Abstract
Objective
To quantify the occurrence of intestinal metaplasia in columnar-lined esophagus (CLE) during endoscopic surveillance and to evaluate the impact of antireflux surgery on the development of intestinal metaplasia.
Summary Background Data
The malignant potential in segments of CLE is mainly restricted to those containing intestinal metaplasia. Patients with segments of CLE in which no intestinal metaplasia can be detected are rarely enrolled in a surveillance program but may still be at increased risk of developing esophageal adenocarcinoma because intestinal metaplasia may be missed or may develop with time.
Methods
The occurrence of intestinal metaplasia on biopsy samples was determined on repeated endoscopies in 177 patients enrolled in a surveillance program for CLE. The incidence of intestinal metaplasia in patients with no evidence of intestinal metaplasia on the two first endoscopies was evaluated on the subsequent endoscopies and compared in patients with medically and surgically treated gastroesophageal reflux disease.
Results
Intestinal metaplasia was found in 53% of the patients (94/177) on their first surveillance endoscopy and was more prevalent in long segments of CLE. The prevalence of intestinal metaplasia increased markedly with increasing number of surveillance endoscopies. Intestinal metaplasia tended to be detected early in patients with long segments of CLE; in patients with shorter segments, intestinal metaplasia was also detected late in the course of endoscopic surveillance. Patients with surgically treated reflux disease were 10.3 times less likely to develop intestinal metaplasia compared with a group receiving standard medical therapy.
Conclusion
Biopsy samples from a single endoscopy, despite an adequate biopsy protocol, are insufficient to rule out the presence of intestinal metaplasia. Patients in whom biopsy specimens from a segment of CLE show no intestinal metaplasia have a significant risk of having undetected intestinal metaplasia or of developing intestinal metaplasia with time. Sampling error is probably the reason for the absence of intestinal metaplasia in segments of CLE longer than 4 cm, whereas development of intestinal metaplasia is common in patients with shorter segments of CLE. Antireflux surgery protects against the development of intestinal metaplasia, possibly by better control of reflux of gastric contents.
The rationale for endoscopic surveillance in patients with columnar-lined esophagus (CLE) is to detect progression of disease toward cancer and to allow early intervention while cure is still likely. In the late 1950s, the term “Barrett’s esophagus” was understood to mean anesophagus linked by columnar mucosa. This vague definition persisted until the 1980s, when a requirement of at least 3 cm of columnar mucosa above the perceived gastroesophageal junction was introduced. 1 The “3-cm rule” was introduced to avoid overdiagnosis of Barrett’s esophagus resulting from failure to recognize a tubularized portion of a hiatal hernia or from failure to allow for the “normal” 1 to 2 cm of columnar mucosa in the distal esophagus reported by Hayward. 2 Although the goblet cell had been described long before, 3,4 the importance of this component of the metaplastic epithelium was recognized in 1976, when Paull et al described the intestinal goblet cell as part of the mosaic of esophageal columnar mucosa along with cardiac and fundic mucosa. 5 Their recognition of intestinal metaplasia was important because many had considered Barrett’s esophagus to represent gastric tissue in the esophagus. In addition to the association with reflux disease, the association between adenocarcinoma and Barrett’s esophagus was well established by the 1970s. 6–8 By the late 1980s it was clear that specialized intestinal metaplasia was the epithelial type that especially predisposes patients to cancer development. 9–11 These observations lead to further refinement of the definition of Barrett’s esophagus to include only patients with intestinal metaplasia in segments of columnar lining of 3 cm or more. In the 1990s the parallel increase in the incidence of adenocarcinoma of the esophagus and the esophagogastric junction 12 led to an interest in metaplastic processes in the junctional area. Today it is generally accepted that dysplasia and adenocarcinoma also occur in segments of intestinal metaplasia shorter than 3 cm. This has led to the present definition of Barrett’s esophagus, which includes patients with intestinal metaplasia in a segment of CLE of any length.
Because of its association with adenocarcinoma, only patients with intestinal metaplasia are recommended for regular endoscopic and histologic surveillance. 13,14 To assess the magnitude of the risk for malignancy, it is essential to know the histologic type of the columnar mucosa. Patients in whom no intestinal metaplasia is found are generally not recommended for endoscopic surveillance. However, many of these patients may still be at increased risk of esophageal adenocarcinoma because intestinal metaplasia may be missed as a result of sampling error. Others may develop intestinal metaplasia with time, subjecting the patient to an increased risk of malignancy.
