Abstract
Objective
To quantitate disease-specific hospital-based medical costs in 34 patients with chronic pancreatitis before and after treatment by either duodenal-preserving pancreatic head resection (DPPHR) or pylorus-preserving pancreaticoduodenectomy (PPPD).
Summary Background Data
Pancreatic head resection in selected patients with chronic pancreatitis provides pain relief and improves quality of life, but the effect on healthcare costs is unknown.
Methods
This observational cohort study comprised 34 selected patients with chronic pancreatitis followed up exclusively at the authors’ institution treated by either DPPHR or PPPD between 1992 and 1997.
Results
Twenty-one patients had DPPHR and 13 had PPPD. Patients in the PPPD group were slightly older, but other clinical characteristics were similar. Before surgery, the mean number of admissions per patient per year, days in the hospital per patient per year, and disease-specific hospital-based medical costs per patient per year were not significantly different between groups. After surgery, those three variables were similar between the groups but significantly less than preoperative values. Pain control remained significantly improved after 36 months of follow-up.
Conclusions
In selected patients with chronic pancreatitis, DPPHR and PPPD are equally effective in providing long-term pain relief and decreasing disease-specific hospital-based costs.
Patients with chronic pancreatitis who have severe recurrent abdominal pain as the predominant symptom often require in-hospital treatment, including intravenous narcotic analgesics, gut rest, total parenteral nutrition, celiac plexus blocks, endoscopic stent therapy, pancreatic lithotripsy, and occasionally surgical resection or drainage. 1 All of these interventions are associated with hospital-based medical costs. Pancreatic head resection, either pylorus-preserving pancreaticoduodenectomy (PPPD) or duodenal-preserving pancreatic head resection (DPPHR), are the operations of choice to achieve pain relief and improve quality of life in the selected group of patients with chronic pancreatitis who have a nondilated pancreatic duct (<6 mm) and an enlarged pancreatic head. 2 Recent studies evaluating the safety and efficacy of surgery in patients with chronic pancreatitis have focused on perioperative complication and death rates, 3–7 maintenance of pancreatic function, 3,4,6,8,9 and patient outcome as assessed by either pain scores 3–6 or quality of life indices. 3,4,6 Although DPPHR and PPPD have been shown to be equivalent operations in terms of safety and efficacy, 3,5 their effect on healthcare costs is unknown. The primary aim this study was to assess the impact of surgery on the disease-specific hospital-based medical costs incurred by a selected group of patients with chronic pancreatitis undergoing pancreatic head resection.
METHODS
From 1992 until 1997, 74 consecutive patients with chronic small duct pancreatitis and an inflammatory mass (>30 mm in diameter) in the head of the pancreas who were seen, evaluated, and treated by a pancreaticobiliary group (T.J.H., S.S., G.A.L., E.F.) at our institution underwent DPPHR or PPPD as the primary surgical procedure for their chronic pancreatitis. All patients were ethanol-free at the time of surgery, all had severe abdominal pain requiring narcotic analgesics, and most required recurrent hospital admissions for gut rest, intravenous hydration, and pain control. From this group of patients, 34 qualified for this study based on the following inclusion criteria: all medical care and hospital stays for at least 12 months before surgery and at least 12 months after surgery were done exclusively at Indiana University Medical Center, for which complete financial (IBAX system) and clinical data (PHAMIS system) were available; patients completed a visual analog pain scale 10 both before surgery and at specific times during the postoperative follow-up; and there was histopathologic confirmation of chronic pancreatitis.
Patient medical records and office charts were used to obtain demographic data, etiology of pancreatitis, preoperative diabetes, need for pancreatic enzyme replacement, surgical indications, and postoperative complications. The type of operation done was nonrandomized and was based on patient preferences after a thorough discussion of the risks and benefits of each procedure. A Beger-type resection (Fig. 1) was performed in 8 patients (38%) and a Frey-type resection (Fig. 2) in the remaining 13 (62%). A pylorus-preserving pancreaticoduodenectomy (Fig. 3) was done in all 13 patients in the PPPD group. All patients when questioned reported that they were admitted to the hospital and underwent endoscopic or surgical procedures exclusively at Indiana University Medical Center during their periods of observation. A visual analog pain scale was administered before surgery and at 6, 12, 24, 36, 48, 60, and 72 months after surgery by outpatient clinic personnel or direct mailing. Patients were followed up before surgery for a mean of 26 months (range 12–36) and after surgery for a mean of 41 months (range 12–73).
