Abstract
Objective
To determine the variation in number, size, and symptoms in patients with polypoid lesions of the gallbladder.
Summary Background Data
A polypoid lesion is any elevated lesion of the gallbladder mucosa. Several studies have been reported in patients undergoing cholecystectomy, but little information exits regarding the natural history of these lesions in nonoperated patients.
Methods
A total of 111 patients with ultrasound diagnosis of polypoid lesions smaller than 10 mm were followed up by clinical evaluation and ultrasonography. Twenty-seven patients underwent cholecystectomy.
Results
There was no difference in terms of gender. Nearly 80% of the lesions were smaller than 5 mm; they were single in 74%. In nonoperated patients, 50% remained of similar size at the late follow-up, 26.5% increased in number and size, and 23.5% shrank or disappeared. Among the operated patients, 70% corresponded to cholesterol polyps. None of the patients developed symptoms of biliary disease or gallstones or adenocarcinoma.
Conclusions
Ultrasound is useful in the follow-up of patients with polypoid lesions of the gallbladder. Lesions smaller than 10 mm do not progress to malignancy or to development of stones, and none produced symptoms or complications of biliary disease.
Polypoid lesions of the gallbladder (PLGs) correspond to any elevated lesion of the mucosal surface of the gallbladder wall. 1,2 These lesions can be benign or malignant. 3 Christensen and Ishak 4 established a classification for benign tumors of the gallbladder: epithelial tumors or adenomas (tubular, papillary, or mixed types); mesenchymal tumors (hemangioma, lipoma, leiomyoma); and pseudotumors (cholesterol polyps, inflammatory polyps, adenomyoma, and adenomatous hyperplasia). All these lesions can be easily diagnosed by ultrasonography with a sensitivity of 90.1% and a specificity of 93.9%. 2,5,6
Although there are several studies describing the prevalence of these PLGs, 7–12 their eventual malignant potential, 10,13 and their relationship with gallstones, 3 based on resected gallbladders, there has been only one previous report concerning the natural history and behavior of nonoperated PLGs smaller than 10 mm. 14
The purpose of the present prospective study was to perform a late follow-up of patients with PLG by clinical and ultrasonographic evaluation to determine the appearance of biliary symptoms, the eventual modifications in number or size of the lesions, and the relationship with the appearance of stones or adenocarcinoma of the gallbladder.
METHODS
Patients
The group included 111 patients in whom PLGs were detected by ultrasonography with a size less than 10 mm and who were followed up by clinical evaluation and repeated ultrasonograms. This was a prospective study that started with the first patient in January 1987 and ended with the last patient in December 1996. The ultrasonographic follow-up ended on December 1999, when the study was completed and closed. Patients with polypoid lesions larger than 10 mm were excluded (four patients) as were patients with concomitant gallstones and PLG (two patients).
Ultrasonographic Evaluation
The definition of PLG by ultrasonography corresponds to an image with similar echogenicity to the gallbladder wall that projects into the lumen, is fixed, lacks displacement, may or may not have a pedicle, and lacks an acoustic shadow. 15,16 The exact number and size of these lesions were recorded by the same radiologist to avoid interpersonal differences between readers. Changes in size were defined when an increase or decrease of 3 mm was recorded. When two or more lesions were found, the size of the larger lesion was used for analysis.
Clinical Evaluation
All clinical and ultrasonographic findings were maintained in a computer program designed for this study. All patients were clinically followed up and were questioned about the presence of cholic pain, jaundice, pancreatitis, loss of weight, and so forth.
Statistical Analysis
For statistical analysis, the Student t test was used and P < .05 was taken as significant.
RESULTS
The mean age at the entry of the study was 49 years in men and 45 years in women (P < .3). The age range was 17 to 80. The relation of sex was 1.1:1. No patients had biliary symptoms at diagnosis; the PLGs were found on ultrasonography performed for gastrointestinal dyspeptic symptoms or for a routine examination. Of these patients, 13 underwent surgery at their request for different reasons, even those who were asymptomatic. Therefore, 98 patients remained for late follow-up.