Barrett’s esophagus has been postulated to be the result of damaged squamous epithelium being replaced by cardiac or junctional-type mucosa within which, over time and with persistent inflammation, intestinal metaplasia develops. 15–20 Patients with segments of pure cardiac mucosa, over time and under the proper luminal conditions, may develop intestinal metaplasia and its associated risk of progression to dysplasia and adenocarcinoma. If this holds true, the process of goblet cell formation could be stopped with abolition of reflux.
The aim of this study was to evaluate the prevalence of intestinal metaplasia in patients with varying lengths of CLE during long-term endoscopic and histologic surveillance. Further, we wanted to determine whether complete prevention of reflux with antireflux surgery is more effective than standard medical treatment in preventing the development of intestinal metaplasia in esophageal columnar mucosa.
PATIENTS AND METHODS
The endoscopy unit at Lund University Hospital maintains a registry of patients prospectively enrolled in a surveillance program for CLE. As of July 31, 2000, 177 patients with CLE and at least three surveillance endoscopies were included in the database. The patients were diagnosed with CLE between 1979 and 1998, and surveillance endoscopies were performed at an interval of 1 to 2 years.
The presence of specialized intestinal metaplasia in the CLE was determined at baseline endoscopy. The endpoint of this study was the detection of intestinal metaplasia, and further evaluation of the surveillance program was not undertaken.
Patients in whom no intestinal metaplasia could be found despite multiple biopsy samples from the CLE on the two first endoscopies were considered to be free from intestinal metaplasia. The formation of goblet cells, the hallmark of intestinal metaplasia and Barrett’s esophagus, was studied in 69 such patients by evaluating the occurrence of intestinal metaplasia during the subsequent surveillance endoscopies. The occurrence of intestinal metaplasia was compared between patients with medically and surgically treated gastroesophageal reflux disease.
Endoscopy
All patients underwent a complete systematic endoscopic examination of the duodenum, pylorus, stomach, and esophagus. A CLE was suspected when the squamocolumnar junction or any part of its circumference extended above the gastric rugal folds. This included an irregular squamocolumnar junction with tongues of columnar mucosa extending into the esophagus. The presence of a CLE was confirmed on histologic evaluation of the biopsy specimens. The extent of the CLE segment was defined as the distance from the gastroesophageal junction to the location of the highest point of the squamocolumnar junction. The histologic type of the columnar mucosa was evaluated on multiple biopsy samples of the CLE on each surveillance endoscopy. No specific biopsy protocol was used before 1984, when a policy of four-quadrant biopsy samples every 2 cm along the length of the metaplastic mucosa was introduced. Between 1979 and 1984, four patients were included in the study.
Histology
The biopsy specimens were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and mounted on slides using standard technique. Slides were stained with hematoxylin and eosin and analyzed for the histologic type of the epithelium. Fundic mucosa was characterized by glands that contained parietal and chief cells but were devoid of mucous cells, except those lining the surface and foveolar region. Cardiac mucosa was characterized by glands composed entirely of mucous cells without any parietal or chief cells. Intestinal metaplasia, which was identified by the presence of a columnar epithelium with a villiform surface, mucous glands, and well-defined goblet cells, was distinguished from fundic and cardiac mucosa.
Postoperative pH Monitoring
All patients undergoing antireflux surgery had the effectiveness of the procedure evaluated by postoperative 24-hour pH monitoring 6 months after surgery. Abnormal esophageal acid exposure was diagnosed when more than 3.4% of the monitored time was spent at less than pH 4.0.
Statistical Analysis
Values are reported as medians and interquartile ranges unless otherwise stated. The Fisher exact test was used to compare proportions between two groups. The Mann-Whitney test was used to compare continuous data between two groups. Survival curves were constructed using the Kaplan-Meier method, counting the cases of intestinal metaplasia detection as events and the remainder as censored as of the last day of endoscopic surveillance. Survival curves were compared by the log-rank method. Binary logistic regression analysis was performed to estimate relative risks for the development of intestinal metaplasia. The factors evaluated in the logistic regression were age, gender, body mass index, history of tobacco use, duration of reflux symptoms, number of surveillance endoscopies, duration of surveillance, median surveillance interval, previous cholecystectomy, length of hiatal hernia, length of CLE, and previous antireflux surgery.