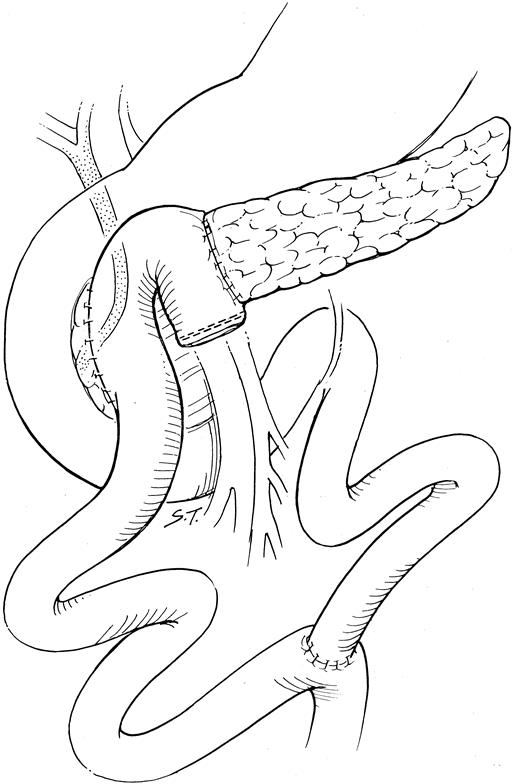
Figure 1. Beger-type procedure: subtotal resection of the pancreatic head and uncinate process, maintaining the blood supply to the duodenum via a small rim of remaining pancreas along the bile duct and duodenum. Reconstruction is by an end-to-side pancreaticojejunostomy to the body and tail of the pancreas, and a side-to-side pancreaticojejunostomy to the remnant pancreatic head.
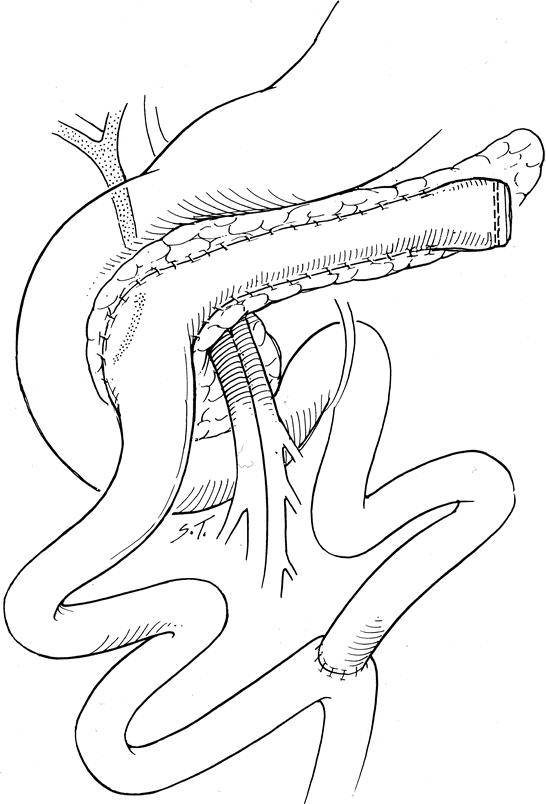
Figure 2. Frey-type procedure: local excision of the pancreatic head overlying the ducts of Wirsung and Santorini and the uncinate, along with their tributary ducts, and decompressing the intrapancreatic portion of the common bile duct. Drainage is improved by opening the main pancreatic duct in the body and tail of the pancreas. The locally resected head and the opened main pancreatic duct in the body and tail are then drained into a Roux-en-Y limb of jejunum.