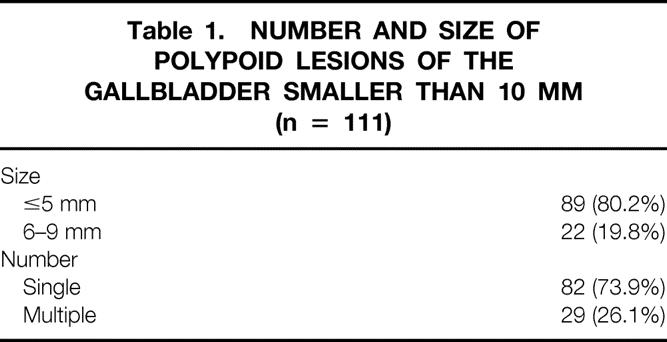
The initial ultrasonographic findings with respect to number and size of PLG are shown in Table 1. Nearly 80% were smaller than 5 mm; 74% of them were single lesions. The mean number of multiple lesions was three, with a maximum of seven lesions.
Table 1. NUMBER AND SIZE OF POLYPOID LESIONS OF THE GALLBLADDER SMALLER THAN 10 MM (n = 111)
The mean clinical and ultrasonographic follow-up of 98 patients was 71 months (range 24–144), with 100% follow-up. None of the patients had symptoms of biliary disease such as cholic pain, acute cholecystitis, or jaundice. None of them developed gallstones or adenocarcinoma.
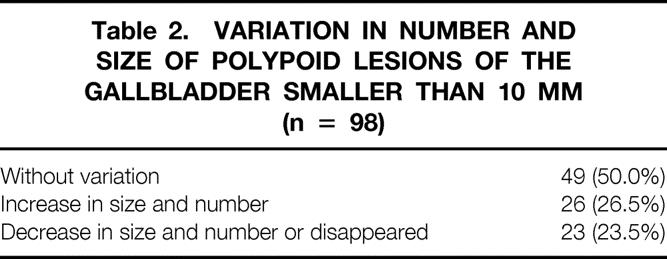
Table 2 shows the ultrasonographic variations in number and size of the PLGs. Half showed no variation; 25% increased in number or size; and 25% decreased in size or number or disappeared.
Table 2. VARIATION IN NUMBER AND SIZE OF POLYPOID LESIONS OF THE GALLBLADDER SMALLER THAN 10 MM (n = 98)
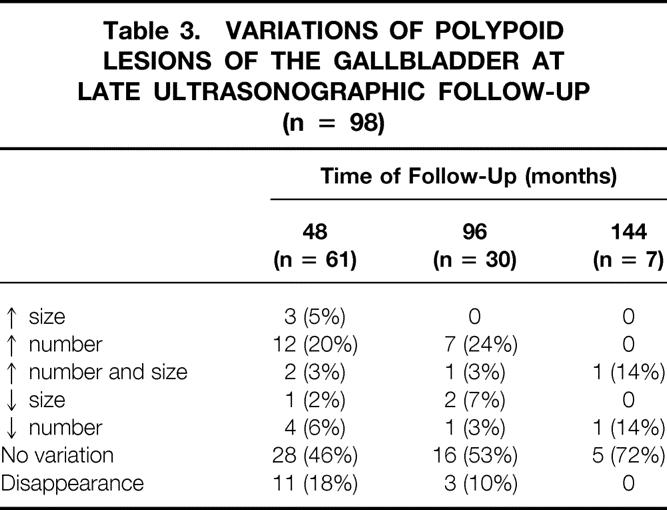
Detailed variations in size or number are shown in Table 3. The maximal increase in size was 8 mm; the increase in number ranged from one to four, whereas the decrease in number ranged from one to five. In 50% of the patients there was no variation in size or number up to 144 months of follow-up. Disappearance of PLGs can occur up to 96 months of follow-up. During the follow-up 14 patients underwent surgery because of variations in the number or size of the PLGs but without clinical symptoms.
