Abstract
Objective
To determine whether surgical residency training has influenced the occurrence of common bile duct injuries during laparoscopic cholecystectomy, and to asses the anatomic and technical details of bile duct injuries from the practices of surgeons trained in laparoscopic cholecystectomy after residency versus surgeons trained in laparoscopic cholecystectomy during residency.
Summary Background Data
Shortly after the introduction of laparoscopic cholecystectomy, the rate of injury to the common bile duct increased to 0.5%, and injuries were more commonly reported early in each surgeon’s experience. It is not known whether learning laparoscopic cholecystectomy during surgery residency influences this pattern.
Methods
An anonymous questionnaire was mailed to 3,657 surgeons across the United States who completed an Accreditation Council for Graduate Medical Education (ACGME)-approved residency between 1980 and 1990 (group A) or 1992 and 1998 (group B). All surgeons in group A learned laparoscopic cholecystectomy after residency, and all those in group B learned laparoscopic cholecystectomy during residency. Information obtained included practice description, number of laparoscopic cholecystectomies completed since residency, postgraduate training in laparoscopy, and annual volume of laparoscopic cholecystectomy in the surgeon’s hospital. In addition, technical details queried included the completion of a cholangiogram, the interval between injury and identification, the method of repair, and the site of definitive treatment. The primary endpoint was the occurrence of a major bile duct injury during laparoscopic cholecystectomy (bile leaks without a major bile duct injury were not tabulated).
Results
Forty-five percent (n = 1,661) of the questionnaires were completed and returned. Mean practice experience was 13.6 years for group A and 5.4 years for group B. At least one injury occurrence was reported by 422 surgeons (37.6%) in group A and 143 surgeons (26.5%) in group B. Forty percent of the injuries in group A occurred during the first 50 cases compared with 22% in group B. Thirty percent of bile duct injuries in group A and 32.9% of all injuries in group B occurred after a surgeon had performed more than 200 laparoscopic cholecystectomies. Independent of the number of laparoscopic cholecystectomies completed since residency, group A surgeons were 39% more likely to report one or more biliary injuries and 58% more likely to report two or more injuries than their counterparts in group B.
Bile duct injuries were more likely to be discovered during surgery if a cholangiogram was completed than if cholangiography was omitted (80.9% vs. 45.1%). Sixty-four percent of all major bile duct injuries required biliary reconstruction, and most injuries were definitively treated at the hospital where the injury occurred. Only 14.7% of injuries were referred to another center for repair.
Conclusions
Accepting that the survey bias underestimates the true frequency of bile duct injuries, residency training decreases the likelihood of injuring a bile duct, but only by decreasing the frequency of early “learning curve” injuries. If one accepts a liberal definition of the learning curve (200 cases), it appears that at least one third of injuries are not related to inexperience but may reflect fundamental errors in the technique of laparoscopic cholecystectomy as practiced by a broad population of surgeons in the United States. Intraoperative cholangiography is helpful for intraoperative discovery of injuries when they occur. Most injuries are repaired in the hospital where they occur and are not universally referred to tertiary care centers.
Laparoscopic cholecystectomy (LC) is the most commonly performed operation on the digestive tract. Many reports have cited increased use of cholecystectomy after the introduction of laparoscopy. 1 Between 1991 and 1997, residents’ experience with LC increased by 64%, while the rate of open cholecystectomies performed by residents decreased by 63%. 2 Concomitantly, it has been well established that as LC was gaining popularity, the number of bile duct injuries increased. 3–7 In one statewide audit, the number of bile duct repairs almost tripled between 1988 and 1992. 8 In response to data substantiating an increased rate of biliary injury occurring during a surgeon’s early experience with LC, the term “learning curve” was coined. 9–12
Most surgeons initially learned LC through 1- to 3-day postgraduate courses and often incorporated it into their practices without supervision. Proctoring, although encouraged, was not mandatory. Consequently, during the first several procedures, many surgeons became anatomically confused, unwittingly transecting the bile duct early in the dissection. Although it is clear that inexperience was responsible for many early biliary injuries, it is distressing that continued reports documenting an increased rate of bile duct injury bear witness to a problem that has not gone away. 13
Although recent studies have suggested that residency training in LC is associated with excellent outcomes, 14 longer operating times for LC were often reported when residents performed the operation. 15,16 Recent reports show that residents are now performing LC with equivalent safety and in equal time as their attendings, and often in patients who are less healthy than were previously operated on laparoscopically. 17,18 Although these reports suggest that the residency training experience might improve surgical outcomes for LC once these residents are in practice, this has not been established.
Technical factors leading to biliary injury are often caused by errors of perception during dissection in Calot’s triangle, including misidentification of anatomy, and failure to recognize injuries when they occur. 19 In addition, acute cholecystitis, a difficult dissection, and bleeding 20 are associated with higher rates of major bile duct injury during LC.
Using routine surgical cholangiography to prevent bile duct injuries is controversial. In 1993, the National Institutes of Health’s consensus conference on LC was unable to resolve the controversy over whether routine (or any) intraoperative cholangiography was warranted. 21 Routine cholangiography during LC is often taught to residents to ensure that they have the opportunity to master the technique and to demonstrate unusual anatomy and common bile duct stones in a learning environment. Often, controlled series reporting excellent results of LC find little use for routine cholangiography, 22 whereas others that focus on bile duct injuries or that report outcomes from broad populations of surgeons support the use of routine cholangiography. 23,24
The aim of this study was to clarify whether residency training has had the desired consequence of attenuating the learning curve and decreasing the severe biliary complications associated with LC. In addition, this study was designed to better understand the relationship of cholangiography to the identification and management of bile duct injuries associated with LC.
METHODS
A two-page, 18-item questionnaire was developed in collaboration with a statistician (D.W.B.). The questionnaire was designed to investigate the educational and anatomic/technical features associated with laparoscopic common bile duct injury. Bilomas occurring as a result of cystic duct stump leaks were not included in the survey.
Demographic data collected included the characteristics of the practice of the surgeon (rural or urban location, private or university practice). Respondents were asked to estimate the number of LCs completed at the time of each reported injury; however, because most surgeons do not know the exact number of procedures completed, either in total or at the time of an injury, categoric responses (e.g., 0–25, 26–50, 51–100, 101–200, >200) were used. Additional questions in the questionnaire included:
Number of LCs completed during residency
Estimated number of LCs performed annually at the surgeon’s hospital
Attendance at postgraduate courses in advanced laparoscopy, completion of a postgraduate fellowship, or a fellowship in minimally invasive surgery.
