Abstract
Objective
To analyze the impact on prognosis of the number of lymph node metastases detected by ultrasound and endoscopic ultrasound in patients with esophageal carcinoma.
Summary Background Data
Ultrasound and endoscopic ultrasound are useful for diagnosing tumor depth and lymph node metastasis in patients with esophageal carcinoma. However, the clinical significance of the number of lymph node metastases before surgery has not been elucidated.
Methods
The authors evaluated lymph node metastases using preoperative ultrasound and endoscopic ultrasound in 329 consecutive patients who underwent esophagectomy with lymphadenectomy. TNM classification and one-to-one comparison of lymph node metastasis was performed between the preoperative and histologic diagnosis. The number of lymph node metastases was subdivided into four groups: zero, one to three, four to seven, and eight or more.
Results
The accuracy of preoperative ultrasound and endoscopic ultrasound diagnosis exceeded 70% in each category of TNM classification. The incidence of lymph node metastasis determined by preoperative and histologic diagnosis was 69.0% (234/339) and 59.3% (201/339), respectively. The correlation between preoperative and histologic diagnosis was significant (P < .0001). According to the subdivision of number of lymph node metastases, the accuracy rates associated with nodal involvement of zero, one to three, four to seven, and eight or more were 83.8%, 59.7%, 43.3%, and 96.0%, respectively. The clinical outcome between ultrasound and endoscopic ultrasound diagnosis and histologic diagnosis in stage grouping was almost similar. The 5-year survival rates of patients with zero, one to three, four to seven, and eight or more lymph node metastases determined by ultrasound and endoscopic ultrasound were 53.3%, 33.8% 17.0%, and 0%, respectively. The differences among groups were statistically significant. The survival curves associated with preoperative and histologic diagnosis were similar.
Conclusions
Not only the stage grouping of TNM classification but also the number of lymph node metastases determined by ultrasound and endoscopic ultrasound before surgery may be useful for predicting prognosis in patients with esophageal carcinoma.
Lymph node metastasis is an important factor in determining the prognosis of patients with cancer. Lymph node groupings according to anatomic sites and the number of lymph node metastases have been assessed and correlated with the prognosis of patients with gastrointestinal carcinoma. 1–5 The TNM classification of the International Union Against Cancer 6 has recently applied the number of lymph node metastases to carcinoma of the stomach, colon and rectum, pancreas, and breast. In the Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus by the Japanese Society for Esophageal Disease, 7 lymph node grouping has been revised according to the number of lymph node metastases.
Developments in imaging techniques, especially in ultrasonography and endoscopic ultrasonography, have improved the presurgical diagnosis of lymph node metastasis in gastrointestinal tumors. Both procedures are considered reliable for the preoperative staging of esophageal carcinoma. 8–10 We also reported that ultrasound and endoscopic ultrasound are useful tools for diagnosing lymph node metastasis. 11–13 The presence or absence of lymph node metastasis should be evaluated before surgery to determine the suitable treatment strategy.
The present study evaluated the accuracy of ultrasound and endoscopic ultrasound in the presurgical diagnosis of tumor depth and lymph node metastasis, and especially in the impact of the number of lymph node metastases on the prognosis of patients with esophageal carcinoma.