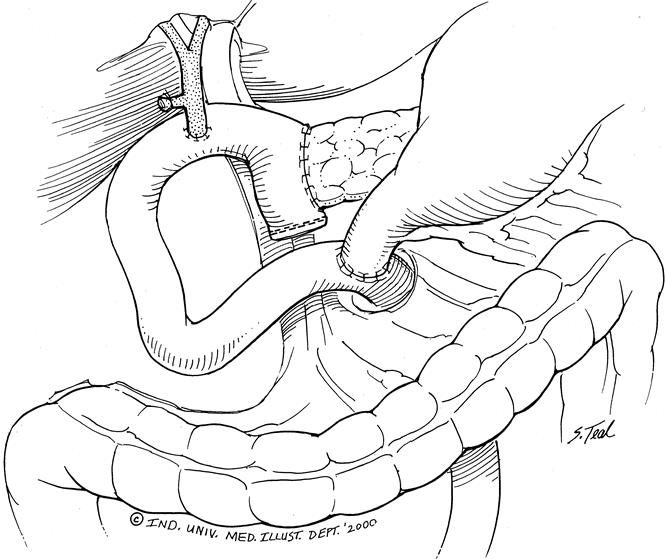
Figure 3. Pylorus-preserving pancreaticoduodenectomy: the head of the pancreas and uncinate process, duodenum, and distal common bile duct are resected. Gastrointestinal continuity is reestablished by an end-to-side pancreaticojejunostomy, end-to-side choledochojejunostomy, and end-to-side pylorojejunostomy.
Our economic analysis used a societal perspective focusing on the disease-specific hospital-based medical costs incurred in this patient population. The hospital-based medical costs used in this study included variable costs, fixed costs, and indirect costs. The primary economic outcomes of this study included both the hospital and physician costs for the disease-specific procedures used to treat chronic pancreatitis directly, such as celiac plexus blocks, endoscopic retrograde cholangiopancreatography (ERCP) with or without sphincterotomy and pancreatic duct stent placement, and abdominal surgery that was directly related to chronic pancreatitis (DPPHR and PPPD) or that was necessary to treat a complication of surgery (drainage of abscess or fistula, pancreatitis, incisional hernia repair, small bowel obstruction) or recurrent pain (revision of pancreaticojejunostomy, completion pancreatectomy).
Hospital cost data were collected from TSI integration of the inpatient clinical (PHAMIS) and financial (IBAX) database systems at Indiana University Medical Center. These databases contain information on admission and discharge dates, length of stay, Diagnostic Related Group (DRG), principal procedure, and hospital-based direct costs. Hospital-based direct costs consisted of operating room and recovery room, hospital room, ERCP suite, radiology, pharmacy, laboratory, and ancillary costs. All hospital-based costs were adjusted for inflation to 1996 dollars using the medical care consumer price index. Physician costs were approximated using the 1996 Medicare resource-based relative value scale for fees. Physician fees were calculated using relative value units (RVUs) for the appropriate CPT codes for operation, endoscopic therapy, or celiac plexus block multiplied by the 1996 Medicare conversion factor for surgical services priced at $40.799. Practice cost RVUs and professional liability insurance RVUs were not included in this analysis. Total disease-specific hospital-based medical costs were defined as the sum of the hospital cost and physician costs. Continuous data were analyzed using one-way analysis of variance and nominal data using the Fisher exact test. Cost data were assessed for normality of distribution. Normally distributed cost data were analyzed by analysis of variance (parametric test). Cost data with an abnormal distribution were analyzed by the Wilcoxon signed-rank test (nonparametric test). 9 Significance was defined as P < .05.