Location of the injury along the biliary tree
Whether a cholangiogram was completed during a case in which an injury occurred
Whether the injury was identified during surgery
Whether the surgeon believed the case in which an injury occurred was difficult
Intervention used to treat the injury
Location of initial and definitive treatment of the injury.
In collaboration with eight United States Surgical Corporation Centers of Excellence, the questionnaire was mailed to 3,657 surgeons throughout the United States between July and October 1999. Addresses were obtained from the American College of Surgeons 1998 yearbook. Inclusion required completion of an ACGME-approved surgical residency between 1980 and 1998. Based on the year that surgical residency was completed, surgeons were divided into two groups for data analysis. Group A comprised surgeons who completed residency between 1980 and 1990 and who had no experience with LC in residency, and group B comprised surgeons who completed residency between 1991 and 1998, all of whom were trained in laparoscopy as part of their residency.
Group A surgeons were chosen by dividing the country into eight geographic regions and then randomly selecting an equivalent number of surgeons within each region who filled the above entry criteria. All surgeons in the American College of Surgeons yearbook with residency completion dates between 1991 and 1998 were included in group B. This was necessary because the pool of surgeons available was smaller in the younger population.
A letter from the senior author (J.G.H.) was mailed with each survey. Surgeons who completed their training at one of the collaborating centers also received a letter from a senior member of the faculty at that center. The letters encouraged the recipients to respond to the questionnaire and outlined the study hypothesis. No remuneration was offered for responding.
All survey recipients were assured that their responses would remain anonymous. Each respondent was assigned a code number affixed to the return envelope, which allowed analysis by group. All mailings were sent and received at one location. Questionnaires were mailed to each surgeon only once, and no follow-up letters were sent; however, questionnaires that were returned for the wrong address were mailed again if the correct address could be found. If the correct address could not be found (n = 202) or if the surgeon indicated that LC was not part of his or her practice, the questionnaire was not analyzed (n = 164). Envelopes returned with the code number removed (n = 11) were also eliminated. The data were entered into a Microsoft Access 2000 database (9.0.2720) (Microsoft Corp., Redmond, WA). Questionnaires were collected until January 2000.
The data were analyzed using a statistical software package, Stata 5.0 (Stata Corp., College Station, TX). A Pearson chi-square test was used to assess differences in variable proportions across categories. Pairwise comparisons in variable means across categories were made using a two-tailed t test. The Fisher exact test was used in the presence of a contingency table cell with five or fewer observations. Years of surgical experience were calculated for each group as a mean, and conditional means were calculated to determine the likelihood of a surgeon reporting a second injury if he or she had reported one, controlling for the experience of the surgeons within the group. Regression analysis of mean number of injuries was used to calculate the relative risk of an injury occurring in either group.
RESULTS
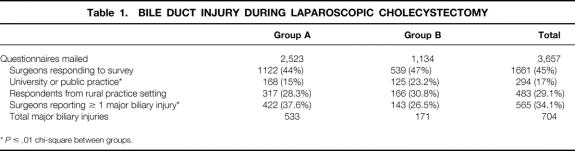
A total of 3,657 questionnaires were mailed, 2,523 (69%) to surgeons in group A and 1,134 (31%) to surgeons in group B. A total of 1,661 surgeons (45%) returned completed surveys, 1,122 (44%) from group A and 539 (47%) from group B (Table 1). The only demographic difference between groups (except age) was that surgeons in group B were more likely to practice in a university or in a public practice (e.g., the Indian Health Service) than surgeons in group A.
Table 1. BILE DUCT INJURY DURING LAPAROSCOPIC CHOLECYSTECTOMY
*P ≤ .01 chi-square between groups.
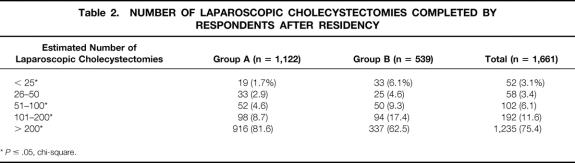
Surgeons in group A had a mean of 13.6 years of surgical experience, compared with 5.4 years for group B surgeons (P < .05, two-sided t test). In addition, a higher proportion of group A surgeons had completed more than 200 LCs after surgical residency than group B surgeons (Table 2). The hospitals where group A surgeons practiced were more likely to have a yearly LC volume of more than 200 cases than the hospitals where group B surgeons practiced (73% vs. 68.3%, P < .05).
Table 2. NUMBER OF LAPAROSCOPIC CHOLECYSTECTOMIES COMPLETED BY RESPONDENTS AFTER RESIDENCY
*P ≤ .05, chi-square.
Instruction or training in LC for all group A surgeons was completed in postgraduate courses. Ninety percent of group B surgeons, all of whom learned the operation during residency, completed more than 25 LCs during residency. There was no difference between groups with respect to completion of a fellowship in a surgical subspecialty (19.3%, group A; 16.3%, group B); however, fewer group A surgeons had completed a fellowship in laparoscopy than group B surgeons (0.8% vs. 3.5%, P < .05).
A total of 565 of the 1,661 (34%) respondents reported 704 biliary tract injuries. In group A, 422 of 1,122 (38%) respondents reported 533 injuries; in group B, 143 of 539 (26%) respondents reported 171 biliary tract injuries (P = .05) (see Table 1). Only one bile duct injury was reported by 447 (79%) surgeons: 329 (78%) from group A and 118 (82%) from group B. More than one injury was reported by 118 (21.9%) respondents (Fig. 1): 75 (17%) surgeons in group A and 22 (15.3%) surgeons in group B reported two injuries, 17 (4%) surgeons in group A and 3 (<1%) surgeons in group B reported three injuries, and 1 surgeon in group A reported four injuries. A conditional mean was calculated to determine the mean number of injuries per surgeon for those surgeons in both groups who reported at least one injury. The mean number of injuries per surgeon for surgeons reporting at least one injury was 1.3 ± 0.5 for group A and 1.2 ± 0.4 for group B (P = NS).