METHODS
Patients
Between 1988 and 1998, 475 consecutive patients with carcinoma of the esophagus were admitted to the First Department of Surgery of Kagoshima University Hospital. Surgical or endoscopic procedures were performed in 380 patients. Of these, 329 who underwent esophagectomy with lymphadenectomy were included in this study, and 51 with endoscopic mucosal resection or blunt dissection without lymphadenectomy were excluded. The 307 male patients and 22 female patients ranged in age from 36 to 85 years (mean 63.7). Thirty-eight tumors were located in the upper third of the esophagus, 170 in the middle third, and 121 in the lower third. Based on the TNM classification, 6 T1 tumors were found in 128 patients, T2 in 35, T3 in 134, and T4 in 61. Histologically, 309 tumors were squamous cell carcinoma and the remaining 20 were miscellaneous, such as carcinosarcoma, basaloid, and undifferentiated carcinoma. Esophagectomy by right thoracotomy was performed in 277 patients, left thoracotomy in 22, and blunt dissection with lymphadenectomy in 30. The number of resected nodes per patient ranged from 12 to 141 (median 48.1). Preoperative radiation, chemotherapy, or both was given to 49 patients and postoperative therapy was given to 111. All patients were followed up after discharge as follows: radiographic examination every 1 to 3 months, computed tomography every 3 to 6 months, and ultrasonography every 6 months. Bronchoscopic and endoscopic examinations were performed when necessary. The clinical course of the patients was monitored for at least 2 years after discharge. Follow-up data after surgery were available for all patients, and the median follow-up period was 33.6 months (range 1–145).
Ultrasonographic Examination
Ultrasound examination was carried out using the following linear-array scanners: Toshiba SLA-50A, SSA-90A (Toshiba Medical Systems, Carson, CA), Yokogawa U-sonic Model RT-2000 and Aloka SSD-256 (Aloka Co., Tokyo, Japan) with a 3.75- or 5-mHz transducer. Endoscopic ultrasound examination was performed with a 7.5-mHz linear-array scanner (Machida, EPE-703FL, and Toshiba, SSA-90A).
The sites of lymph nodes were classified according to the nomenclature and code number of the Japanese Society for Esophageal Diseases (Fig. 1). 7 One-to-one comparisons between the preoperative diagnosis by ultrasound and endoscopic ultrasound and histologic diagnosis were carried out based on the above nomenclature. Mediastinal lymph nodes were examined by endoscopic ultrasound and cervical and abdominal lymph nodes by ultrasound. Mediastinal lymph nodes were scanned along large vessels, such as the brachiocephalic trunk, the ascending aorta, the pulmonary artery and vein, or the azygos vein. Anatomic structures such as the trachea, the spine, the pleura, and the heart were reliably identified by endoscopic ultrasound. Similarly, abdominal lymph nodes near large arteries such as the celiac axis (e.g., the left gastric artery nodes, the common hepatic artery nodes, or the celiac artery nodes) were easily identified. Cervical lymph nodes were also assessed along the common carotid artery, the jugular vein, the trachea, or the thyroid gland.
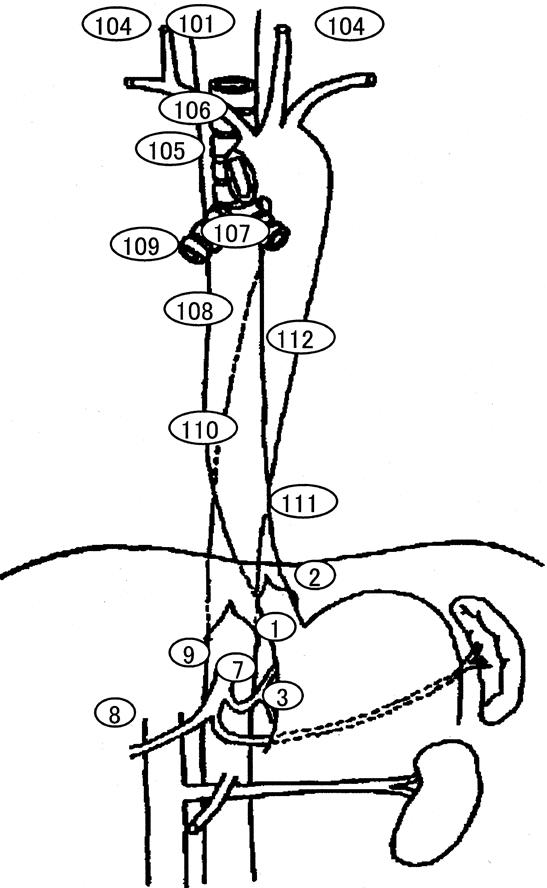
Figure 1. Regional lymph nodes of the esophagus based on the nomenclature and code numbers of the Japanese Society for Esophageal Disease. Lymph nodes were classified as follows: cervical paraesophageal (#101) and supraclavicular (#104) as the cervical nodes; upper thoracic paraesophageal (#105), thoracic paratracheal (#106), bifurcation (#107), middle thoracic paraesophageal (#108), pulmonary hilar (#109), lower thoracic paraesophageal (#110), diaphragmatic (#111), and posterior mediastinal (#112) as the mediastinal nodes; and right cardiac (#1), left cardiac (#2), lesser curvature (#3), left gastric artery (#7), common hepatic artery (#8), and celiac artery (#9) as the abdominal nodes.