RESULTS
Of the 34 patients available for study, 21 were treated with DPPHR and 13 with PPPD (Table 1). Patients in the DPPHR group, with a mean age of 37 years, were significantly younger than patients in the PPPD group, with a mean age of 50 years (P = .001). This difference may reflect the fact that PPPD is considered the more conventional standard of care, which appeals to the elderly patient, rather than DPPHR, which was viewed as a newer, less comprehensive alternative. Approximately 75% of patients in both groups were alcohol abusers, and the predominant indication for surgery was longstanding abdominal pain. Initial average pain scores using a visual analog pain scale (0, no pain; 10, most severe pain imaginable) were 8.3 for the DPPHR group and 7.8 for the PPPD group. This represented severe pain; in the vast majority of patients in both groups, the pain was regarded as continual rather than intermittent and had been present for an average of approximately 3 years before surgery. All patients in both groups had stopped ethanol use before surgery, but all patients were taking narcotic analgesics to control their pain. The average equianalgesic dose of narcotic (based on a scale assigning codeine a value of 1) used before surgery was 10 ± 8 in the DPPHR group and 8 ± 5 in the PPPD group. Almost all patients in both groups had prior attempts at controlling their abdominal pain with ERCP and sphincterotomy, pancreatic duct stents, somatostatin therapy, or celiac plexus blockade. Less than a quarter of all patients had diabetes requiring exogenous insulin or oral hypoglycemic agents, and approximately one third routinely used pancreatic enzyme replacement.
Table 1. PREOPERATIVE CLINICAL CHARACTERISTICS
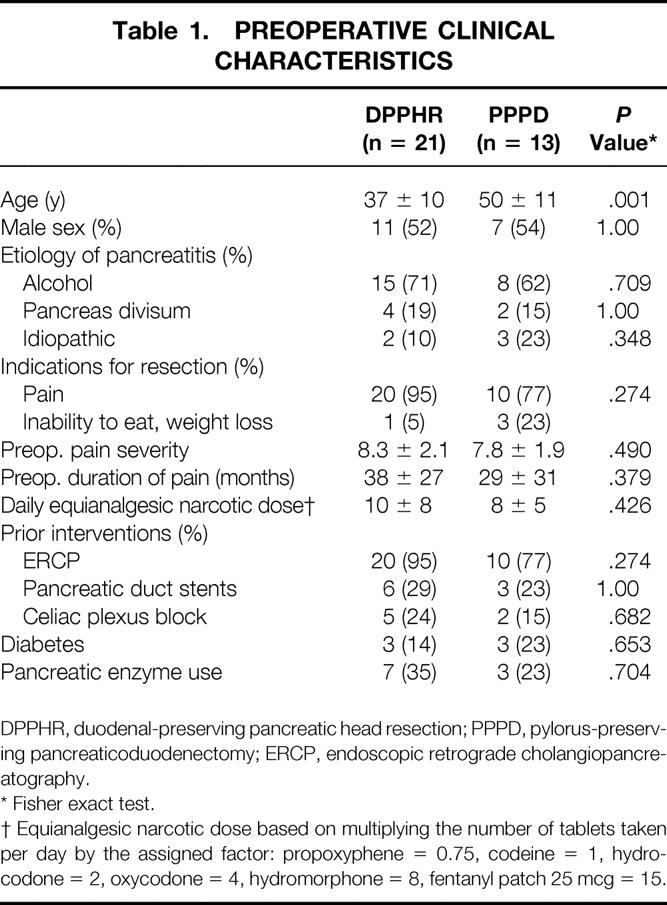
DPPHR, duodenal-preserving pancreatic head resection; PPPD, pylorus-preserving pancreaticoduodenectomy; ERCP, endoscopic retrograde cholangiopancreatography.
* Fisher exact test.
† Equianalgesic narcotic dose based on multiplying the number of tablets taken per day by the assigned factor: propoxyphene = 0.75, codeine = 1, hydrocodone = 2, oxycodone = 4, hydromorphone = 8, fentanyl patch 25 mcg = 15.