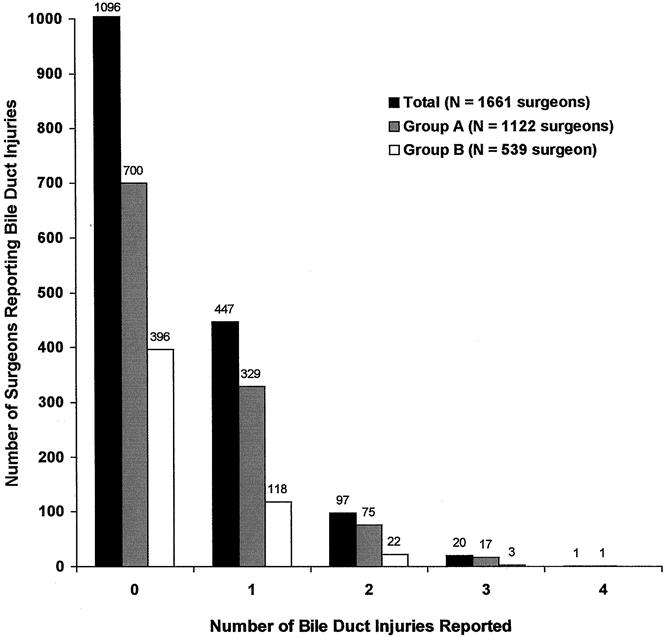
Figure 1. Number of bile duct injuries reported by all respondents. In group A, 422 surgeons reported 533 biliary injuries. In group B, 143 surgeons reported 171 biliary injuries. In group A, 75 surgeons reported two biliary injuries, 17 reported three injuries, and 1 reported four injuries. In group B, 22 surgeons reported two biliary injuries and 3 reported three injuries.
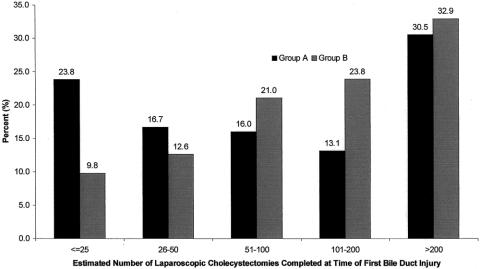
The number of LCs completed at the time of the first bile duct injury is shown in Figure 2. Although a higher percentage of surgeons in group A reported their first bile duct injuries in the first 50 LCs, surgeons in group B reported a greater percentage of total injuries than group A from cases 50 to 200. Combining two surgical volume categories, 40.3% of those in group A and 22.4% of those in group B reported that the injury occurred within the first 50 cases (P < .001) (Fig. 3). Further, the percentage of surgeons reporting their first injury within their first 100 cases was also higher in group A (56% vs. 43%, P = .008). However, the difference between the groups was not significantly different comparing the number of surgeons who reported their first injury within the first 200 cases. After completing 200 cases, the proportions of surgeons reporting their first injury was similar between groups.
Figure 2. Respondents from group A and group B reported the estimated number of laparoscopic cholecystectomies completed at the time of their first bile duct injury. A higher percentage of surgeons in group A reported injuries early in their experience compared with group B; however, the frequency of reported first injuries equalized in the category “more than 200 cases.” Approximately one third of surgeons reporting an injury in either group reported that the injury occurred after having completed 200 cases. Although it appears that the incidence of injury is increasing in group B, correction for the size of the category reveals a decreasing injury rate. Beyond 200 cases, the incidence cannot be calculated because the total experience (denominator) is unknown.
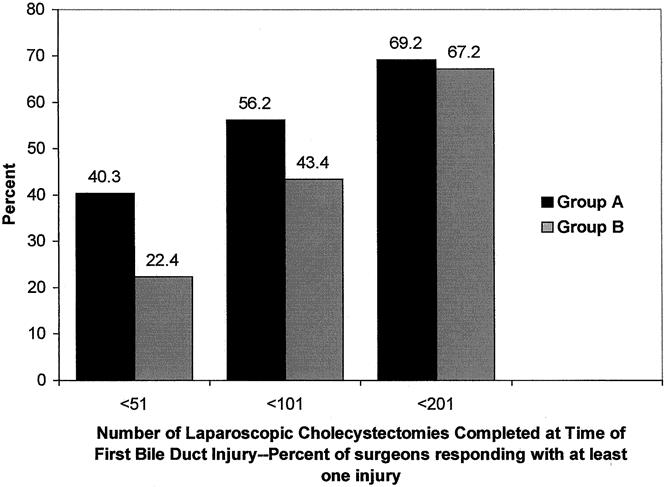
Figure 3. Number of laparoscopic cholecystectomies completed at the time of first reported bile duct injury, combining response categories of surgeons reporting at least one injury. More surgeons in group A than group B reported injuries within the first 50 cases completed. This observation was also true when calculating the number of surgeons reporting their first injury in the first 100 cases. The percentage of surgeons reporting their first injury within 200 cases, however, was similar between groups.
Independent of the number of LCs completed since residency, surgeons from group A were 39% more likely to report at least one injury than group B surgeons (odds ratio = 1.39, 95% confidence interval = 1.10–1.175). Further, compared with group B surgeons, group A surgeons were 58% more likely to report two or more injuries after adjustment for the number of LCs completed since residency (odds ratio = 1.58, 95% confidence interval = 1.00–2.50).
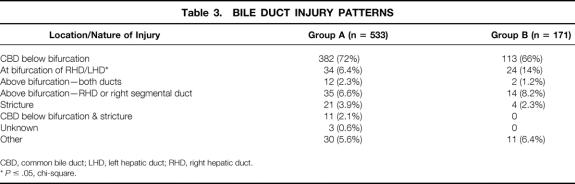
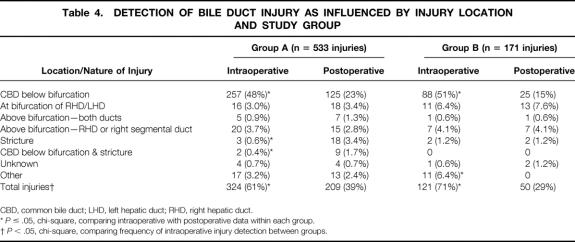
Approximately two thirds of reported bile duct injuries in both groups occurred below the bifurcation of the common bile ducts (Table 3). Group B surgeons reported more injuries at the bifurcation of the bile ducts (15% vs. 7.6%, P ≤ .05), and these injuries were more commonly discovered after surgery (Table 4). All other injury patterns were reported with similar frequency between groups. Regardless of group, more injuries below the bifurcation of the bile duct were identified during surgery than after surgery, and more strictures were identified after surgery than during surgery (P < .05) In addition, more surgeons in group B reported identifying injuries during surgery than surgeons in group A (71% vs. 61%, P ≤ .05).
Table 3. BILE DUCT INJURY PATTERNS
CBD, common bile duct; LHD, left hepatic duct; RHD, right hepatic duct.
*P ≤ .05, chi-square.
Table 4. DETECTION OF BILE DUCT INJURY AS INFLUENCED BY INJURY LOCATION AND STUDY GROUP
CBD, common bile duct; LHD, left hepatic duct; RHD, right hepatic duct.
*P ≤ .05, chi-square, comparing intraoperative with postoperative data within each group.