As we reported, 11–13 lymph nodes were classified into three types according to the boundary and internal echoes on the basis of the ultrasound appearance. Type 1 lymph nodes had poorly defined boundaries and diffuse homogeneous internal echoes. Type 2 lymph nodes had well-defined boundaries and weak and relatively sonolucent internal echoes. Type 3 lymph nodes had well-defined boundaries and often displayed notching and strong internal echoes. Type 1 lymph nodes were considered to be nonmetastatic; type 2 or type 3 nodes were considered metastatic. The accuracy and prognosis by ultrasound and endoscopic ultrasound diagnosis were compared with the histologic diagnosis after the number of metastatic nodes was divided into four subclassifications: zero, one to three, four to seven, and eight or more.
The normal esophageal wall structure consists of five layers. The first (hyperechoic) layer corresponds to the boundary between the epithelium and balloon; the second (hypoechoic) layer corresponds to the deep mucosa and muscularis mucosae; the third (hyperechoic) layer corresponds to the submucosa; the fourth (hypoechoic) layer corresponds to the muscularis propria; and the fifth (hyperechoic) layer corresponds to the adventitia. The depth of tumor invasion was estimated by a change in appearance or discontinuity in each layer.
Statistical Analysis
The data were evaluated using the StatView 5.0 software package (SAS Institute, Inc., Cary, NC) and the CDC computer system of the Leibniz Rechenzentrum, Munich. Survival data were analyzed using the Kaplan-Meier survival model and are expressed as observed overall survival excluding postoperative deaths (deaths within 30 days of surgery). The log-rank test was used to assess statistical differences between the groups. P < .05 was considered significant.
RESULTS
Stage Grouping
When the depth of tumor invasion was compared between preoperative diagnosis and histologic diagnosis, the accuracy rate of preoperative diagnosis was 71.4% (235/329) (Table 1). Regional lymph node metastasis was histologically found in 201 of 329 patients. Of these, 88.1% (177/201) of patients were correctly diagnosed by ultrasound and endoscopic ultrasound examination. The accuracy rate of ultrasound and endoscopic ultrasound diagnosis was 80.2% (264/329) (Table 2). The incidence of distant lymph node metastasis by preoperative diagnosis and histologic diagnosis was 26.4% (87/329) and 25.3% (85/329), respectively. The accuracy rate of preoperative diagnosis was 91.5% (301/329) (Table 3). Accordingly, the accuracy rate of preoperative ultrasound and endoscopic ultrasound diagnosis in stage grouping was 74.5% (245/329) (Table 4).