Table 3. VARIATIONS OF POLYPOID LESIONS OF THE GALLBLADDER AT LATE ULTRASONOGRAPHIC FOLLOW-UP (n = 98)
The histologic findings from the 27 operated patients are shown in Table 4. In nearly 70%, PLGs corresponded to cholesterol polyps, which usually were 4 to 5 mm and were multiple. Associated histologic changes were cholesterolosis in 44.4%.
Table 4. HISTOLOGIC FINDINGS IN 27 PATIENTS WITH POLYPOID LESIONS OF THE GALLBLADDER UNDERGOING CHOLECYSTECTOMY

DISCUSSION
Our results suggest that PLGs smaller than 10 mm do not correspond to adenocarcinoma and do not develop into it. All of them are asymptomatic and remain so for a long period of follow-up. They do not develop into gallstones, and in 50% of patients, they do not vary in size or number up to 12 years of follow-up.
There have been several studies about the prevalence of PLGs. Jorgensen and Jensen 7 reported in a population of 3,608 subjects a prevalence of 4.6% for men and 4.3% for women. Chen et al, 8 in 3,510 subjects, found a rate of 6.9%. In patients undergoing cholecystectomy, Ozmen et al 9 reported a prevalence of 1.3% among 3,718 patients; Koga et al, 10 among 411 operated patients, reported a rate of 9.7%. The prevalence of cholesterol polyps was 6.3% in men and 3.5% in women among 21,771 subjects studied by Segawa et al. 11 The incidence of adenoma in the general population is unknown, but during cholecystectomies, it is around 1%3,13; in Chile it was around 0.09% among 12,153 cholecystectomies studied by Smok et al. 12
In the present study we found a similar prevalence in men and women. This is in contrast to other publications, 7,8 which found a greater prevalence among men.
There has been only one previous paper concerning the natural history of nonoperated patients with PLGs. 14 Those authors found no change in size in 88% at 5 years of follow-up, with no apparent correlation between change in diameter and the patient’s age or sex. Apparently all of the patients remained asymptomatic during the observation period.
In our study, with up to 12 years of follow-up, we did not see any patient presenting with biliary symptoms. It is a frequent surgical opinion that patients with PLGs should undergo surgery at the time of diagnosis, because a polyp can loosen and may obstruct or prolapse into the cystic duct, producing an acute cholecystitis or even jaundice from obstruction of the common bile duct. However, our results and those of others 17–20 do not support this general opinion and actually show the contrary. We cannot, however, exclude a remote possibility of such complications.
What seems more important is the size of the largest lesion, because it may be a malignant lesion; the dilemma of whether to perform a cholecystectomy or to take a conservative approach is frequently discussed among surgeons. 21
The observation of the natural history of PLGs in one study showed that 95% of the lesions with a probable diagnosis of cholesterol polyps remained the same size or were shown to be benign lesions after cholecystectomy; in the other 5%, two thirds turned out to be adenoma or adenocarcinoma when they were resected. 22
The probable relationship between gallbladder adenoma and adenocarcinoma has been sustained by several authors, including Kosuka and others, 13,23 who in the histopathologic analysis of resected gallbladders determined the presence of transition from adenoma to carcinoma, the association of all carcinomas in situ with adenomas, the high frequency of residual adenoma in patients with invasive carcinoma, and an increase in size of lesions transforming from adenoma to carcinoma (benign adenomas were <12 mm, adenomas with carcinoma in situ were >12 mm, and invasive carcinomas were >30 mm). Koga et al 10 made a comparative analysis between benign and malignant lesions and found that 94% of benign lesions are smaller than 10 mm, whereas 88% of malignant lesions are larger than 10 mm. Therefore, cholecystectomy is indicated when PLGs are larger than 10 mm, 6,24–27 when ultrasound findings suggest malignancy (e.g., thickening of the gallbladder wall or rapid increase in size), 14,23,28–30 or when there are stones 31–35 or biliary symptoms. 1,31
We included only PLGs smaller than 10 mm because we have not found any early carcinomas less than 10 mm, although we have the highest incidence of gallbladder cancer in the world among women. 36,37 It seems that the size of PLGs (larger than or smaller than 10 mm) is crucial with respect to the indication for cholecystectomy and is an useful discriminator for malignancy. Two recent studies confirm our opinion in the sense that lesions larger than 10 mm can be malignant, but below this value it is rare to find an adenocarcinoma. 38,39 The approach to the eventual surgical treatment of PLG is laparoscopic cholecystectomy. However, if there is a suspicion of malignancy, we advocate open cholecystectomy so that a more extensive procedure can be performed if necessary.