†P < .05, chi-square, comparing frequency of intraoperative injury detection between groups.
Fifty-one percent of surgeons in group A and 48% of surgeons in group B completed a cholangiogram during an operation in which an injury occurred (P = NS). More group A surgeons obtained static cholangiograms in cases in which an injury occurred (24% vs. 17%, P = .05).
Respondents were asked whether they believed the operation in which an injury occurred was “routine” or “difficult.” Group B surgeons reported more frequently that the operation was “difficult” (71% vs. 60%, P ≤ .05) (Table 5).
Table 5. CASES IN WHICH AN INJURY OCCURRED: PERFORMANCE OF CHOLANGIOGRAPHY AND DISSECTION DIFFICULTY
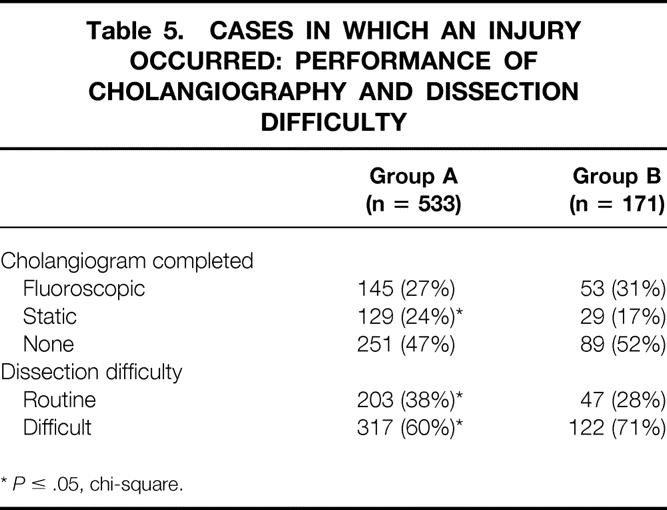
*P ≤ .05, chi-square.
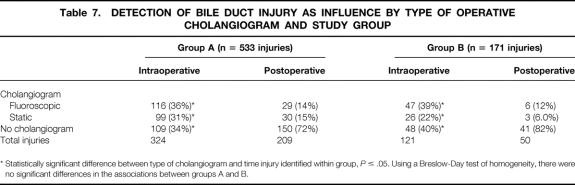
Independent of group, the performance of cholangiography increased the likelihood of injury recognition. Eighty-one percent of surgeons from either group who obtained a cholangiogram discovered the bile duct injury during surgery, compared with 45% of surgeons who did not obtain a cholangiogram (P ≤ .05) (Table 6). Likewise, surgeons within each group were more likely to discover an injury during surgery if a cholangiogram was performed compared with surgeons within their own group who did not obtain a cholangiogram. There was no difference, however, in the likelihood of discovering an injury during surgery and the use of cholangiography between groups A and B (Table 7).
Table 6. DETECTION OF BILE DUCT INJURY AS INFLUENCED BY PERFORMANCE OF OPERATIVE CHOLANGIOGRAM (ANY TYPE, ALL CASES)
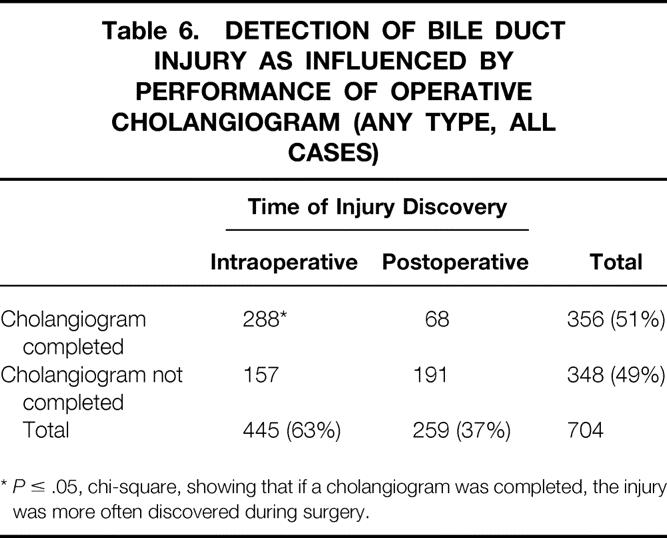
*P ≤ .05, chi-square, showing that if a cholangiogram was completed, the injury was more often discovered during surgery.
Table 7. DETECTION OF BILE DUCT INJURY AS INFLUENCE BY TYPE OF OPERATIVE CHOLANGIOGRAM AND STUDY GROUP
* Statistically significant difference between type of cholangiogram and time injury identified within group, P ≤ .05. Using a Breslow-Day test of homogeneity, there were no significant differences in the associations between groups A and B.
Interventions used to treat bile duct injuries and the frequency of their use are shown in Table 8. Of the 704 injuries reported, 450 (64%) required biliary reconstruction; however, only 15% of these repairs were completed at a referral hospital, and 85% were completed at the hospital where the injury occurred. Bile duct injuries occurring in rural practices were referred to another hospital for definitive treatment 24.1% of the time; those from urban practices were referred 10.2% of the time (P ≤ .05).
Table 8. INTERVENTIONS FOR BILE DUCT INJURIES AND LOCATION OF TREATMENT
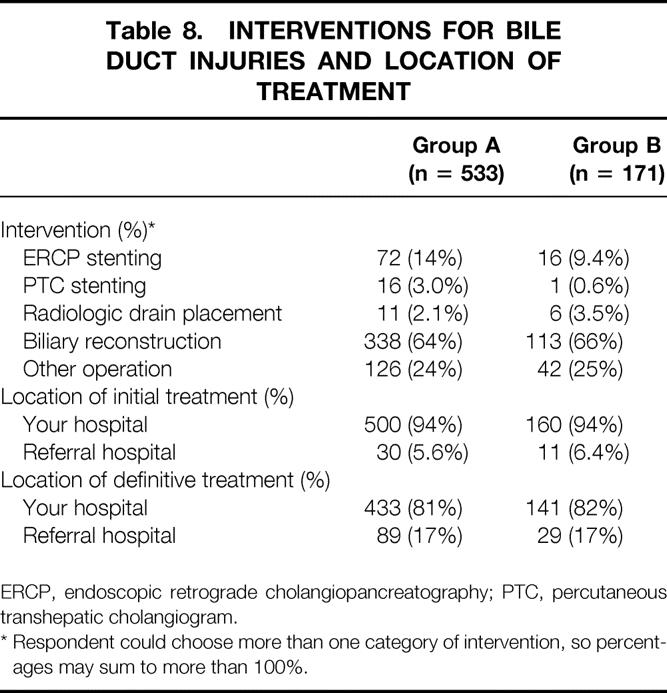
ERCP, endoscopic retrograde cholangiopancreatography; PTC, percutaneous transhepatic cholangiogram.
