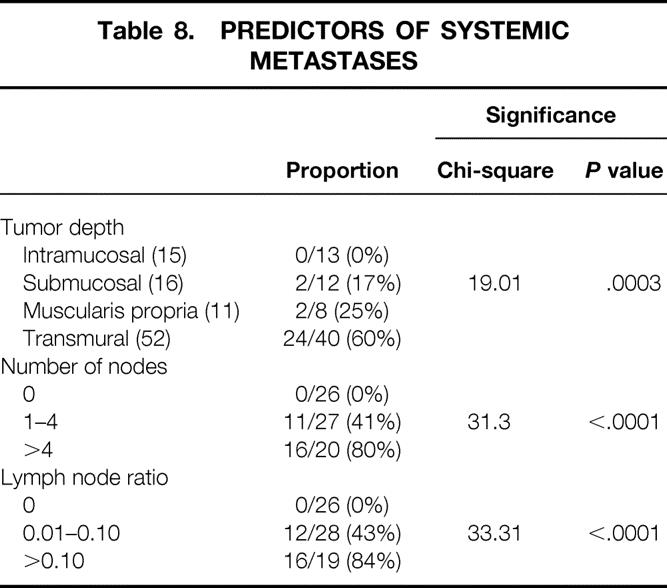
Distant organ metastases were identified in 31 patients. The median time to systemic recurrence was 10 months (IQR 5–21.5). To investigate factors that predicted the risk of developing systemic disease, only the 73 patients who underwent surgery before November 1998 were used for the calculations listed in Table 8. This date was chosen to ensure at least 2 years of follow-up, a period during which more than 75% of all systemic recurrences would have been expected to occur. This reduction of the study sample was necessary to avoid inclusion of patients who had not yet been followed a sufficient length of time to manifest systemic recurrence. Inclusion of these patients would skew the risk estimate calculations. Table 8 shows that the risk for development of systemic metastases progressively increased with the depth of tumor invasion, the number of involved nodes, and an increasing ratio of involved to uninvolved nodes. The highest risk was seen with transmural invasion (60%), more than four involved nodes (80%), or a ratio of involved to uninvolved nodes of more than 10% (84%).
Table 8. PREDICTORS OF SYSTEMIC METASTASES
Latent Nodal Recurrence
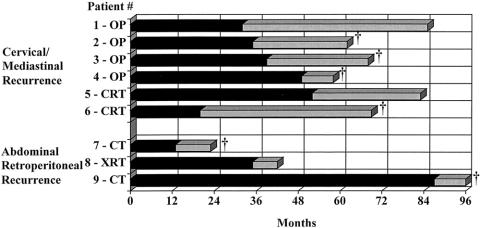
Latent lymph node recurrence outside the field of the en bloc resection, in the absence of systemic metastases, occurred in nine patients (Fig. 6). The median time to the emergence of the latent nodal recurrences was 35 months (IQR 25.5–50). This was significantly longer than the median time to systemic organ recurrence of 10 months observed in the 73 patients analyzed in the preceding paragraph for the risk of systemic recurrence (U statistic 46.5, P = .0027). Six of the nine patients developed cervical and/or superior mediastinal nodal recurrence, and three developed abdominal infrapancreatic retroperitoneal nodal recurrence. In four of these nine patients, the initial surgical specimen showed no involved nodes in the en bloc specimen; two had one to four involved nodes, and three had more than four involved nodes.
Figure 6. Time interval from initial surgery to treatment of latent nodal recurrence (dark bar) and outcome after treatment (light bar). †Death. OP, surgery; CT, chemotherapy; CRT, chemoradiotherapy; XRT, radiation therapy.
A second resection was attempted in five of the six patients with cervical and/or superior mediastinal nodes, and the operation was successful in four. Both the unoperated patient and the patient who could not be successfully resected received chemoradiotherapy. Three of the four patients who underwent a successful resection and the two unresected patients developed systemic recurrences at a median of 30.5 months after the detection of the latent nodal disease. Four of the six patients have died, and two are alive.