RESULTS
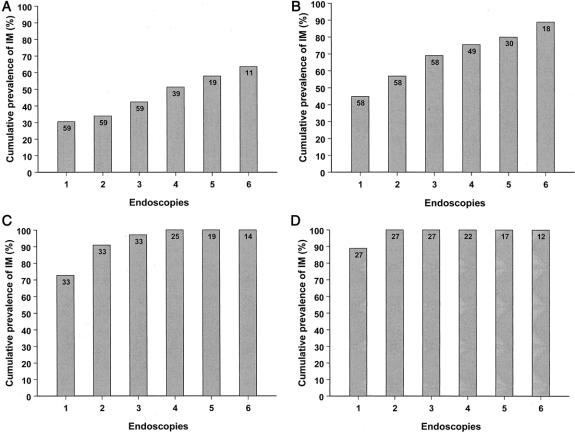
Demographic and clinical data of the study population are shown in Table 1. Fifty-two percent (89/177) of the patients had intestinal metaplasia found on the first surveillance endoscopy. A median of eight biopsy specimens was obtained per endoscopy. The prevalence of intestinal metaplasia on the first endoscopy was 30.5% in patients with short segments (1–2 cm) of CLE and increased with increasing length of CLE; it was 88.9% in segments longer than 6 cm (Fig. 1).
Table 1. DEMOGRAPHIC AND CLINICAL DATA
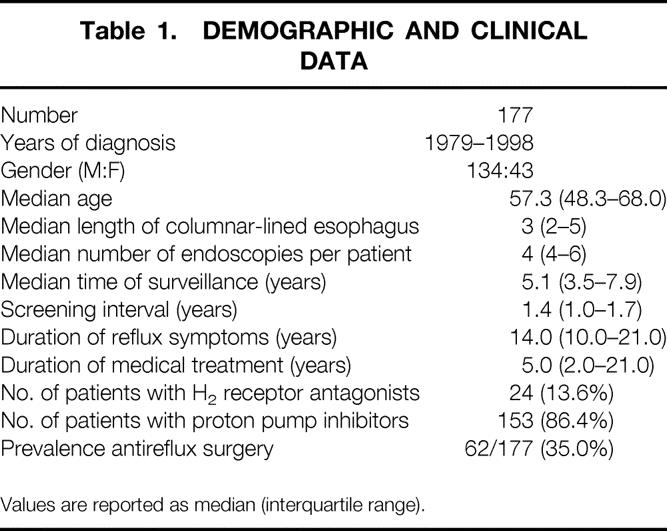
Values are reported as median (interquartile range).
Figure 1. Cumulative prevalence of intestinal metaplasia during endoscopic surveillance (endoscopies one through six) in patients with varying lengths of esophageal columnar lining. The lengths of columnar lining were 1 to 2 cm (A), 3 to 4 cm (B), 5 to 6 cm (C), and longer than 6 cm (D). The number of patients at each endoscopy is denoted within the bars.
The prevalence of intestinal metaplasia increased with increasing number of surveillance endoscopies. Figure 1 shows the cumulative prevalence of intestinal metaplasia for the first six endoscopies. The cumulative prevalence of intestinal metaplasia in patients with CLE segments of 1 to 2 cm and 3 to 4 cm increased from 30.5% and 44.8% to 63.6% and 88.9%, respectively, after six endoscopies. Intestinal metaplasia was found in all patients with segments exceeding 4 cm in length after two to four endoscopies. Figure 2 shows the relationship between the time to detect intestinal metaplasia and the extent of CLE. Intestinal metaplasia tended to be detected early in patients with long segments of CLE, whereas in patients with shorter segments, intestinal metaplasia was also detected late in the course of endoscopic surveillance.
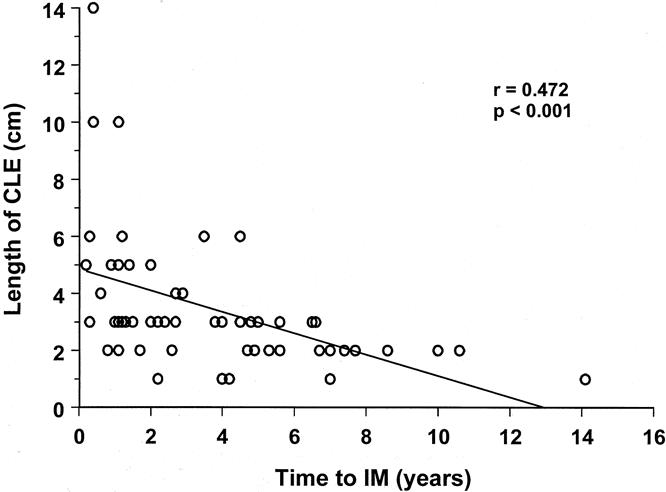
Figure 2. The relationship between the length of esophageal columnar lining and the time to detect intestinal metaplasia in patients with no intestinal metaplasia on the first endoscopy.