* Respondent could choose more than one category of intervention, so percentages may sum to more than 100%.
DISCUSSION
This study shows that LC training during residency is associated with fewer learning curve injuries to the bile duct but has no effect on injuries occurring after 200 cases have been performed. Further, cholangiography performed during surgery increases the likelihood of intraoperative detection of bile duct injuries. This study did not attempt to determine whether the performance of cholangiography prevented bile duct injury, because it focused exclusively on the cases in which an injury occurred. In addition, we could not calculate the incidence of bile duct injury for either group because we did not attempt to collect the total experience of any surgeon. We believed that if we asked a surgeon to tabulate and validate his or her entire experience, the task would be sufficiently onerous to the conscientious surgeon that the questionnaire would be discarded. The common tendency of humans to exaggerate their experience would have made any other method of gathering total experience less accurate than the categorical methodology we used. The size of each category was different from all others to sample carefully at the early part on a surgeon’s experience and to decrease the compound error of recollection as experience was accrued.
While exaggerating their experience, surgeons are likely to underestimate the frequency of complications; however, this memory bias is unlikely to affect the recollection of catastrophic complications with great emotional impact, such as biliary tract injury. Some critics might impugn a 45% response rate as insufficient to draw conclusions, but given the sensitive nature of these injuries and the fear, by many, of discovery, we believed this response was better than expected. Because the response rate was equivalent between groups, statistical comparisons are valid. Nonetheless, it is likely that these data underestimate the magnitude of the problem, because surgeons injuring bile ducts would be expected to reply less frequently than those free of such injury.
Laparoscopic Training and Learning Curve
Surgeons in group A were more likely than those in group B to report the occurrence of a biliary injury within the first 100 LCs performed in their practice; however, after 200 LCs, the number of biliary injuries reported had become nearly equivalent for the two groups. These results suggest two conclusions: first, residency training in LC allowed the surgeon to gain the experience needed to avoid the early injuries that plagued surgeons who learned LC through postgraduate courses. We do not know whether resident trainees were involved in cases during residency that resulted in biliary injury. If this happened, we assume the responsibility for the injury belonged to the attending physician (and may have been counted in group A). Regardless, after resident trainees had completed a surgical residency that included LC training, their likelihood of injuring a bile duct was attenuated but not eliminated compared with those who did not receive such training during residency.
Nearly one third of injuries occurred after a surgeon had performed 200 LCs, regardless of his or her training. This may indicate that late injuries are related to the technique of the operation itself rather than to the training of the surgeon. Another recent population-based report found that increasing experience (during a 5-year period) was unrelated to the incidence of biliary tract injury during LC. 25 Likewise, a community-based survey of surgeons in one city found that the learning curve was not important in determining when, in a surgeon’s experience, an injury to the biliary tree would occur during LC. 26 Further study of the technical details of these late injuries is needed to determine whether any correctable patterns exist that could change LC technique or could assist in remedial training for surgeons who experience a late biliary injury in their practice.
Although surgeons in group A had more years of surgical experience and had completed more LCs since learning the operation than surgeons in group B, if one controlled for the number of LCs completed since learning the operation, group A surgeons were 39% more likely than group B surgeons to report at least one biliary injury and 58% more likely to report two more injuries compared with group B surgeons. This finding suggests that residency training has led to improvement in the safety of LC. It also suggests that the occurrence of one injury should prompt a surgeon to review his or her LC technique to lessen the possibility of another injury.
Anatomy and Treatment of Injuries
As in previous reports, 27 injuries to the bile ducts during LC were seen along the length of the extrahepatic biliary tree, but the most frequent site reported in the present study was the common bile duct below the bifurcation of the right and left hepatic ducts (65%). Surgeons in group B reported a higher percentage of injuries at the bifurcation of the bile ducts, but these injuries represented a minority of the total (15.4%). Otherwise, the distribution of injuries along the biliary tree was similar between groups, indicating that the pattern of injury may be a function of the operation itself rather than the training of the surgeon. In other words, once anatomic confusion has led the surgeon astray, injury tends to occur in relatively predictable locations along the biliary tree no matter who is doing the operation.
The results of the questionnaire show that two thirds of bile duct injuries required biliary reconstruction. Although the operation used for repair was not ascertained, hepaticojejunostomy is usually necessary, because the proximal location of injuries and the intense inflammatory reaction at the site of the injury makes more distal repair prone to stricture formation. 28,29 Because treatment of an injury after it occurs is not usually a function of laparoscopic training or skill, it is not surprising that there were no differences between groups in the management strategy and the frequency of referral for bile duct reconstruction.
Cholangiography
Despite mounting evidence that biliary injury is less likely when routine cholangiography is used, 24,30–33 respondents completed a cholangiogram in half of the operations in which a bile duct injury occurred. Surgeons in group B were no more likely to have performed a cholangiogram during an index case than surgeons in group A. Despite this, group B surgeons discovered injuries during surgery more frequently than surgeons in group A. This finding may reflect a better understanding of laparoscopic biliary anatomy, a greater vigilance for bile leaks, better videooptics, or one of several other factors. This survey did not attempt to assess the routine use of cholangiography, but it is likely that the rate is similar to the 50% utilization reported in the biliary injury cases. From these data it appears that residency training has little effect on the application of cholangiography, at least in cases in which a bile duct injury occurs.
Despite the apparent benefits of cholangiography, there are equal numbers of surgeons who decry its use as time-consuming, expensive, the cause of injuries, and unnecessary for the discovery of asymptomatic choledocholithiasis. 34–36 If one excludes from analysis the prevention or detection of low-frequency events such as bile duct injury, then selective cholangiography is usually found to be a more cost-effective approach. 34 None of the studies advocating selective cholangiography have been large enough to pick up statistical differences in bile duct injury patterns between selective and routine cholangiography. In addition, the results of series by expert surgeons may not represent the practices of an unselected population.