The three patients with latent abdominal retroperitoneal node recurrence were treated with chemotherapy (n = 2) and radiation therapy (n = 1). Of the three patients, two have died and one is alive.
DISCUSSION
The findings of this study on 100 consecutive en bloc resections performed as primary therapy for esophageal adenocarcinoma have defined the clinical biology of adenocarcinoma and determined what an operation based on classical principles of surgical oncology can accomplish. The results allow the assessment of outcomes of newly proposed treatment regimens and technologies.
It has been previously reported that the likelihood of nodal metastases in esophageal cancer depends on the depth to which the tumor invades the esophageal wall. 24,25 Our observations clearly support this concept. Tumor depth predicts not only the prevalence of nodal involvement but also the number of nodes involved. This observation has major implications regarding management of early tumors that are not visible on endoscopic ultrasound. In particular, when a tumor is limited to the submucosa, only 19% of the patients will have lymph node metastases, with only 3% having more than four nodes involved. Only 1 of the 32 patients with tumor extension limited to the submucosa had more than four involved nodes, and this same patient was the only patient with distant nodal, subcarinal and paratracheal, involvement. These findings indicate that an extended transhiatal resection 26 including a complete lymphadenectomy of the upper abdominal region and lower mediastinum performed through an enlarged hiatus would be adequate for complete removal of all disease in 97% of patients with tumor penetration limited to the submucosa.
Only 1 of the 16 patients with an intramucosal tumor had lymph node involvement, and in this patient a single node was involved. Recently, we have described that in patients with no visible lesion on endoscopy and a biopsy showing intramucosal cancer, the probability of lymph node metastases is small. 27 In such patients, it appears that resection can be limited to removal of the esophagus alone, without a lymph node dissection, sparing the vagal nerves to maintain gut innervation and preserve a functioning gastric reservoir. The esophagus is replaced by interposing a segment of colon between the cervical esophagus and the fundus of an intact innervated stomach. 28 If an intraoperative frozen section shows that the tumor unexpectedly extended into the submucosa than a node disection could be performed through the opened hiatus as described above.
In contrast to intramucosal or intramural/submucosal tumor extension, invasion of the muscularis propria or beyond was associated with lymph node involvement in 75% to 85% of patients, with up to 45% having more than four involved nodes, of which 30% to 40% were distant in location and 23% to 27% involved celiac nodes. These findings require that for tumors that extend into the muscularis propria or beyond, a systematic abdominal and thoracic lymph node dissection be performed. In this situation, a transthoracic exposure is necessary to perform an adequate mediastinal lymph node dissection.
When the depth of the tumor is limited to the muscularis propria, the enbloc procedure is particularly beneficial in that these patients have an outcome survival similar to patients with earlier diseases (Fig. 3). We have observed that the 5-year survival rate in patients with involved distant nodes, including the celiac group, did not differ from the survival rate in those with only local nodal involvement. This suggests that the current staging system for esophageal cancer needs to be adjusted by removing patients with celiac involvement from the category of M1 disease.
Our study found that the lack of local nodal involvement for tumors that invade the muscularis propria or beyond does not provide confidence that nodes more distant from the tumor will also be uninvolved. Although uncommon, we identified three patients in whom celiac nodal metastases were the only site of nodal spread, and seven additional patients in whom distant nodes were involved when four or fewer total nodes were involved. This finding supports the concept of skip nodal metastases that has been reported by others and further suggests that the concept of a sentinel lymph node is probably not applicable to adenocarcinoma of the esophagus. It also indicates that tumors invading the muscularis propria or beyond require a systematic mediastinal and abdominal lymph node dissection to achieve a complete (R0) resection. The observation that the survival of patients with involved nodes in distant sites does not differ from the survival of those with only local nodal involvement, and a 39% 5-year survival rate in patients with lymph node metastases and tumors that extended into muscularis propria or beyond, further supports the utility of an en bloc resection in such circumstances.