Sixty-nine patients were considered to be free from intestinal metaplasia because no evidence of intestinal metaplasia was found on the first two endoscopies despite multiple biopsy samples from the CLE. During the course of endoscopic surveillance, 35 of these patients developed intestinal metaplasia. There was no difference in the median age between patients who developed intestinal metaplasia versus those who did not (56.1 vs. 53.1 years, P = .374). The median duration of reflux symptoms, however, was significantly longer in patients who developed intestinal metaplasia (10.5 vs. 15.0 years, P = .01). Figure 3 shows the intestinal metaplasia-free survival rate in patients with no intestinal metaplasia on the first two endoscopies. The median time to develop intestinal metaplasia was 6.2 years.
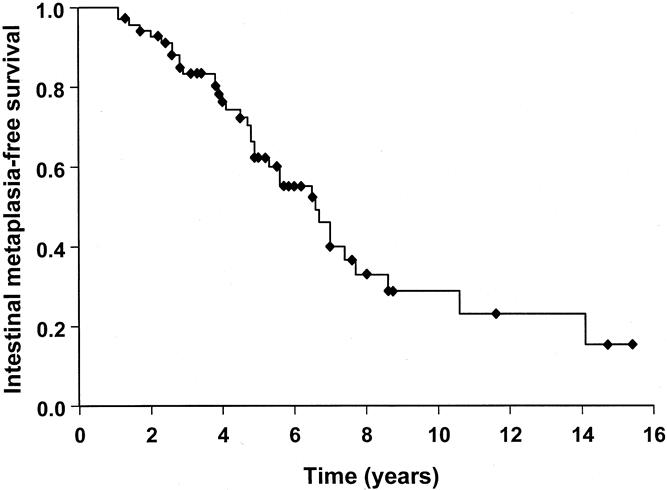
Figure 3. Kaplan-Meier curve for the intestinal metaplasia-free survival during endoscopic surveillance of patients with no evidence of intestinal metaplasia on the first two endoscopies (n = 79).
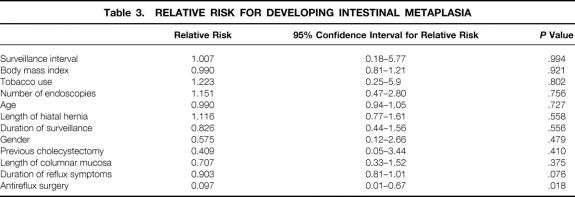
Forty-nine of the 69 patients with no intestinal metaplasia on the first two endoscopies were treated with chronic acid-suppression therapy and 20 patients underwent antireflux surgery (18 open and 2 laparoscopic Nissen fundoplications). All patients undergoing antireflux surgery were receiving acid-suppression therapy before surgery. Demographic and clinical data of these patients are shown in Table 2. The surgically treated patients were significantly younger than the patients receiving medical treatment (P < .01). All patients who underwent antireflux surgery had the effectiveness of the procedure evaluated by 24-hour esophageal pH monitoring 6 months after surgery. The median time spent at less than pH 4.0 after surgery was 0.4%, and esophageal acid exposure was restored to normal in 19 patients (95%); one patient continued to have abnormal esophageal acid exposure 6 months after surgery. The intestinal metaplasia-free survival in patients with medically and surgically treated reflux disease is shown in Figure 4. Intestinal metaplasia occurred significantly less frequently in patients with surgically treated reflux disease. The median time to develop intestinal metaplasia was 5.3 years in patients with medical therapy but could not be calculated in patients who underwent antireflux surgery because only four patients developed intestinal metaplasia. The binary logistic regression analysis showed that no factor was associated with an increased risk of developing intestinal metaplasia, whereas a previous antireflux procedure was independently associated with a decreased risk of intestinal metaplasia development (Table 3). The relative risk was 0.097, equivalent to a 10.3-fold decreased risk of developing intestinal metaplasia in the patients with surgically treated reflux disease.
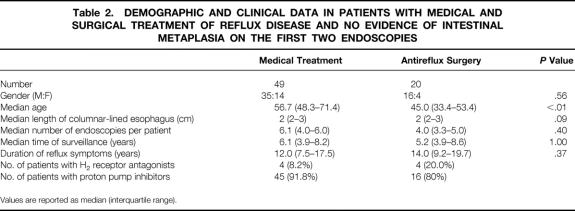
Table 2. DEMOGRAPHIC AND CLINICAL DATA IN PATIENTS WITH MEDICAL AND SURGICAL TREATMENT OF REFLUX DISEASE AND NO EVIDENCE OF INTESTINAL METAPLASIA ON THE FIRST TWO ENDOSCOPIES
Values are reported as median (interquartile range).