Among the 27 patients who underwent cholecystectomy, cholesterol polyps were the most frequent histologic finding, but in three patients at the time of cholecystectomy no polyps were found, presumably because they have a very thin and fragile stalk and could be detached or floating among the bile. 4,12,40,41 These cholesterol polyps are formed by foamy histiocytes that contain cholesterol, covered by a single layer of columnar epithelium. Their cause is not known. Some investigators argue that this lesion is derived from a direct cholesterol deposit from serum cholesterol; others believe that free sterols could be transferred from bile to the gallbladder mucosa and the development of cholesterolosis could be associated with altered hepatic cholesterol synthesis. 42,43
We found that the most frequently associated histopathologic finding was cholesterolosis. Both cholesterol polyps and cholesterolosis seem to belong to the same histopathologic entity. 22 This is not the case when gallstones and cholesterolosis are compared; indeed, a negative association has been postulated because of the mechanical effect of gallstones, which can move and destroy polyps. 7
In conclusion, ultrasonography is useful for the diagnosis and follow-up of PLGs. These PLGs are usually 5 mm or smaller and single. During the late follow-up, no biliary symptoms or complications appeared, and no patients developed stones or adenocarcinoma. In 50% of the patients there was no variation in size up to 76 months of follow-up, and in 25% of the patients the PLGs disappeared. Cholesterol polyps are the most frequent lesions.
Footnotes
Correspondence: Attila Csendes, MD, Dept. of Surgery, Hospital J. J. Aguirre, Santos Dumont 999, Santiago, Chile.
E-mail: acsendes@machi.med.uchile.cl
Accepted for publication February 21, 2001.
References
- 1.Shirai Y, Ohtani T, Hatakeyama K. Is laparoscopic cholecystectomy recommended for large polypoid lesions of the gallbladder? Surg Laparosc Endosc 1997; 7: 435–436. [PubMed] [Google Scholar]
- 2.Yang HL, Sun YG, Wang Z. Polypoid lesions of the gallbladder: diagnosis and indications for surgery. Br J Surg 1992; 79: 227–229. [DOI] [PubMed] [Google Scholar]
- 3.Aldridge MC, Bismuth H. Gallbladder cancer: the polyp–cancer sequence. Br J Surg 1990; 77: 363–364. [DOI] [PubMed] [Google Scholar]
- 4.Christensen AH, Ishak KG. Benign tumors and pseudotumors of the gallbladder. Arch Pathol 1970; 90: 423–432. [PubMed] [Google Scholar]
- 5.Basso L, Tocchj A. Ultrasonography in the diagnosis of gallbladder polyps. Br J Surg 1993; 80: 1083. [DOI] [PubMed] [Google Scholar]
- 6.Johnson CD. Polypoid lesions of the gallbladder. Gut 1997; 41: 578. [PubMed] [Google Scholar]