The observation that survival after en bloc resection depends heavily on the number of involved nodes calls into question the AJCC staging system’s classification with respect to nodal involvement. Rather than categorizing patients as simply N0 and N1 based on the absence or presence of nodal involvement, the staging system should be restructured to include three categories: uninvolved nodes, one to four involved nodes, and more than four involved nodes. 22,29,30 Our study supports this approach in that it provides a better stratification of patients in regard to disease-related survival (see Table 7).
The ratio of involved to uninvolved nodes has also been reported to be a predictor of survival, and various ratios have been proposed as indicators of poor survival. 31,32 We have found that the presence of more than 10% nodal involvement is associated with a poor survival and that higher ratios, suggested by others, are less able to predict survival with confidence.
The value of the number or ratio of lymph node metastases to predict survival is also reflected in their ability to identify patients at risk for the development of systemic metastases after complete resection. This observation can be used as an indication for postoperative adjuvant therapy. We have found that the presence of more than four involved nodes or a lymph node ratio of more than 10% identifies patients with an 80% or greater risk of developing systemic metastases. Our data show that a complete (R0) resection alone is unlikely to be curative in such patients, in that systemic recurrences commonly developed at a median of 10 months. On the basis of this finding, we recommend that patients with more than four involved lymph nodes or a ratio of more than 10% receive adjuvant chemotherapy after surgery, beginning within 2 to 3 months of surgery. Although previous studies have failed to show a benefit to postoperative chemotherapy, 33,34 they may not be applicable in that they were conducted at a time when squamous cell cancer was the dominant cell type, and they included all patients who underwent resection rather than selecting those known to be at high risk for systemic disease. This would have the effect of diluting the apparent benefit of adjuvant chemotherapy by including variable numbers of patients in the treatment arms who were at little risk of recurrence if nothing were done.
The occurrence of latent cervical and superior mediastinal nodal involvement in patients with adenocarcinoma of the esophagus is debated, and controversy persists as to whether a three-field lymph node dissection should be performed. 13,35,36 Adding a third field of dissection has been associated with increased complications 37 and has not been universally accepted. In our study, only six patients to date have developed latent cervical and/or superior mediastinal nodal recurrence, a frequency that makes the complications of a routine three-field dissection difficult to justify. In four patients, resection of the cervical and superior mediastinal lymph nodes was possible at the time of recurrence. The finding that three of these patients have subsequently gone on to develop systemic recurrence between 9 and 41 months after the node resection brings into question the benefits of this approach. The time period between the detection of the latent cervical/superior mediastinal nodal recurrence and the subsequent development of systemic metastases, more than 2 years on average, suggests that the systemic disease originated from the involved nodes. This observation supports the importance of a systematic lymph node dissection as part of the initial operation and would encourage the addition of a third field to the dissection if one were willing to accept the additional complications.
The low incidence of local recurrence in the field of surgery, even when multiple nodes are involved, is clear evidence of the ability of an en bloc resection to control local disease. In contrast, local recurrence has been reported in 14% of patients undergoing an Ivor-Lewis resection, 38 35% after a transhiatal resection, 39 and up to 32% after neoadjuvant chemoradiotherapy and a limited resection. 40 The use of neoadjuvant chemoradiotherapy as a means to reduce the local recurrence does not appear to be as effective as performing an en bloc resection.
The overall 5-year survival rate of 52% and the 34% 5-year survival rate for patients with lymph node metastases and tumors that extend into the muscularis propria or beyond, although encouraging, are not intended to provide evidence for the superiority of the en bloc esophagectomy, or to convince the reader to use the procedure. It is a technically demanding operation that requires considerably more time to complete than a standard esophagectomy. A dedicated team of specialists is necessary to perform the operation and care for the patient afterward to achieve acceptable death and complication rates. The technical expertise required to perform the surgery is a significant factor, and the learning curve is steep. Postoperative care is constant and complex for 10 to 14 days, and on occasion much longer. Recognizing these issues, it is doubtful that the procedure will gain widespread acceptance until a prospective randomized trial is accomplished to show the benefit of the en bloc procedure. If such a study were to show the superiority of the en bloc resection, it should be done only in a few select centers capable of organizing a team to perform the procedure and committed to providing the care after the procedure.