This study shows that intraoperative cholangiography is associated with intraoperative detection of bile duct injuries at a rate almost double that of cases in which no cholangiogram was completed (81% vs. 45%). Neither the perceived difficulty of the operation nor the era of surgical residency training affected this finding. Other large, population-based studies showed similar findings. In Switzerland, a retrospective review of more than 10,000 LCs found that cholangiography led to the intraoperative discovery of bile duct injury in 75% of patients who sustained an injury. 37 In Belgium, a survey that reviewed more than 9,000 LCs found a significantly improved detection rate after intraoperative cholangiography (68% vs. 32%, P ≤ .01); however, cholangiograms were performed in only 34% of the patients in the study. 38
A recent report of liver biopsies in patients referred for repair of bile duct injuries illustrates the benefit of early detection and treatment of bile duct injuries, showing that a delay in referral led to evidence of hepatic fibrosis in 31% of patients. 27 Finally, if injuries are discovered during surgery, the cost of treatment is 43% to 83% less than if discovered later, and hospital stays are reduced by 76%. 39 Based on these studies and the present study, increased use of cholangiography would result in a greater intraoperative detection rate for biliary injuries, which would lead to reduced injury-associated costs and complications. Whether the added costs of routine cholangiography would eliminate this economic benefit is a matter of local cost allocation and operative efficiency.
Referral Patterns
Beyond case reports, no large series of bile duct injury repairs exists outside tertiary referral centers, and the rates of referral to specialized centers for reconstruction were previously unknown. We were surprised to find that the overwhelming majority of injuries were treated without referral. Although surgeons in rural practice referred patients for definitive treatment more frequently than their urban counterparts (24% vs. 10%, P < .05), more than 90% of injuries were initially treated at the hospital where they occurred, and only 20% were referred to another center for definitive treatment. It appears that about half of the patients who were referred represented treatment failures at the first hospital. In addition, a small percentage of patients may have received care at a tertiary referral center without the knowledge of the original surgeon. Because of the devastating psychological impact of a bile duct injury and the fear of litigation, we believe it is unlikely that many surgeons were unaware that their patient had been referred to a tertiary referral center. Although most bile duct injuries may be adequately managed without referral, treatment failures, complex injuries, and surgeon inexperience with the technique of hepaticojejunostomy warrant consultation from a specialized center.
CONCLUSIONS
One of the hallmarks of surgical residency training is graduated responsibility and personal supervision by attending surgeons during operations. After LC was introduced in the United States, most surgeons quickly learned the operation without the benefit of proctoring, and this may have led to the intraoperative errors that resulted in bile duct injuries. Early reports addressing the increasing rate of bile duct injury after cholecystectomy focused on methodical and well-visualized dissection of the gallbladder–cystic duct junction before clips are applied and any structure is cut. 40
Recent emphasis on medical errors by the lay press and in the medical literature indicate that as many as half of medical errors are preventable. 41 Residents learning LC during residency appear to benefit from the experience of their attendings and the process of graduated responsibility and close supervision. Nonetheless, there remains a worrisome proportion (26% in this report) of surgeons trained to do laparoscopy in residency who reported injuring at least one bile duct after residency training. Further refinements of the technique of the operation and in the education of surgeons in training are needed to reduce the number of bile duct injuries. An injury rate of one in a thousand may seem acceptable to some, but it probably seems too high to the 600 to 700 patients in the United States annually who sustain bile duct injuries. The economic impact of this is approximately $40 million (extrapolating from single-center data), before legal expenses are added to the bill.
In this survey, bile duct injuries during LC were more commonly found during surgery if a cholangiogram was completed, regardless of the training of the surgeon; however, fewer than half of the surgeons reporting an injury completed a cholangiogram during the operation. More injuries would likely be discovered in the operating room if the use of cholangiography were increased. The overwhelming majority of reported patients with bile duct injuries were treated locally and not sent to referral centers, even if they required biliary reconstruction. Further investigation into the outcomes of locally treated biliary injuries and investigation into the types of injuries or clinical scenarios that should prompt early referral would be of benefit.
The introduction of LC to general surgery provided a unique perspective on how surgeons acquire new skills and assimilate new technology. It appears that even experienced surgeons were at higher risk of creating a biliary injury early in their LC experience. It is often said that laparoscopic operations must be performed so that the results are at least equivalent to the same operation performed in a conventional fashion. Perhaps it should also be stated that the introduction and learning of new technology and operations should follow a similar dictum that surgeons should be proctored while learning new techniques so that the benefit of another’s experience serves to attenuate the trainee’s learning curve.
Acknowledgments
The authors thank Cyndi Lyon and Ramaz Metrovelli, MD, for help with distribution of the questionnaire. Also, the authors acknowledge the endosurgery fellows who made up the USSC Bile Duct Collaborative Group and who aided in study design and in data collection: Brent Matthews, MD, Ed Chekan, MD, Amjad Ali, MD, Scot Roth, MD, Larry Damore, MD, Omar Bholat, MD, Daniel Scott, MD, Renee Wolff, MD, Dieter Pohl, MD, and Christina Richards, MD.
Discussion
Dr. Lawrence W. Way (San Francisco, California): The premise of this work is that the technique of laparoscopic cholecystectomy is learned better within a residency program – in other words, the result is an attenuation of the learning curve – than in the spontaneous, short, unproven courses, which were the only educational opportunities available for surgeons already in practice when laparoscopic cholecystectomy got started in the early 1990s. Who would argue otherwise? Mountains of evidence show that humans best acquire procedural skills through structured practice under expert guidance, with gradual withdrawal of supervision as technical competency is approached.
To support the hypothesis, the authors surveyed practicing surgeons who had learned this operation in two different environments, using their reported numbers of bile duct injuries while in practice as a measure of expertise. The surgeons who had learned how to perform the operation in the educationally deprived setting (Group A) reported more bile duct injuries than the control group (Group B) did, and the excess was confined to the first 200 cases. The findings suggested that learning the operation during residency avoided the complications associated with inexperience, the so-called learning curve.
I have questions about the design of the experiment, however, relative to its stated aims and conclusions. If the purpose were to determine the effects of residency training on the learning curve of this operation, the initial experience of both groups would have to be measured. The learning curve of Group A took place in post-residency practice; the learning curve of Group B took place within residency. We have data for Group A, but no data were collected for the learning curve period for Group B, so we are unable to make any conclusions regarding the effects of residency training on the learning curve. Do you agree?
The authors also say that their data suggest that residency training has improved the safety of this operation. While I suspect that residency training actually does have that effect, I question whether the survey findings provide empirical evidence either way. Once again, we would need data on the frequency of bile duct injury during residency training in order to know. A further reason for caution is that during the period of this study, the residents were learning the operation under the supervision of surgeons whose experience was typified by Group A. In other words, we could reasonably assume that the bile duct injuries occurring in the practices of Group A surgeons involved cases that formed the learning curves of the Group B surgeons, who at that time were residents.
The authors did not ask their respondents for anything but the crudest estimates of numbers of cases performed, assuming that the reliability of such figures generated from memory would not be worth having. Consequently, rates of injury cannot be calculated, although this would be the most valid index of overall performance.