- 7.Jorgensen T, Jensen KH. Polyps in the gallbladder. A prevalence study. Scand J Gastroenterol 1990; 25: 281–286. [PubMed] [Google Scholar]
- 8.Chen CY, Lu CL, Chang FY, et al. Risk factors for gallbladder polyps in the Chinese population. Am J Gastroenterol 1997; 92: 2066–2068. [PubMed] [Google Scholar]
- 9.Ozmen MM, Patankar RV, Hengirmen S, et al. Epidemiology of gallbladder polyps. Scand J Gastroenterol 1994; 29: 480. [DOI] [PubMed] [Google Scholar]
- 10.Koga A, Watanabe K, Fukuyama T, et al. Diagnosis and operative indications for polypoid lesions in the gallbladder. Arch Surg 1988; 123: 26–29. [DOI] [PubMed] [Google Scholar]
- 11.Segawa K, Arisawa T, Niwa Y, et al. Prevalence of gallbladder polyps among apparently healthy Japanese: ultrasonographic study. Am J Gastroenterol 1982; 87: 630–633. [PubMed] [Google Scholar]
- 12.Smok G, Bentjerodt R, Csendes A. Lesiones polipoideas benignas de la vesícula biliar. Relación con adenocarcinoma vesicular. Rev Med Chile 1992; 120: 31–35. [PubMed] [Google Scholar]
- 13.Kosuka S, Tsubone M, Yasui A, et al. Relation of adenoma to carcinoma in the gallblader. Cancer 1982; 50: 2226–2234. [DOI] [PubMed] [Google Scholar]
- 14.Moriguchi H, Tazawa J, Hayashi Y, et al. Natural history of polypoid lesions in the gallbladder. Gut 1996; 39: 860–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jirón MI, Silva H, Whittle C, et al. Pólipos vesiculares. Segundo taller de consenso de la asociación chilena de hepatología. Rev Med Chile 1994; 122: 1316–1317. [PubMed] [Google Scholar]
- 16.Ozdemir A, Oze NC A, Bozoklu S, et al. Ultrasonography in the diagnosis of gallbladder polyps. Br J Surg 1993; 80: 345. [DOI] [PubMed] [Google Scholar]
- 17.Capell MS, Marks M, Kerschenbaum H. Massive hemobilia and acalculous cholecystitis due to benign gallbladder polyp. Dig Dis Sci 1993; 38: 1156–1161. [DOI] [PubMed] [Google Scholar]
- 18.Takii Y, Shirai Y, Kanehara H, et al. Obstructive jaundice caused by a cholesterol polyp of the gallbladder: report of a case. Surg Today 1994; 24: 1104–1106. [DOI] [PubMed] [Google Scholar]
- 19.Shepard VD, Walsters W, Dockerty MB. Benign neoplasms of the gallbladder. Arch Surg 1942; 45: 1–18. [Google Scholar]
- 20.Niv Y, Kosakov K, Shcolnik B. Fragile papilloma (papillary adenoma) of the gallbladder. Gastroenterology 1986; 91: 999–1001. [DOI] [PubMed] [Google Scholar]
- 21.Boulton RA, Adams DH. Gallbladder polyps: when to wait and when to act. Lancet 1997; 349: 817. [DOI] [PubMed] [Google Scholar]
- 22.Bilhartz LE. Cholesterolosis. In: Sleisenger Fordtran eds. Gastrointestinal disease. Philadelphia: WB Saunders; 1993: 1860–1862.