Our experience with the en bloc resection has provided us with the opportunity to study the clinical biology of esophageal adenocarcinoma in a way that is not possible when less-complete resections are performed. The absence of any preoperative or postoperative therapy in our study population allowed us to define the patterns of spread, the mode of recurrences, and the relationships between various tumor characteristics and outcome. By better defining the risk factors for recurrent disease, we can now identify patients who should be considered for postoperative adjuvant therapy. By defining the extent of disease in patients based on the depth of tumor invasion, we have the justification to use a more limited and less morbid resection in patients with lesions limited to the mucosa and submucosa.
Discussion
Dr. J. Rudiger Siewert (Munich, Germany): I am a little bit surprised that I have the honor to discuss this paper and never have seen the manuscript before. But I will do it on the basis of the presentation. First of all, I would like to congratulate you on this excellent analysis. There are only some smaller points to discuss.
One is: What are the selection criteria for these 100 patients? That means, have you seen in the same time period other patients which were excluded from the esophagectomy?
The second point: I would like to discuss the problem of local recurrences. In my experience it is extremely difficult to identify local recurrences following an esophagectomy. That means to detect local recurrences, you have to do a very careful CAT scan analysis. What have you done in your patient group?
The third point: You are accepting in your lecture a limited type of surgery. I agree absolutely with your suggestion, but I would like to stress that such recommendation is coming only from the retrospective analysis of your data and maybe we should discuss if the prospective analysis is valid enough for such a recommendation or bias?
Indeed, all the recommendations you have given to us were based on retrospective analysis, and I am absolutely convinced that it is very difficult to draw a conclusion only from retrospective analyses. But I think that retrospective analysis is absolutely necessary to ask the right questions in a prospective trial.
Last, but not least, I would like to discuss a little bit your recommendation to bring the group of patients with a lymph node ratio of above 10% in an adjuvant treatment protocol. Once more, that is absolutely unproven. All the meta-analyses of postoperative chemotherapy protocols are unable to present a benefit for the patients, postoperatively with chemotherapy.
But maybe these analyzed groups are too defined. Maybe your suggestion is the right one. Maybe we should look in the future to a very well defined group of patients on the basis of the lymph node ratio. But again, we have to do a prospective analysis to answer this question.
Presenter Dr. Jeffrey A. Hagen (Los Angeles, California): There is no question that the patients included in this series do not represent all of the patients we treated for adenocarcinoma of the esophagus. We use a fairly detailed preoperative assessment which is outlined in the manuscript, including endoscopic ultrasound, CT scan, and laboratory studies to exclude the presence of metastatic disease. In addition, these patients are carefully selected on the basis of their physiologic reserve using cardiopulmonary testing to be sure that they are fit for the procedure.
To monitor for local recurrence, our schedule of follow-up includes an office visit with the operating surgeon every three months for the first three years and every four to six months thereafter. At each of these visits, laboratory studies and CT scans of the chest and abdomen are performed to search for evidence of recurrent disease. With this schedule of careful follow-up, I think that our ability to identify patients with local recurrence and systemic metastases is quite good.
I agree that our data would only suggest the feasibility of more limited resections in patients with tumors confined to the mucosa and submucosa since we don’t have a prospective experience to document the efficacy of such an approach. I look forward to your presentation later today, when I am sure you will tell us a lot more about the efficacy of this type of approach. Until prospective studies are available, however, we are forced to rely on what is known about the biology of this disease to make these types of treatment decisions.
I also agree completely that a prospective trial would be desirable. However, this would be difficult thing to accomplish for several reasons. First, the numbers of patients are still relatively small in any given center, and thus a multicenter trial would be required. Because the operation itself is technically demanding, this type of trial could only be performed in a limited number of centers. Clearly, however, that is what will be necessary to determine for certain whether this operation is a better cancer operation. However, our goals were to clarify the biology of the disease, to define prognostic factors and to identify predictors of recurrence rather than to try to convince people that the en bloc resection was superior.