While the data are soft – something the authors unhesitatingly acknowledge – the experiment is a sincere effort to address a critically important issue, whose facets include how to introduce new procedural technology into post-residency practice, how to define the learning curve, and how much of the curve should be sheltered inside a structured, supervised training program, and how to accomplish these things in a practical, affordable manner.
Presenter Dr. Stephen B. Archer (Atlanta, Georgia): In terms of our being unable to address the learning curve of the Group B surgeons who learned laparoscopy after residency with those who learned during residency, you asked me if I agree or disagree with you. And I disagree.
The reason I disagree is that we have garnered the experience of the people who taught those surgeons in Group A, as you said, but also if we had counted the bile duct injuries of the residents while they were in training we would have been asking for their bile duct injuries as well as the bile duct injuries of the people who trained them, and that would have been a form of double dipping.
But even beyond that, I really believe that the experience of being a resident is a very different experience from being a surgeon out on one’s own and that residents, as they are operating in their residency, wear the cloak of the experience of their teachers. Once that cloak is removed things are very different than they were before. That is why we feel the groups are different.
Dr. Keith D. Lillemoe (Baltimore, Maryland): I think we all agree that learning a procedure during a residency is better than learning it on your own. Specifically for this operation, it is better than the two-day courses that many attended back in the early 1990s. I do have a few questions, however, about this study.
Currently at our institution, lap-chole has become a procedure performed by second- and third-year residents, leaving a two- to three-year gap between their most concentrated experience and when they do the procedure on their own. Obviously, these individuals will be better trained at the end of their five years, but do you think this gap in time may lead to potential problems in the future?
Second of all, having read your manuscript a couple times as well as listened carefully to your discussion, I am still not convinced that experience of your Group B has simply not matured to the point of the surgeons in Group A. That is, have the surgeons in Group B really done enough procedures to know what their true incidence of injury will be?
And finally, I would like to offer a different interpretation of your data. The data shows the highest incidence of injury for the Group A surgeons was in their first 25 cases, likely done between the years 1990 and 1993. I would contend that the knowledge obtained since that time concerning the mechanisms of injury and the appropriate techniques to avoid injury, so nicely defined by your senior authors Drs. Branum and Hunter, plus Bill Meyers, Nat Soper, and Larry Way, has made us all much smarter in how to do this operation safely. I would appreciate your comments concerning this point.
Dr. Stephen B. Archer: Concerning your first question about the gap in experience between a junior resident and a chief resident, one thing I will say is that the chief residents in 1999 completed on average almost 80 laparoscopic cholecystectomies. If there is a gap in the experience between early years and late years, then that could make a difference. And we don’t have a way right now of knowing how that gap would affect the frequency of reported bile duct injuries.
You also asked whether or not the surgeons in Group B might have a frequency of injuries that would be higher because they just haven’t had enough time to have enough cases to show their injuries yet. To that I would say that there were 10% of surgeons in Group B who had completed less than 25 laparoscopic cholecystectomies since completing their surgical residency and in that group the number of injuries was no different statistically than the group who completed more than 25, indicating that the rate of injuries was the same and that the benefit of laparoscopic cholecystectomy was gained in residency and not afterwards.
Finally, you asked whether or not what we are seeing in these data are the experiences of those who worked out the problems with the operation early in the laparoscopic era. I think you are, to some extent, and that the rates of injuries have fallen overall partially as a result of that. But that may also be simply saying that those who teach us have done a good job. And our proposal for introducing new technologies and new techniques in surgery would be to echo the necessity of working out technical problems before we get into the same kind of trouble again.
Footnotes
Presented at the 121st Annual Meeting of the American Surgical Association, April 26–28, 2001, the Broadmoor Hotel, Colorado Springs, Colorado.
The United States Surgical Corporation funded this research through the Emory Endosurgery Center of Excellence grant. The United States Surgical Corporation (USSC) Bile Duct Injury Collaborative Group was composed of the following USSC Centers of Excellence: Emory University; Baylor College of Medicine; Duke University; Ohio State University; Penn State University; University of Texas, Southwestern, Dallas; University of Kentucky; University of Washington; and Vanderbilt University.
Correspondence: Stephen Archer, MD, P.O. Box 5488, Bend, OR 97708.
Accepted for publication April 26, 2001.
References
- 1.Rocco O, Russell JC, Lynch J, et al. Laparoscopic cholecystectomy: a statewide experience. Arch Surg 1993; 128: 494–499. [DOI] [PubMed] [Google Scholar]
- 2.Pars CJ, Organ CH Jr, Barkan H. Changing patterns of resident operative experience from 1990 to 1997. Arch Surg 2000; 135: 570–573. [DOI] [PubMed] [Google Scholar]
- 3.Adamsen S, Hansen OH, Funch-Jensen P, et al. Bile duct injury during laparoscopic cholecystectomy: a prospective nationwide series. J Am Coll Surg 1997; 184: 571–578. [PubMed] [Google Scholar]
- 4.Merrie AE, Booth MW, Shah A, et al. Bile duct imaging and injury: a regional audit of laparoscopic cholecystectomy. Aust NZ J Surg 1997; 67: 706–711. [DOI] [PubMed] [Google Scholar]
- 5.Legorreta AP, Silber JH, Costantino GN, et al. Increased cholecystectomy rate after the introduction of laparoscopic cholecystectomy. JAMA 1993; 270: 1429–1432. [PubMed] [Google Scholar]
- 6.Shea JA, Berlin JA, Bachwich DR, et al. Indications for outcomes of cholecystectomy: a comparison of the pre and postlaparoscopic eras. Ann Surg 1998; 227: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman GR, Hunter JG. Laparoscopic cholecystectomy: results from a university hospital. Am J Surg 1991; 162: 576–579. [DOI] [PubMed] [Google Scholar]