Table 1. COMPARISON BETWEEN PREOPERATIVE AND HISTOLOGIC DIAGNOSIS ACCORDING TO TUMOR DEPTH

Table 2. COMPARISON BETWEEN PREOPERATIVE AND HISTOLOGIC DIAGNOSIS ACCORDING TO LYMPH NODE METASTASIS
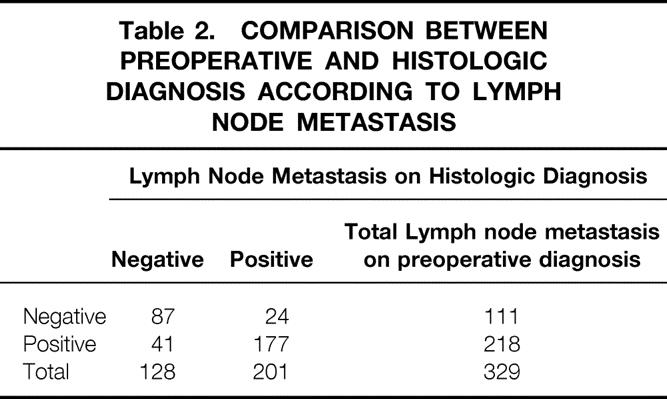
Table 3. COMPARISON BETWEEN PREOPERATIVE AND HISTOLOGIC DIAGNOSIS ACCORDING TO DISTANT LYMPH NODE METASTASIS
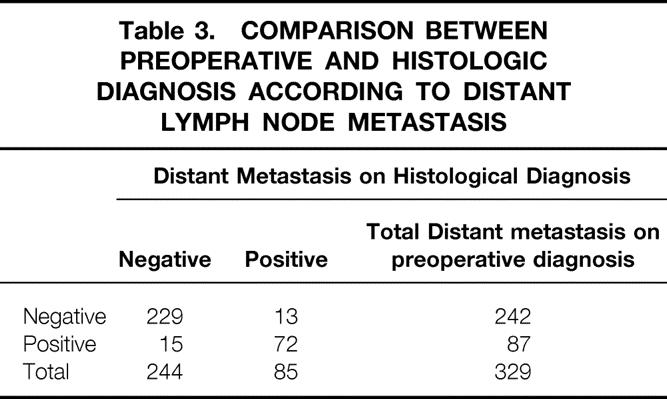
Table 4. COMPARISON BETWEEN PREOPERATIVE AND HISTOLOGIC DIAGNOSIS ACCORDING TO THE STAGE GROUPING

Lymph Node Metastasis
Ultrasound and endoscopic ultrasound revealed regional and distant lymph node metastasis in 71.1% (234/329), whereas lymph node metastasis was histologically confirmed in 61.1% (201/329). Ultrasound and endoscopic ultrasound correctly diagnosed the presence of lymph node metastasis in 184 of 201 patients (91.5%) with histologically proven nodal involvement. The total number of metastatic nodes identified by histology and by ultrasound and endoscopic ultrasound was 1,228 and 846, respectively. Figure 2 shows the relationship of the number of lymph node metastases between diagnosis by ultrasound and endoscopic ultrasound and by histologic diagnosis. The correlation between them was significant (P < .0001).

Figure 2. Correlation of the number of lymph node metastases between ultrasound and endoscopic ultrasound diagnosis and histologic diagnosis. A significant difference was found (P < .0001; correlation coefficient [r] = 0.856).
Subdivision of the Number of Metastatic Nodes
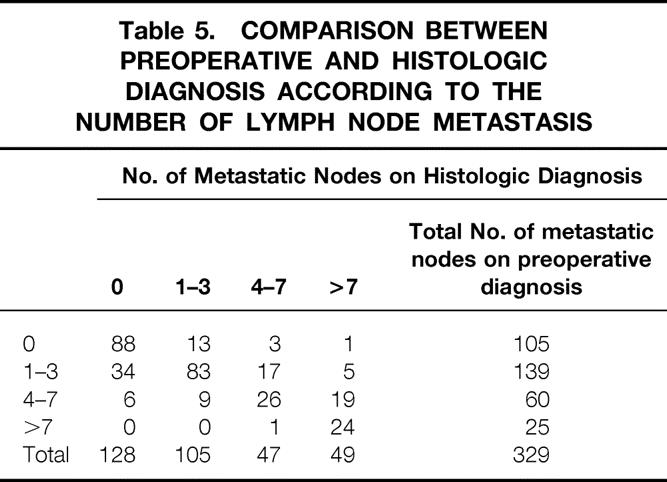
The number of metastatic nodes was classified into subdivisions of zero, one to three, four to seven, and eight or more (Table 5). Among 105 patients in whom lymph node metastasis was undetectable by ultrasound and endoscopic ultrasound, 88 (83.8%) were diagnosed as being free of nodal involvement by histology. Although 59.7% (83/139) of the patients who had one to three 3 lymph node metastases were correctly diagnosed by ultrasound and endoscopic ultrasound, 34 were underestimated. The accuracy rate of ultrasound and endoscopic ultrasound diagnosis was 43.3% among patients with four to seven involved nodes. Histology confirmed 24 of 25 patients (96.0%) with eight or more involved nodes diagnosed by ultrasound and endoscopic ultrasound.