With respect to your comments regarding adjuvant therapy, I agree that the studies that have been done in the past have not shown a benefit to therapy administered after resection. There are, however, a couple of differences in terms of the approach we propose. One major difference is that past trials did not include a complete resection and lymph node dissection. This leads to questions regarding both the adequacy of local therapy and the accuracy of staging. Second, these studies included essentially all patients after surgery rather than identifying patients at high risk for the development of metastases, which could have a big impact on the outcome of the adjuvant therapy.
Dr. Alex G. Little (Las Vegas, Nevada): I would like to begin by expressing my admiration for what I can only call the fortitude of this group in persisting with an attempt to find a way to cure these patients surgically. I can attest from personal experience that this is a real commitment of time and energy and really is a continuation of a very impressive Western pedigree that runs through Logan and Skinner and on in a line of surgeons that really have made a significant commitment.
Having said that, I would like to agree a little bit with Dr. Siewert in terms of the conclusions that can be drawn from a single approach to a disease and point out that while there is nothing really deceptive about the data, it is a little dangerous, or can at least lead to unfortunate implications if people simply look at the overall five-year survival of nearly 50%. Obviously you really have to really look at survival for specific stages.
While excellent survival outcomes come from the Stage 1 and Stage 2 patients, if you look at the Stage 3, the T3N1 patients, who unfortunately are still the majority in most of our experiences, their survival is not very different from that obtained with a range of different operations and approaches.
So I would follow up with Dr. Siewert. I personally am still uncertain about when to apply a more or less aggressive approach, and I struggle with it with my patients. And, Dr. Hagen, I would suggest there are enough institutions that would participate in a well-designed focused prospective randomized comparison, with or without neoadjuvant therapy, of a radical to a less radical operation. I think the time has come to do that. And I would encourage your leadership in trying to get that to take place.
And I am just curious about one thing, the number of stage 1 patients you are fortunate enough to have. Did those come from surveillance of Barrett’s patients? I hope you aren’t taking them out of Las Vegas. Please give them back if you are.
Dr. Jeffrey A. Hagen: Thank you very much, Dr. Little, for your comments. We are indeed, I think, indebted to those giants who have done a lot of work in this area before us, such as Drs. Logan and Skinner as you mentioned. Again, I agree completely that it would be desirable to perform a prospective randomized trial, and maybe that is something we need to look at now, to try to answer the question about the extent of resection for esophageal adenocarcinoma.
With respect to the patients with stage 1 tumors, the majority of them were identified in the course of Barrett’s surveillance programs. About one-quarter were incident cancers detected at the first endoscopy during which Barrett’s was detected.
Dr. Murray F. Brennan (New York, New York): If I understand correctly, you conclude that five-year survival for stage 4 disease is 27% and you conclude that celiac nodes and remote nodes are not a factor in survival. And yet you conclude that greater than four nodes is a factor in survival, do I misunderstand?
Dr. Jeffrey A. Hagen: That is correct. Our data clearly show that it is the number of involved nodes, and not their location, that is of prognostic importance. In this series patients operated on for stage IV disease were stage by virtue of celiac node involvement as opposed to systemic metastases. Our demonstration that there is no difference in survival between patients with celiac node involvement and those with nodal involvement elsewhere points out the need for change in the staging system. We suggest a change in ‘N’ status to reflect the impact of the number of nodes, or the lymph node ratio, and in the ‘N‘ status to include only patients with metastases in sites other than the regional nodes.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association, April 26–28, 2001, the Broadmoor Hotel, Colorado Springs, Colorado
Correspondence: Tom R. DeMeester, MD, Professor and Chairman, Department of Surgery, Keck School of Medicine, University of Southern California, 1510 San Pablo St., Suite 514, Los Angeles, CA 90033.
E-mail: demeester@surgery.hsc.usc.edu
Accepted for publication April 26, 2001.