- 8.Rutledge R, Fakhry SM, Baker CC, et al. The impact of laparoscopic cholecystectomy on the management and outcome of biliary tract disease in North Carolina: a statewide, population-based, time-series analysis. J Am Coll Surg 1996; 183: 31–45. [PubMed] [Google Scholar]
- 9.Deziel DJ, Millikan KW, Economou SG, et al. Complications of laparoscopic cholecystectomy: a national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg 1993; 165: 9–14. [DOI] [PubMed] [Google Scholar]
- 10.Hunter JG, Sackier JM, Berci G. Training laparoscopic cholecystectomy: quantifying the learning curve. Surg Endosc 1994; 8: 28–31. [DOI] [PubMed] [Google Scholar]
- 11.Hunter JG. The learning curve in laparoscopic cholecystectomy. Minimally Invasive Therapy & Allied Technologies 1997; 6: 24–25. [Google Scholar]
- 12.A prospective analysis of 1518 laparoscopic cholecystectomies. The Southern Surgeons Club. N Engl J Med 1991; 324:1073–1078. [DOI] [PubMed]
- 13.Windsor JA, Pong J. Laparoscopic biliary injury: more than a learning curve problem. Aust NZ J Surg 1998; 68: 186–189. [DOI] [PubMed] [Google Scholar]
- 14.Ferzli GS, Fiorillo MA, Hayek NE, et al. Chief resident experience with laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech 1997; 7: 147–150. [DOI] [PubMed] [Google Scholar]
- 15.Traverso LW, Koo KP, Hargrave K, et al. Standardizing laparoscopic procedure time and determining the effect of patient age/gender and presence or absence of surgical residents during operation. A prospective multicenter trial. Surg Endosc 1997; 11: 226–229. [DOI] [PubMed] [Google Scholar]
- 16.Hodgson WJ, Byrne DW, Savino JA, et al. Laparoscopic cholecystectomy. The early experience of surgical attendings compared with that of residents trained by apprenticeship. Surg Endosc 1994; 8: 1058–1062. [DOI] [PubMed] [Google Scholar]
- 17.Wu JS, Dunnegan DL, Luttmann DR, et al. The evolution and maturation of laparoscopic cholecystectomy in an academic practice. J Am Coll Surg 1998; 186: 554–561. [DOI] [PubMed] [Google Scholar]
- 18.Matthews BD, Williams GB. Laparoscopic cholecystectomy in an academic hospital: evaluation of changes in perioperative outcomes. Journal of the Society of Laparoscopic Surgery 1999; 3: 9–17. [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart L, Way L. Bile duct injuries during laparoscopic cholecystectomy: factors that influence results of treatment. Arch Surg 1995; 130: 1123–1128. [DOI] [PubMed] [Google Scholar]
- 20.Davidoff AM, Papas TN, Murray AE, et al. Mechanisms of major biliary injury during laparoscopic cholecystectomy. Ann Surg 1992; 215: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institutes of Health consensus development conference statement of gallstones and laparoscopic cholecystectomy. Am J Surg 1993; 165:390–398. [DOI] [PubMed]
- 22.Taylor OM, Sedman PC, Jones BM, et al. Laparoscopic cholecystectomy without operative cholangiogram: 2038 cases over a 5-year period in two district general hospitals. Ann R Coll Surg Engl 1997; 79: 376–380. [PMC free article] [PubMed] [Google Scholar]
- 23.Moossa AR, Mayer AD, Stabile B. Iatrogenic injury to the bile duct. Who, how, where? Arch Surg 1990; 125: 1028–1030. [DOI] [PubMed] [Google Scholar]
- 24.Fletcher DR, Hobbs MS, Tan P, et al. Complications of cholecystectomy: risks of the laparoscopic approach and protective effects of operative cholangiography: a population-based study. Ann Surg 1999; 229: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvete J, Sabater L, Camps B, et al. Bile duct injury during laparoscopic cholecystectomy: myth or reality of the learning curve? Surg Endosc 2000; 14: 608–611. [DOI] [PubMed] [Google Scholar]
- 26.Jones Monahan K, Gruenberg JC. Bile duct injuries during laparoscopic cholecystectomy: a community’s experience. Am Surg 1998; 64: 638–642. [PubMed] [Google Scholar]
- 27.Moossa AR, Easter DW, van Sonnenberg E, et al. Laparoscopic injuries to the bile duct. A cause for concern. Ann Surg 1992; 215: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson SR, Koehler A, Pennington LK, Hanto DW. Long-term results of surgical repair of bile duct injuries following laparoscopic cholecystectomy. Surgery 2000; 128: 668–677. [DOI] [PubMed] [Google Scholar]
- 29.Lilliemoe KD, Melton GB, Cameron JL, et al. Postoperative bile duct strictures: management and outcome in the 1990s. Ann Surg 2000; 232: 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter JG. Laparoscopic cholecystectomy and the common bile duct. Surg Endosc 1994; 8: 285–286. [DOI] [PubMed] [Google Scholar]
- 31.Way LW. Bile duct injury during laparoscopic cholecystectomy. Ann Surg 1992; 215: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vezakis A, Davides D, Ammori BJ, et al. Intraoperative cholangiography during laparoscopic cholecystectomy. Surg Endosc 2000; 14: 1118–1122. [DOI] [PubMed] [Google Scholar]
- 33.Olsen D. Bile duct injuries during laparoscopic cholecystectomy. Surg Endosc 1997; 11: 133–138. [DOI] [PubMed] [Google Scholar]
- 34.Soper NJ, Denegan DL. Routine versus selective intra-operative cholangiography during laparoscopic cholecystectomy. World J Surg 1992; 16: 1133–1140. [DOI] [PubMed] [Google Scholar]
- 35.Silverstein JC, Wavak E, Millikan KW. A prospective experience with selective cholangiography. Am Surg 1998; 64: 654–658. [PubMed] [Google Scholar]
- 36.Snow LL, Weinstein LS, Hannon JK, et al. Evaluation of operative cholangiography in 2043 patients undergoing laparoscopic cholecystectomy. A case for the selective operative cholangiogram. Surg Endosc 2001; 15: 14–20. [DOI] [PubMed] [Google Scholar]
- 37.Z’graggen K, Wehrli H, Metzger A, et al. Complications of laparoscopic cholecystectomy in Switzerland. A prospective 3-year study of 10,174 patients. Swiss Association of Laparoscopic and Thoracoscopic Surgery. Surg Endosc 1998; 12: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 38.Gigo J, Etienne J, Aerts R, et al. The dramatic reality of biliary tract injury during laparoscopic cholecystectomy. An anonymous multicenter Belgian survey of 65 patients. Surg Endosc 1997; 11: 1171–1178. [DOI] [PubMed] [Google Scholar]
- 39.Savader SJ, Lilllemoe KD, Prescott CA, et al. Laparoscopic cholecystectomy-related bile duct injuries: a health and financial disaster. Ann Surg 1997; 225: 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunter JG. Avoidance of bile duct injury during laparoscopic cholecystectomy. Am J Surg 1991; 161: 71. [DOI] [PubMed] [Google Scholar]
- 41.Kohn LT, Corrigan JM, Donaldson MS, eds. To err is human: building a safer health system. Washington DC: The National Academy of Sciences; 2000. [PubMed]