Table 5. COMPARISON BETWEEN PREOPERATIVE AND HISTOLOGIC DIAGNOSIS ACCORDING TO THE NUMBER OF LYMPH NODE METASTASIS
Impact on Prognosis
The survival rate was compared between preoperative and histologic diagnosis in stage grouping. The 5-year survival rates of patients with stage 1, 2A, 2B, 3, and 4 based on preoperative diagnosis were 64.5%, 33.8%, 51.3%, 20.7%, and 12.8%, respectively. However, the 5-year survival rates of patients with pathologic stage 1, 2A, 2B, 3, and 4 were 65.4%, 46.7%, 46.7%, 15.0%, and 7.0%, respectively.
Patient survival was analyzed according to the number of lymph node metastases determined by ultrasound and endoscopic ultrasound. The 5-year survival rates of patients with zero, one to three, four to seven, and eight or more lymph node metastases diagnosed by ultrasound and endoscopic ultrasound were 53.3%, 33.8%, 17.0%, and 0%, respectively. The difference among groups was significant (Fig. 3). However, the 5-year survival rate according to histologic diagnosis was 55.5% in patients without nodal involvement, 30.5% in those with one to three metastases, 14.0% in those with four to seven metastases, and 4.8% in those with eight or more metastases (Fig. 4).

Figure 3. Five-year survival curves according to the number of lymph node metastases by ultrasound and endoscopic ultrasound. A significant difference was found among each group.

Figure 4. Five-year survival curves according to the number of lymph node metastases by histologic examination.
DISCUSSION
Ultrasound and endoscopic ultrasound offer useful information about lymph node metastasis and the depth of tumor invasion before surgery in patients with esophageal carcinoma. When preoperative ultrasound and endoscopic ultrasound diagnosis was compared with histologic diagnosis based on TNM classification, the accuracy rate of preoperative diagnosis exceeded 70% in each category. However, the diagnosis of T4 tumor was insufficient in this series because the endoscope could not be inserted as a result of rigid tumor stenosis in some patients with advanced carcinoma.
When diagnosing lymph node metastasis by ultrasound and endoscopic ultrasound, it is important to examine not only the size of the nodes but also the morphologic findings. Roundness and the absence of an echogenic hilum are generally considered ultrasonographic clues of malignancy. 14–16 In our study, ultrasonographic images of lymph nodes were classified on the basis of boundary and internal echoes. 11–13 We diagnosed a patient as having positive lymph nodes when the detected lymph nodes met the criteria of both boundary and internal echoes. Freimanis 17 reported that most lymph nodes are anechoic, although internal echoes are often seen in certain metastases. Both the anatomic sites of lymph node metastases and the number of metastases should be taken into consideration. The number of lymph node metastases may reflect the tumor volume. Therefore, estimating the number of lymph node metastases before surgery is thought to be crucial for deciding on the surgical treatment strategy and for predicting the prognosis. In this series, three-field lymphadenectomy was performed between 1988 and 1990 to compare the accuracy between ultrasound and endoscopic ultrasound diagnosis and histologic findings. After 1991, lymph node dissection, especially in the cervical region, was prospectively chosen based on the preoperative ultrasound and endoscopic ultrasound diagnosis. Further, after 1993, neoadjuvant chemotherapy was used in some patients with advanced cancer, particularly those with eight or more involved nodes diagnosed by ultrasound and endoscopic ultrasound. The rate of histologic effect for lymph node metastasis was 36.4% (8/22). Recently, we have used preoperative chemoradiation in patients with many nodal metastases by ultrasound and endoscopic ultrasound.