References
- 1.Wang HH, Antonioli DA, Goldman H. Comparative features of esophageal and gastric adenocarcinomas: recent changes in type and frequency. Hum Pathol 1986; 17: 482–487. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, Devesa SS, Fraumeni JFJ. Continuing climb in rates of esophageal adenocarcinoma: an update [letter]. JAMA 1993; 270: 1320–1321. [PubMed] [Google Scholar]
- 3.Sabile J, Rice TW, Goldblum JR, et al. Superficial esophageal carcinoma. Ann Thorac Surg 1995; 60: 896–902. [DOI] [PubMed] [Google Scholar]
- 4.Sampliner RE, Jaffe P. Malignant degeneration of Barrett’s esophagus: the role of laser ablation and photodynamic therapy. Dis Esoph 1995; 8: 104–108. [Google Scholar]
- 5.Takeshita K, Tani M, Inoue H, et al. Endoscopic treatment of early oesophageal or gastric cancer. Gut 1997; 40: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. World J Surg 1992; 16: 1104–1109. [DOI] [PubMed] [Google Scholar]
- 7.Le Prise EL. Cancer of the esophagus: outcome of neoadjuvant therapy on surgical morbidity and mortality [in French]. Cancer Radiother 1998; 2: 763–770. [DOI] [PubMed] [Google Scholar]
- 8.Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepato-Gastroenterology 1994; 41: 391–393. [PubMed] [Google Scholar]
- 9.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996; 335: 462–467. [DOI] [PubMed] [Google Scholar]
- 10.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997; 337: 161–167. [DOI] [PubMed] [Google Scholar]
- 11.Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001; 19: 305–331. [DOI] [PubMed] [Google Scholar]
- 12.Skinner DB, Dowlatshahi KD, DeMeester TR. Potentially curable cancer of the esophagus. Cancer 1982; 50 (11 Suppl): 2571–2575. [PubMed] [Google Scholar]
- 13.Altorki NK, Girardi L, Skinner DB. En bloc esophagectomy improves survival for stage III esophageal cancer. J Thorac Cardiovasc Sur 1997; 114: 948–956. [DOI] [PubMed] [Google Scholar]
- 14.Bumm R, Feussner H, Bartels H, et al. Radical transhiatal esophagectomy with two-field lymphadenectomy and endodissection for distal esophageal adenocarcinoma. World J Surg 1997; 21: 822–831. [DOI] [PubMed] [Google Scholar]
- 15.Stark SP, Romberg MS, Pierce GE, et al. Transhiatal versus transthoracic esophagectomy for adenocarcinoma of the distal esophagus and cardia. Am J Surg 1996; 172: 478–481. [DOI] [PubMed] [Google Scholar]
- 16.Orringer MB. Transhiatal esophagectomy without thoracotomy for carcinoma of the thoracic esophagus. Ann Surg 1984; 200: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagen JA, DeMeester TR. En bloc oesophagectomy for cancer of the distal oesophagus, cardia and proximal stomach. In Jamieson GG, Debas HT, eds. Surgery of the upper gastrointestinal tract. London: Chapman & Hall; 1994: 214–229.
- 18.Casson AG, Rusch VW, Ginsberg RJ, et al. Lymph node mapping of esophageal cancer [letter]. Ann Thorac Surg 1994; 58: 1569–1570. [DOI] [PubMed] [Google Scholar]
- 19.AJCC cancer staging manual. Philadelphia: Lippincott-Raven; 1997:65–69.