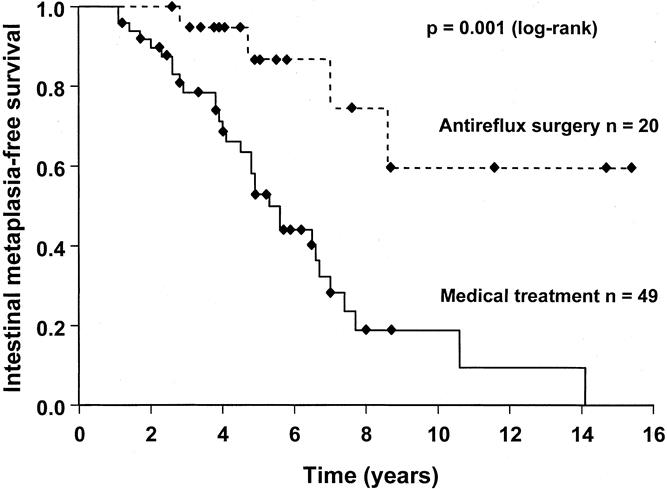
Figure 4. Kaplan-Meier curve comparing the intestinal metaplasia-free survival during endoscopic surveillance of patients with medically and surgically treated reflux disease. The patients had no evidence of intestinal metaplasia on the first two endoscopies.
Table 3. RELATIVE RISK FOR DEVELOPING INTESTINAL METAPLASIA
DISCUSSION
This is the first study to quantify the occurrence of intestinal metaplasia during endoscopic surveillance of patients with CLE. In this study the cumulative prevalence of intestinal metaplasia increased with increasing number of surveillance endoscopies. In patients with 1 to 2 cm of CLE, the prevalence increased markedly from 30.5% to 63.6% after six endoscopies. The prevalence of intestinal metaplasia increased with increasing length of the CLE. In patients with columnar mucosa exceeding 4 cm in length, the cumulative prevalence of intestinal metaplasia rapidly reached 100% during endoscopic surveillance.
Detection of intestinal metaplasia in patients in whom no intestinal metaplasia was found on the first endoscopy can be due to two factors. First, it may be the result of sampling error—that is, intestinal metaplasia is present but missed on biopsy because of its patchy and focal distribution. Second, goblet cells, the hallmark of intestinal metaplasia and Barrett’s esophagus, may develop with time within nonintestinalized segments of esophageal columnar mucosa. The observations that intestinal metaplasia is generally found early in the course of surveillance in patients with long segments of CLE, and that these patients rapidly reach a cumulative prevalence of 100%, suggest that they have intestinal metaplasia at baseline endoscopy that is missed because of sampling error. Although intestinal metaplasia was also detected early in patients with CLE less than 4 cm in length, it was also found late in the course of endoscopic surveillance in many of these patients. This observation suggests that intestinal metaplasia develops with time in many patients with short CLE segments.
In patients with CLE, it has been recommended that only the subgroup of patients with intestinal metaplasia should undergo a surveillance program. 14 However, the results of this study suggest that biopsy samples from a single endoscopy, despite an adequate biopsy protocol, are insufficient to rule out the presence of intestinal metaplasia. Based on our observations, it can be concluded that patients with CLE exceeding 4 cm in length almost invariably harbor intestinal metaplasia. These patients could be recommended for continued endoscopic surveillance, regardless of the type of mucosa found on the histopathologic evaluation of the biopsy specimens. The management of patients with shorter lengths of columnar mucosa and no intestinal metaplasia is more challenging because intestinal metaplasia may develop with time. The risk of malignant transformation in patients with short segments of intestinal metaplasia is still unclear, but if the results of future studies suggest a significant risk for malignancy, additional endoscopies in patients with no intestinal metaplasia should be considered.
The formation of goblet cells within nonintestinalized columnar mucosa was studied. To avoid the effect of sampling error, this was done by determining the occurrence of intestinal metaplasia during endoscopic surveillance of patients in whom no intestinal metaplasia could be found despite multiple biopsy samples on the two first endoscopies. Patients with surgically treated reflux disease developed intestinal metaplasia significantly less frequently than patients with medically treated disease. The protective effect of antireflux surgery on the development of intestinal metaplasia suggested by log-rank analysis was strengthened on binary logistic regression analysis, in which patients with previous antireflux surgery were 10.3 times less likely to develop intestinal metaplasia.
The patient with abnormal esophageal acid exposure after surgery has thus far not developed intestinal metaplasia. However, the postoperative follow-up is only 1.5 years, and it is therefore likely that this patient is subject to the same high risk for intestinal metaplasia development as the patients in the medically treated group. Four patients in the surgically treated group developed intestinal metaplasia despite normal results on postoperative esophageal pH monitoring. One of these patients was found to have intestinal metaplasia on the second postoperative endoscopy; the remaining three patients were found to have it on the third postoperative endoscopy. None of the patients had symptoms suggestive of recurrent reflux disease, and the reasons for intestinal metaplasia in these patients remain unclear.