- 23.Koga A, Yamauchi S, Izumi Y, et al. Ultrasonographic detection of early and curable carcinoma of the gallbladder. Br J Surg 1985; 72: 728–730. [DOI] [PubMed] [Google Scholar]
- 24.Kubota K, Bandai Y, Otomo Y, et al. Role of laparoscopic cholecystectomy in treating gallbladder polyps. Surg Endosc 1994; 8: 42–46. [DOI] [PubMed] [Google Scholar]
- 25.Majeed AW, Johnson AG. Gallbladder polyps are a common ultrasonographic finding. J Am Coll Surg 1995; 181: 189. [PubMed] [Google Scholar]
- 26.Shinkai H, Kimura W, Muto T. Surgical indications for small polypoid lesions of the gallbladder. Am J Surg 1998; 175: 114–117. [DOI] [PubMed] [Google Scholar]
- 27.Chijiiwa K, Tanaka M. Polypoid lesion of the gallbladder: indications of carcinoma and outcome after surgery for malignant polypoid lesion. Int Surg 1994; 79: 106–109. [PubMed] [Google Scholar]
- 28.Reck T, Kockerling F, Heydern N, et al. Polypoid lesions of the gallbladder—preventive cholecystectomy? Chirurgie 1992; 63: 506–510. [PubMed] [Google Scholar]
- 29.Ukai C, Akita Y, Mizuno S, et al. Cholesterol polyp of the gallbladder showing growth and atypical changes. A case report. Hepato-Gastroenterology 1992; 39: 371–373. [PubMed] [Google Scholar]
- 30.Kyriacou E. Natural history of polypoid lesions of the gallbladder. Gut 1997; 41: 577–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tinsley AR, Mulkerin LE, Van Der Linde JM, et al. Polypoid lesions of the acalculous gallbladder. South Med J 1975; 68: 958–962. [DOI] [PubMed] [Google Scholar]
- 32.Smok G, Cervilla K, Bosch H, et al. Lesiones precursoras del carcinoma invasor de vesícula biliar. Rev Med Chile 1986; 114: 954–958. [PubMed] [Google Scholar]
- 33.Bivins BA, Meeker WR, Weiss DL, et al. Carcinoma in situ of the gallbladder: a dilemma. South Med J 1975; 68: 297–300. [DOI] [PubMed] [Google Scholar]
- 34.Albores-Saavedra J, Alcantara-Vasquez A, Cruz-Ortiz H, et al. The precursor lesions of invasive gallbladder carcinoma. Hyperplasia, atypical hyperplasia and carcinoma in situ. Cancer 1980; 45: 919–927. [DOI] [PubMed] [Google Scholar]
- 35.Edelman DS. Carcinoma of a gallbladder polyp treated by laparoscopic laser cholecystectomy. Surg Laparosc Endosc 1993; 3: 142–143. [PubMed] [Google Scholar]
- 36.Csendes A, Becerra M, Smok G, et al. Prevalence of gallbladder cancer in cholecystectomies. Rev Med Chile 1991; 119: 887–890. [PubMed] [Google Scholar]
- 37.Csendes A, Becerra M, Morales E. Gallbladder carcinoma. Trib Med 1991; 84: 131–136. [Google Scholar]
- 38.Mainprize KS, Gould SWT, Gilbert JH. Surgical management of polypoid lesions of the gallbladder. Br J Surg 2000; 87: 414–417. [DOI] [PubMed] [Google Scholar]
- 39.Terzi C, Sokmen S, Seckin S, et al. Polypoid lesions of the gallbladder: Report of 100 cases with special reference to operative indications. Surgery 2000; 127: 7622–7627. [DOI] [PubMed] [Google Scholar]
- 40.Astete G, Lynch O, Madariaga J, et al. Lesiones elevadas de la vesícula biliar. Rev Chilena de Cirugía 1999; 51: 159–163. [Google Scholar]
- 41.Collett J, Allan R, Chisholm R, et al. Gallbladder polyps: prospective study. J Ultrasound Med 1998; 17: 207–211. [DOI] [PubMed] [Google Scholar]
- 42.Furukawa H, Kosuge T, Shimada K, et al. Small polypoid lesions of the gallbladder. Differential diagnosis and surgical indications by helical computed tomography. Arch Surg 1998; 133: 735–739. [DOI] [PubMed] [Google Scholar]
- 43.Wu SS, Lin KC, Soon ME, et al. Ultrasound-guided percutaneous transhepatic fine needle aspiration cytology study of gallbladder polypoid lesions. Am J Gastroenterol 1996; 91: 1591–1594. [PubMed] [Google Scholar]