- 20.Korst RJ, Rusch VW, Venkatraman E, et al. Proposed revision of the staging classification for esophageal cancer. J Thorac Cardiovasc Surg 1998; 115: 660–669. [DOI] [PubMed] [Google Scholar]
- 21.Ellis FH Jr, Heatley GJ, Krasna MJ, et al. Esophagogastrectomy for carcinoma of the esophagus and cardia: a comparison of findings and results after standard resection in three consecutive eight-year intervals with improved staging criteria. J Thorac Cardiovasc Surg 1997; 113: 836–846. [DOI] [PubMed] [Google Scholar]
- 22.Skinner DB, Little AG, Ferguson MK, et al. Selection of operation for esophageal cancer based on staging. Ann Surg 1986; 204: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–462. [Google Scholar]
- 24.Nigro JJ, Hagen JA, DeMeester TR, et al. Prevalence and location of nodal metastases in distal esophageal adenocarcinoma confined to the wall: implications for therapy. J Thorac Cardiovasc Surg 1999; 117: 16–23. [DOI] [PubMed] [Google Scholar]
- 25.Rice TW, Zuccaro G Jr, Adelstein DJ, et al. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thor Surg 1998; 65: 787–792. [DOI] [PubMed] [Google Scholar]
- 26.Alderson D, Courtney SP, Kennedy RH. Radical transhiatal oesophagectomy under direct vision. Br J Surg 1994; 81: 404–407. [DOI] [PubMed] [Google Scholar]
- 27.Nigro JJ, Hagen JA, DeMeester TR, et al. Occult esophageal adenocarcinoma: extent of disease and implications for effective therapy. Ann Surg 1999; 230: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akiyama H, Tsurumaru M, Ono Y, et al. Esophagectomy without thoracotomy with vagal preservation. J Am Coll Surg 1994; 178: 83–85. [PubMed] [Google Scholar]
- 29.Hagen JA, Peters JH, DeMeester TR. Superiority of extended en bloc esophagogastrectomy for carcinoma of the lower esophagus and cardia. J Thorac Cardiovasc Surg 1993; 106: 850–859. [PubMed] [Google Scholar]
- 30.Ellis FH, Watkins E, Krasna MJ, et al. Staging of carcinoma of the esophagus and cardia: a comparison of different staging criteria. J Surg Oncol 1993; 52: 231–235. [DOI] [PubMed] [Google Scholar]
- 31.Roder JD, Busch R, Stein HJ, et al. Ratio of invaded to removed lymph nodes as a predictor of survival in squamous cell carcinoma of the oesophagus. Br J Surg 1994; 81: 410–413. [DOI] [PubMed] [Google Scholar]
- 32.Holscher AH, Bollschweiler E, Bumm R, et al. Prognostic factors of resected adenocarcinoma of the esophagus. Surgery 1995; 118: 845–855. [DOI] [PubMed] [Google Scholar]
- 33.Ando N, Iizuka T, Kakegawa T, et al. A randomized trial of surgery with and without chemotherapy for localized squamous carcinoma of the thoracic esophagus: the Japan Clinical Oncology Group Study. J Thorac Cardiovasc Surg 1997; 114: 205–209. [DOI] [PubMed] [Google Scholar]
- 34.Roth JA, Pass HI, Flanagan MM, et al. Randomized clinical trial of preoperative and postoperative chemotherapy with cisplatinum, vindesine, and bleomycin for carcinoma of the esophagus. J Thorac Cardiovasc Sur 1988; 96: 242–248. [PubMed] [Google Scholar]
- 35.Akiyama H, Tsurumaru M, Udagawa H, Kajiyawa Y. Principles of surgical treatment for carcinoma of the esophagus: analysis of lymph node involvement. Ann Surg 1981; 194: 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice TW. Superficial oesophageal carcinoma: is there a need for three-field lymphadenectomy? Lancet 1999; 354: 792–794. [DOI] [PubMed] [Google Scholar]
- 37.Fujita H, Kakegawa T, Yamana H, et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg 1995; 222: 654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King RM, Pairolero PC, Trastek VF, et al. Ivor-Lewis esophagogastrectomy for carcinoma of the esophagus: early and late functional results. Ann Thorac Surg 1987; 44: 119–122. [DOI] [PubMed] [Google Scholar]
- 39.Hulscher JB, van Sandick JW, Tijssen JG, et al. The recurrence pattern of esophageal carcinoma after transhiatal resection. J Am Coll Surg 2000; 191: 143–148. [DOI] [PubMed] [Google Scholar]
- 40.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998; 339: 1979–1984. [DOI] [PubMed] [Google Scholar]