We recognize the concern that intestinal metaplasia still may have been missed because of its patchy distribution. However, the vast majority of these patients had short segments of columnar mucosa, from which biopsy samples are easy to obtain, so we believe that a systematic biopsy protocol with multiple samples of the columnar mucosa on two subsequent occasions would find intestinal metaplasia if it were present. Also, it is unlikely that the difference in the detection of intestinal metaplasia in patients with surgically and medically treated reflux disease is explained by sampling error, given that the same biopsy protocol was applied in both groups.
Barrett’s esophagus is believed to be the result of injured squamous epithelium being replaced by cardiac-type mucosa within which, over time and persistent inflammation, intestinal metaplasia develops. 15–17 It is possible that patients undergoing successful antireflux surgery have better control of reflux of gastric contents than patients receiving standard medical treatment. This may result in less inflammation and halting of the process of goblet cell formation. In support of this hypothesis, it has recently been shown that using symptom relief as the endpoint of medical therapy of patients with Barrett’s esophagus is unreliable. Katzka and Castell 21 and Ouato-Lascar and Triadafilopoulos 22 have shown that 40% to 80% of patients with Barrett’s esophagus continue to have abnormal esophageal acid exposure despite receiving high doses of proton pump inhibitors. Complete control of reflux of duodenal juice may be an important goal when treating patients with CLE. A recent study by Öberg et al 19 found that patients with intestinal metaplasia in short segments of CLE, in addition to longer duration of reflux symptoms, had a significantly greater prevalence of abnormal duodenoesophageal reflux than patients without intestinal metaplasia. It was suggested that the presence of duodenoesophageal reflux and the duration of reflux might be important in the pathogenesis of intestinal metaplasia. Acid-suppression therapy decreases pathologic acid reflux, but reflux of duodenal juice, although reduced, still exceeds the normal range in many patients. 23 Antireflux surgery has been shown to result in a more reliable elimination of reflux of both acid and duodenal contents, 24 thus offering a theoretical advantage in the management of these patients. The importance of complete abolition of reflux in patients with CLE has been emphasized by Fitzgerald et al, 25 who recently suggested that the dynamic effects of acid exposure may effect columnar cell proliferation and differentiation. Using cultured human Barrett’s specimens, they showed that continuous exposure to acidic media at pH 3.5 resulted in increased villin expression, which is a marker for cell differentiation, and reduced cell proliferation. A dramatic increase in proliferation occurred when the tissue was exposed to short pulses of pH greater than 3.5. Consequently, acid exposure may lead to altered growth properties and may contribute to the process of intestinalization of columnar mucosa. They implied that acid-suppression therapy needs to be powerful and continuous enough to abolish any acid pulses and inhibit cell proliferation to allow a more differentiated state in the Barrett’s epithelium. Ouatu-Lascar et al 26 confirmed the hypothesis that effective intraesophageal acid suppression favors differentiation and decreases proliferation in biopsy samples from Barrett’s mucosa.
The pathogenesis of intestinal metaplasia is incompletely understood, but it has been suggested that intestinal metaplasia develops with increasing age. 27,28 Although patients in the medically treated group were significantly older than the patients undergoing antireflux surgery, we do not believe that the difference in goblet cell development was due to the difference in age. First, the process of metaplasia is believed to be a consequence of chronic inflammation, 29 so it is unlikely that intestinal metaplasia develops with age alone. Second, when the 35 patients who developed intestinal metaplasia were compared with those who did not, there was no difference in age. The duration of reflux is a more likely factor in the development of intestinal metaplasia, because the median duration of reflux symptoms was significantly longer in the patients who developed intestinal metaplasia.
The results of this study support the hypothesis that the development of Barrett’s esophagus is a two-step process. Intestinal metaplasia has been shown to occur in the presence of inflamed cardiac-type mucosa only and never in the rare situation of cardiac-type mucosa that was not inflamed. 30 This suggests that there is an intestinalization of cardiac-type mucosa from repetitive injury to gastric juice, which appears to give the mucosa an increased ability to withstand damage from reflux, because the fully intestinalized mucosa commonly shows minimal inflammation or reactive change. It is hypothesized that under this stimulus there is an induced change of the cardiac-type mucosa in which the cells first undergo enlargement and distention with neutral or Alcian blue-negative mucin. This is followed by a progressive change from neutral to acidic mucin, manifested by variable positive staining of the hypertrophic cardiac mucous cells with Alcian blue; at this stage they are commonly referred to as “columnar blue cells.” Eventually these cells may progress to well-formed goblet cells, which have a rounded mucin globule that is strongly Alcian blue-positive and is separated from the cell surface by eosinophilic cytoplasm. Mendes de Amedia et al 31 provided biochemical support for this hypothesis by showing no differences in intestinal-type proteins between cardiac-type mucosa and intestinal metaplasia in patients with Barrett’s esophagus. Their observation defined an intestinal pattern of protein expression common to these two morphologic types of mucosa, suggesting that they were derived from a common origin.