The clinical outcome of patients associated with preoperative ultrasound and endoscopic ultrasound diagnosis and histologic diagnosis was similar. Although the 5-year survival rate was not significantly different between stage 2A and 2B by both preoperative and histologic diagnosis, a significant difference was found in the remaining stages. It was suggested that preoperative ultrasound and endoscopic ultrasound diagnosis according to TNM classification was useful to estimate the prognosis of patients with esophageal carcinoma.
A close relationship between the number of lymph node metastases and the clinical outcome of patients with esophageal carcinoma has been reported. However, most reports were based on histologic examinations of lymph node metastasis after surgery. In the present study, we prospectively evaluated the number of lymph node metastases by ultrasound and endoscopic ultrasound before surgery and analyzed them by one-to-one correspondence between preoperative and histologic diagnosis. The correlation between the number of lymph node metastases diagnosed before surgery and by histology was significant. We divided the number of involved nodes into groups of zero, one to three, four to seven, and eight or more according to the Guidelines for Clinical and Pathologic Studies on Carcinoma of the Esophagus by the Japanese Society for Esophageal Disease. 7 Among patients without metastasis and those with eight or more, the accuracy rate by ultrasound and endoscopic ultrasound was high. However, ultrasound and endoscopic ultrasound overestimated some patients with one to three lymph node metastases, and these patients had false-positive results. Some patients with four to seven nodal metastases underestimated by ultrasound and endoscopic ultrasound had false-negative results.
Problems associated with ultrasound and endoscopic ultrasound diagnosis include the following: with a larger number of metastases, the metastases were small and had a small metastatic area; some lymph node metastases that interfered with tracheal echo in the mediastinal region were undetectable by endoscopic ultrasound; and the echo pattern of inflammatory lymph nodes with fibrosis or calcification was sometimes similar to that of metastatic nodes. Other imaging methods such as computed tomography and magnetic resonance imaging may be helpful for diagnosing such lymph nodes. 18
The 5-year survival rates of patients were significantly different based on the four subdivisions of preoperative lymph node metastasis by ultrasound and endoscopic ultrasound. In this series, the survival curves obtained after diagnosis by ultrasound and endoscopic ultrasound were similar to those obtained by histologic diagnosis. These results suggested that the prognosis of patients with esophageal carcinoma can be predicted by the number of lymph node metastases based on preoperative ultrasound and endoscopic ultrasound diagnosis.
In conclusion, there was a close relationship in the TNM classification, especially in the number of lymph node metastases, between preoperative ultrasound and endoscopic ultrasound diagnosis and histologic diagnosis. Therefore, ultrasound and endoscopic ultrasound are useful when estimating the prognosis of patients with esophageal carcinoma. In the future, the preoperative number of lymph node metastases may be used in the staging system.
Footnotes
Correspondence: Shoji Natsugoe, MD, First Department of Surgery, Kagoshima University School of Medicine, 8-35-1 Sakuragaoka, Kagoshima 890-8520, Japan.
Accepted for publication November 29, 2001.