The length of hospital stay and cost data for the two surgical procedures evaluated are shown in Table 2. The mean total hospital-based medical costs per patient for DPPHR were $3,914 less than for PPPD (P = .018). This trend toward lower cost for DPPHR was the result of both a lower hospital cost (roughly proportional to the length of stay) and lower total RVUs assigned to the procedure itself, resulting in a decrease in calculated physician costs. Early and late postoperative complications are shown in Table 3. The types of postoperative complications and their respective incidence were comparable between the two groups as well as similar series reported in the literature. 3–5,10,11 There were no early postoperative deaths in the PPPD group and one early postoperative death in the DPPHR group. This death occurred in a 56-year-old woman in whom critical celiac stenosis was unrecognized and the pancreaticoduodenal arcade was disrupted during the procedure, resulting in antral ischemia requiring reoperation. During anesthetic induction for the reoperation, aspiration occurred, resulting in acute respiratory distress syndrome and death.
Table 2. HOSPITAL LENGTHS OF STAY AND AVERAGE DISEASE-SPECIFIC MEDICAL COSTS PER PATIENT
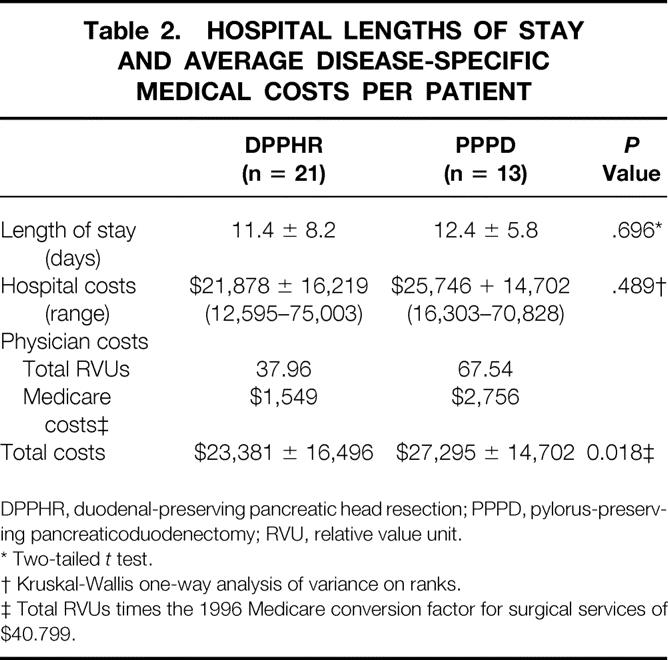
DPPHR, duodenal-preserving pancreatic head resection; PPPD, pylorus-preserving pancreaticoduodenectomy; RVU, relative value unit.
* Two-tailed t test.
† Kruskal-Wallis one-way analysis of variance on ranks.
‡ Total RVUs times the 1996 Medicare conversion factor for surgical services of $40.799.
Table 3. COMPLICATIONS AND DEATHS
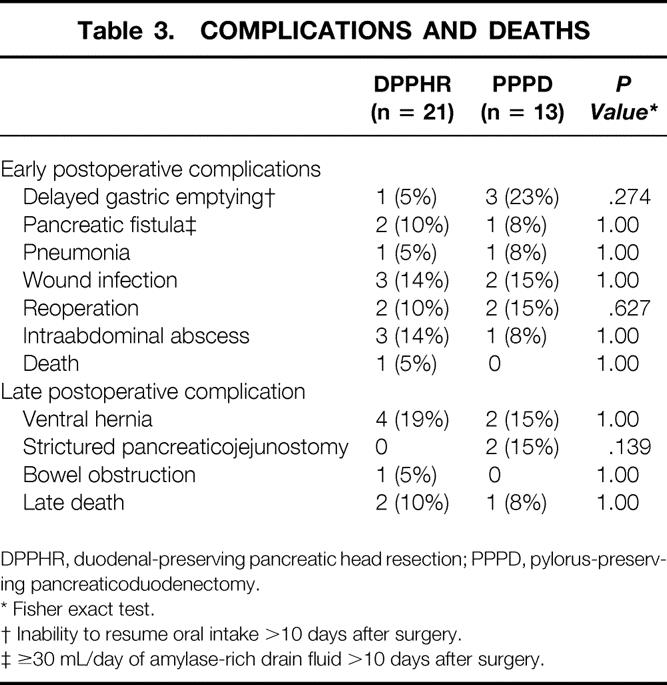
DPPHR, duodenal-preserving pancreatic head resection; PPPD, pylorus-preserving pancreaticoduodenectomy.