The hypothesis that there is a sequential development from squamous epithelium to cardiac-type mucosa to intestinal metaplasia in response to acid-induced injury is also supported by the observations of Hamilton and Yardley. 32 They reported on the development of Barrett’s mucosa in the esophagus above the anastomosis in three patients after esophagogastrectomy. In two, they documented progression from squamous epithelium to cardiac mucosa and subsequently intestinal metaplasia over 6.3 and 10 years.
The aim of a surveillance program in patients with CLE is to identify dysplastic changes and malignancy in an early, potentially curable stage. Because the risk for malignancy is primarily limited to those with intestinal metaplasia, careful histologic examination of the biopsy specimens is essential to select patients for regular endoscopic and histologic surveillance. The results of this study indicate that biopsy samples from a single endoscopy, despite an adequate biopsy protocol, are insufficient to rule out the presence of intestinal metaplasia. Patients with CLE segments exceeding 4 cm in length almost invariably harbor intestinal metaplasia and should be recommended for continued endoscopic surveillance, regardless of the type of mucosa found on the histopathologic evaluation of the biopsy specimens. The management of patients with shorter CLE lengths and no intestinal metaplasia is more challenging because intestinal metaplasia may develop with time. In this study the median time to develop intestinal metaplasia in these patients was 5.3 years. Possibly, patients with nonintestinalized CLE segments should undergo additional endoscopy after 5 years to assess the risk of malignancy and to evaluate the need for further endoscopic surveillance. Patients with surgically treated reflux disease developed intestinal metaplasia significantly less frequently than patients receiving standard medical treatment. Possibly, patients undergoing successful antireflux surgery have better control of reflux of gastric contents. This may result in less inflammation and halting of the process of goblet cell formation.
Footnotes
Correspondence: Stefan Öberg, MD, Department of Surgery, Lund University Hospital, 231 41 Lund, Sweden.
E-mail: stefan.oberg@skane.se
Accepted for publication March 13, 2001.
References
- 1.Skinner DB, Walther BC, Riddell RH, et al. Barrett’s esophagus. Comparison of benign and malignant cases. Ann Surg 1983; 198: 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayward J. The lower end of the oesophagus. Thorax 1961; 16: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosher LH, Taylor FH. Heterotopic gastric mucosa in the esophagus with ulceration and stricture formation. J Thorac Surg 1951; 21: 306–312. [PubMed] [Google Scholar]
- 4.Morson BC, Belcher JR. Adenocarcinoma of the oesophagus and ectopic gastric mucosa. Br J Cancer 1952; 6: 127–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paull A, Trier JS, Dalton MD, et al. The histologic spectrum of Barrett’s esophagus. N Engl J Med 1976; 295: 476–80. [DOI] [PubMed] [Google Scholar]
- 6.Adler RH. The lower esophagus lined by columnar epithelium: its association with hiatal hernia, ulcer, stricture and tumor. J Thorac Cardiovasc Surg 1963; 45: 13–32. [PubMed] [Google Scholar]
- 7.Hawe A, Payne WS, Weiland LH. Adenocarcinoma in the columnar epithelial lined lower (Barrett) esophagus. Thorax 1973; 28: 511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haggitt RC, Tryzelaar J, Ellis FH. Adenocarcinoma complicating columnar epithelium-lined (Barrett’s) esophagus. J Clin Pathol 1979; 70: 1–5. [DOI] [PubMed] [Google Scholar]
- 9.Hameeteman W, Tytgat GN, Houthoff HJ, et al. Barrett’s esophagus: development of dysplasia and adenocarcinoma. Gastroenterology 1989; 96: 1249–1256. [DOI] [PubMed] [Google Scholar]
- 10.Reid BJ, Weinstein WM. Barrett’s esophagus and adenocarcinoma. Ann Rev Med 1987; 38: 477–492. [DOI] [PubMed] [Google Scholar]
- 11.Reid BJ, Weinstein WM, Lewin KJ, et al. Endoscopic biopsy can detect high-grade dysplasia or early adenocarcinoma in Barrett’s esophagus without grossly recognizable neoplastic lesions. Gastroenterology 1988; 94: 81–90. [DOI] [PubMed] [Google Scholar]