References
- 1.Baba M, Aikou T, Yoshinaka H, et al. Long-term results of subtotal esophagectomy with three-field lymphadenectomy for carcinoma of the thoracic esophagus. Ann Surg 1994; 219: 310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimaki T, Suzuki T, Suzuki S, et al. Outcome of extended radical esophagectomy for thoracic esophageal cancer. J Am Coll Surg 1998; 186: 306–312. [DOI] [PubMed] [Google Scholar]
- 3.Kawahara K, Maekawa T, Okabayashi K, et al. The number of lymph node metastases influences survival in esophageal cancer. J Surg Oncol 1998; 67: 160–163. [DOI] [PubMed] [Google Scholar]
- 4.Kodera Y, Yamamura Y, Shimizu Y, et al. The number of metastatic nodes: a promising prognostic determinant for gastric carcinoma in the latest edition of the TNM classification. J Am Coll Surg 1998; 187: 597–603. [DOI] [PubMed] [Google Scholar]
- 5.Lee WJ, Lee PH, Yue SC, et al. Lymph node metastases in gastric cancer: significance of positive number. Oncology 1995; 52: 45–50. [DOI] [PubMed] [Google Scholar]
- 6.International Union Against Cancer. TNM classification of malignant tumors, 5th ed. New York: Wiley-Liss; 1997.
- 7.Esophageal Disease Research Society. Guidelines for the clinical and pathologic studies on carcinoma of the esophagus, 9th ed. Tokyo: Kanehara; 1999.
- 8.Tio TL, Cohen P, Coene PP, et al. Endosonography and computed tomography of esophageal carcinoma. Preoperative classification compared to new (1987) TNM system. Gastroenterology 1989; 96: 1478–1486. [DOI] [PubMed] [Google Scholar]
- 9.Hiele M, Leyn PD, Schurmans P, et al. Relation between endoscopic ultrasound findings and outcome of patients with tumors of the esophagus or esophagogastric junction. Gastrointest Endosc 1997; 45: 381–386. [DOI] [PubMed] [Google Scholar]
- 10.Vickers J, Alderson D. Oesophageal cancer staging using endoscopic ultrtasonography. Br J Surg 1998; 85: 994–998. [DOI] [PubMed] [Google Scholar]
- 11.Yoshinaka H, Nishi M, Kajisa T, et al. Ultrasonic detection of lymph node metastases in the regional around the celiac axis in esophageal and gastric cancer. J Clin Ultrasound 1985; 13: 153–160. [DOI] [PubMed] [Google Scholar]
- 12.Natsugoe S, Yoshinaka H, Morinaga T, et al. Ultrasonographic detection of lymph node metastases in superficial carcinoma of the esophagus. Endoscopy 1996; 28: 674–679. [DOI] [PubMed] [Google Scholar]
- 13.Natsugoe S, Yoshinaka H, Shimada M, et al. Assessment of cervical lymph node metastasis in esophageal carcinoma using ultrasonography. Ann Surg 1999; 229: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon PB, Gilks B. Sonographic appearance of normal intramammary lymph nodes. J Ultrasound Med 1988; 7: 545–548. [DOI] [PubMed] [Google Scholar]
- 15.Catalano MF, Sivak MV, Rice T, et al. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc 1994; 40: 442–446. [DOI] [PubMed] [Google Scholar]
- 16.Doldi SB, Lattuada E, Zappa MA, et al. Ultrasonographic evaluation of the cervical nodes in preoperative staging of esophageal neoplasms. Abdom Imaging 1998; 23: 275–277. [DOI] [PubMed] [Google Scholar]
- 17.Freimanis AK. Echographic diagnosis of lesions of the abdominal aorta and lymph nodes. Radiol Clin North Am 1975; 13: 557–572. [PubMed] [Google Scholar]
- 18.Van Overhagen H, Lameris JS, Berger MY, et al. Improved assessment of supraclavicular and abdominal metastases in oesophageal and gastro-oesophageal junction carcinoma with the combination of ultrasound and computed tomography. Br J Radiol 1993; 66: 203–208. [DOI] [PubMed] [Google Scholar]