* Fisher exact test.
† Inability to resume oral intake >10 days after surgery.
‡ ≥30 mL/day of amylase-rich drain fluid >10 days after surgery.
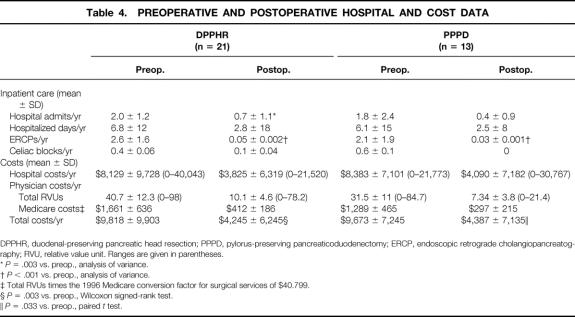
The mean number of preoperative hospital admissions per patient per year was 2.0 ± 1.2 (range 1–8) for the DPPHR group and 1.8 ± 2.4 (range 0–5) for the PPPD group (Table 4). The average time spent in the hospital per patient per year was 6.8 ± 12 days for the DPPHR group and 6.1 ± 15 days for the PPPD group. Patients were treated with an average of 2.6 and 2.1 ERCPs in each group and 0.4 and 0.6 celiac blocks, respectively. These similarities in utilization and hospital admission rates were reflected in the comparable average hospital costs ($8,129 and $8,383), physician RVUs (40.7 and 31.5), and total disease-specific hospital-based medical costs ($9,818 and $9,673) per patient per year.
Table 4. PREOPERATIVE AND POSTOPERATIVE HOSPITAL AND COST DATA
DPPHR, duodenal-preserving pancreatic head resection; PPPD, pylorus-preserving pancreaticoduodenectomy; ERCP, endoscopic retrograde cholangiopancreatography; RVU, relative value unit. Ranges are given in parentheses.
*P = .003 vs. preop., analysis of variance.
†P < .001 vs. preop., analysis of variance.
‡ Total RVUs times the 1996 Medicare conversion factor for surgical services of $40.799.
§P = .003 vs. preop., Wilcoxon signed-rank test.
∥P = .033 vs. preop., paired t test.
In the postoperative period, hospital-based resource utilization declined dramatically. The average number of hospital admissions per patient per year was 0.78 ± 1.1 (P = .003 vs. preoperative) in the DPPHR group and 0.4 ± 0.9 (P = .053 vs. preoperative) in the PPPD group. Correspondingly, the average number of hospital days per patient per year (2.8 and 2.5), ERCPs (0.05 and 0.03), and celiac blocks (0.1 and 0.05) also declined during the postoperative period. Total disease-specific hospital-based costs (DPPHR $4,245, PPPD $4,387) were similar between groups, but both were significantly less than accrued before surgery (DPPHR, P = .003; PPPD, P = .033). Further, the majority of hospital admissions and hospital costs incurred in the postoperative period, 77% in the DPPHR group and 68% in the PPPD group, were due to a procedure-related complication, such as delayed gastric emptying, incisional hernia repair, or small bowel obstruction, rather than recurrent episodes of pancreatitis or pain (see Table 4).
The improvement in the visual analog scale pain score at 6 months, from an average of 8.3 to 2.1 in the DPPHR group and 7.8 to 1.7 in the PPPD group, deteriorated slightly at 24 months (DPPHR 3.4, PPPD 3.1) but still remained significantly improved compared with preoperative values (P < .001;Fig. 4). Four patients (10%) in the DPPHR group and two patients (15%) in the PPPD group returned to ethanol use after the procedure. Despite this improvement in pain score, narcotic analgesic use continued in 9 of 21 patients (43%) in the DPPHR group and 4 of 13 patients (31%) in the PPPD group, albeit at a significantly lower equianalgesic dose (DPPHR 3.3 ± 0.7, PPPD 2.4 ± 1.6;P < .001) than was being used before surgery. Eight patients (38%) in the DPPHR group and four patients (31%) in the PPPD group were completely pain-free after surgery. In the DPPHR group before surgery 17 patients were employed or worked in the home, 2 were receiving disability, and 2 were retired. After surgery, 14 remained employed or worked in the home, 5 were receiving disability, and 2 were retired. In the PPPD group, eight were employed before surgery, two were disabled, and three were retired. After surgery six remained employed, four were disabled, and three were retired.