- 12.Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991; 265: 1287–1289. [PubMed] [Google Scholar]
- 13.Kim SL, Waring JP, Spechler SJ, et al. Diagnostic inconsistencies in Barrett’s esophagus. Department of Veterans Affairs Gastroesophageal Reflux Study Group. Gastroenterology 1994; 107: 945–949. [PubMed] [Google Scholar]
- 14.Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 1890; 93: 1028–1032. [DOI] [PubMed] [Google Scholar]
- 15.Goldman MC, Beckman RC. Barrett syndrome: case report with discussion about concepts of pathogenesis. Gastroenterology 1960; 39: 104–110. [PubMed] [Google Scholar]
- 16.Bremmer CG, Lynch VP, Ellis FHJ. Barrett’s esophagus: congenital or acquired? An experimental study of esophageal mucosal regeneration in the dog. Surgery 1970; 68: 209–216. [PubMed] [Google Scholar]
- 17.Hage E, Pederson SA. Morphological characteristics of the columnar epithelium lining the lower esophagus in patients with Barrett’s syndrome. Virchows Arch 1972; 357: 219–229. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein WM, Ippoliti AF. The diagnosis of Barrett’s esophagus: goblets, goblets, goblets. Gastrointest Endosc 1890; 44: 91–95. [DOI] [PubMed] [Google Scholar]
- 19.Öberg S, Peters JH, DeMeester TR, et al. Determinants of intestinal metaplasia within the columnar-lined esophagus. Arch Surg 2000; 135: 651–655. [DOI] [PubMed] [Google Scholar]
- 20.Weston AP, Krmpotich P, Makdisi WF, et al. Short segment Barrett’s esophagus: clinical and histological features, associated endoscopic findings, and association with gastric intestinal metaplasia. Am J Gastroenterol 1996; 91: 981–986. [PubMed] [Google Scholar]
- 21.Katzka DA, Castell DO. Successful elimination of reflux symptoms does not insure adequate control of acid reflux in patients with Barrett’s esophagus. Am J Gastroenterol 1994; 89: 989–991. [PubMed] [Google Scholar]
- 22.Ouatu-Lascar R, Triadafilopoulos G. Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal acid reflux in patients with Barrett’s esophagus. Am J Gastroenterology 1998; 93: 711–716. [DOI] [PubMed] [Google Scholar]
- 23.Champion G, Richter JE, Vaezi MF, et al. Duodenogastroesophageal reflux: relationship to pH and importance in Barrett’s esophagus Gastroenterology 1994; 107: 747–754. [DOI] [PubMed] [Google Scholar]
- 24.Stein HJ, Kauer WK, Feussner H, et al. Bile reflux in benign and malignant Barrett’s esophagus: effect of medical acid suppression and Nissen fundoplication. J Gastrointest Surg 1998; 2: 333–341. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald RC, Omary MB, Triadafilopoulos G. Dynamic effects of acid on Barrett’s esophagus. An ex vivo proliferation and differentiation model. J Clin Invest 1996; 98: 2120–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouatu-Lascar R, Fitzgerald RC, Triadafilopoulos G. Differentiation and proliferation in Barrett’s esophagus and the effects of acid suppression. Gastroenterology 1999; 117: 327–335. [DOI] [PubMed] [Google Scholar]
- 27.Qualman SJ, Murray RD, McClung HJ, et al. Intestinal metaplasia is age related in Barrett’s esophagus. Arch Pathol Lab Med 1990; 114: 1236–1240. [PubMed] [Google Scholar]
- 28.Gulizia JM, Wang H, Antonioli D, et al. Proliferative characteristics of intestinalized mucosa in the distal esophagus and gastroesophageal junction (short-segment Barrett’s esophagus): a case control study. Hum Pathol 1999; 30: 412–418. [DOI] [PubMed] [Google Scholar]
- 29.Cameron AJ, Kamath PS, Carpenter HA. Prevalence of Barrett’s esophagus and intestinal metaplasia at the gastroesophageal junction. Gastroenterology 1999; 112: A82. [Google Scholar]
- 30.Öberg S, Peters JH, DeMeester TR, et al. Inflammation and specialized intestinal metaplasia of cardiac mucosa is a manifestation of gastroesophageal reflux disease. Ann Surg 1997; 226: 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendes de Almeida JC, Chaves P, Pereira D, et al. Is Barrett’s esophagus the precursor of most adenocarcinomas of the esophagus and cardia? A biochemical study. Ann Surg 1997; 226: 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton SR, Yardley JH. Regenerative of cardiac type mucosa and acquisition of Barrett mucosa after esophagogastrostomy. Gastroenterology 1977; 72: 669–675. [PubMed] [Google Scholar]