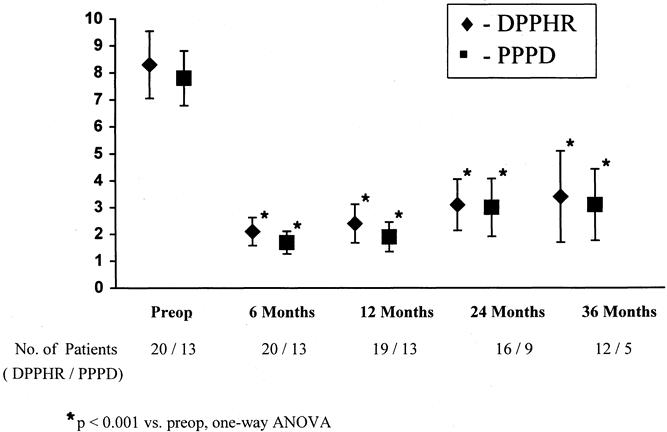
Figure 4. Pain score before surgery and at 6, 12, 24, and 36 months after surgery. DPPHR, duodenal-preserving pancreatic head resection; PPPD, pylorus-preserving pancreaticoduodenectomy. *P < .001 versuspreoperative, one-way analysis of variance.
DISCUSSION
Interest in the economic impact of healthcare interventions has increased dramatically in recent years. 12 Both public and private payers have grown increasingly aware of the costs of chronic conditions and the disproportionate use of resources by this minority of patients. 13 Patients with chronic pancreatitis and chronic abdominal pain consume a large portion of healthcare resources and are a difficult and costly group to treat. Multiple interventions, including ERCP with or without endoscopic sphincterotomy, pancreatic stent placement, and/or pancreatic lithotripsy, 14,15 total parenteral nutrition, 16 celiac plexus blocks, 17 and somatostatin therapy, 18 have all been used with varying degrees of success. Despite concerns about the costs of managing patients with chronic conditions, there are few sources of data that allow us to evaluate one course of treatment versus another or to weigh the economic impact and benefit of these differing treatment options.
Pancreatic head resection, either by DPPHR as advocated by Beger et al. 6 or Frey and Akimura, 10 or PPPD, 11 is the operation of choice to treat patients with chronic small duct pancreatitis and an enlarged pancreatic head (>30 mm in diameter). These operations have been shown in multiple studies to improve the quality of life and decrease pain in patients with chronic pancreatitis. 1–7,11 Implicit in these outcome studies is the assumption that patients who have less or no pain and are experiencing an improved quality of life will not utilize healthcare resources. Despite its apparent logic, to our knowledge this important relationship has never been shown.
Our primary aim in this study was to quantify the impact of surgery on the disease-specific hospital-based medical costs incurred by patients with chronic small duct pancreatitis and an enlarged pancreatic head. We focused on disease-specific costs associated with pancreatitis and postoperative complications to avoid medical costs incurred for other conditions (i.e., trauma, chronic obstructive pulmonary disease, coronary artery disease, diabetes) that were not directly affected by the operations under study. Hospital-based medical costs were used because high-quality data were readily available by integrating currently running inpatient clinical (PHAMIS) and financial (IBAX) databases at our institution. Outpatient costs and indirect costs of care, such as opportunity costs resulting from lost time from work, travel costs to and from the hospital and clinic, or the costs of family members providing home care, were not included in this analysis because we lacked high-quality data on outpatient costs, and quantifying indirect costs of care remains an inexact science. Despite their absence from this analysis, these costs should be relatively small when compared with the hospital-based costs studied, and their distribution should be similar across treatment groups. Based on the total number of outpatient clinic visits made by patients in each group (data not shown), these assumptions appear valid.
We hypothesized that the initial healthcare costs associated with a major operation in this selected group of patients would be offset after surgery by a reduction in both hospital readmission rates and need for disease-specific hospital-based intervention (i.e., ERCP, celiac block). A secondary objective was to assess the impact of surgery on pain score, analgesic use, and return to employment. Our data show that both DPPHR and PPPD are similar operations in term of efficacy and safety. In terms of efficacy, both were able to decrease abdominal pain significantly, as assessed by a visual analog pain scale, and to maintain this pain control for the 36 months of follow-up. With regard to safety, both operations had similar postoperative complication and death data. Both of these observations have been shown previously in two prospective, randomized clinical trials. 3,5 Average hospital costs were $21,878 for DPPHR and $25,746 for PPPD. These hospital costs were slightly more than the average total hospital costs of $17,252 reported in the 1995 study of pancreaticoduodenectomy by Holbrook et al. 19 These cost differences can be attributed to variable accounting practice between the two facilities as well as the indexing of all of our cost data to 1996 dollars based on the medical care consumer price index. The $3,914 savings for DPPHR over PPPD in our study was the result of a reduction in both hospital and physician costs. Delayed gastric emptying occurred in 23% of patients in the PPPD group and contributed to their prolonged average postoperative stay (12.4 vs. 11.4 days) compared with the DPPHR group. Although not specifically analyzed in this study, postoperative complications did prolong the postoperative stay and contributed to an increase in overall hospital costs, as has been pointed out previously. 19 Although statistically significant in this small cohort of patients, these cost savings were relatively small and may not hold up over analysis of a larger group of patients.
After surgery, both DPPHR and PPPD significantly reduced the pain score, hospital admission rate, and the average yearly hospital cost per patient with chronic pancreatitis. In the early postoperative period (6 months), the pain score decreased approximately 75% in both groups (P < .001) and remained significantly decreased during 36 months of follow-up. These results compare favorably with those of Izbicki et al, 3 who found a median decrease in pain score of 90% in the DPPHR group and 75% in the PPPD group. In contrast to our findings, Büchler et al 5 reported that although 75% of patients were pain-free after DPPHR, only 40% of patients were pain-free after PPPD. In our experience, complete pain relief is an uncommon outcome for most patients with chronic pancreatitis irrespective of the type of pancreatic head resection performed. Complete relief of pain occurred in only 25% of the DPPHR group and 23% of the PPPD group in this series. The visual analog pain scale is a standardized, quick, and easy method for quantifying the amount of pain a patient is experiencing at a given point in time both before and after surgery in patients with chronic pancreatitis. One limitation of this study is that a more robust quality of life assessment using a general symptom index, working ability, or a financial strain scale might have identified differences in quality of life not discerned using a simple pain score. 20
Average yearly disease-specific hospital-based costs decreased after surgery by 57% in the DPPHR group and 55% in the PPPD group. Further, these costs shifted from those used to treat abdominal pain, nausea, and recurrent bouts of pancreatitis toward costs used to treat postoperative complications such as ventral hernia repair, small bowel obstruction, and revision of strictured pancreaticojejunostomies. This total decrease in disease-specific medical costs and the shift in resource utilization reinforce the improvements in postoperative pain scores by documenting a concomitant decrease in hospital admission rates for recurrent abdominal pain. The durability of the pain relief in both surgical groups was a welcome surprise and had been confirmed by others. 1,3,6,7,11,21,22 Of more concern with our particular series is that despite an improvement in pain score and decrease in hospital admission rates, narcotic analgesic use continued in 43% of the DPPHR group and 31% of the PPPD group, albeit at lower doses than consumed before surgery. The occupational rehabilitation rates of 74% for the DPPHR group and 60% for the PPPD group also compare favorably with the 68% for DPPHR and 43% for PPPD reported by Izbicki et al, 3 but lag behind the superb 96% occupational rehabilitation rate reported after the Whipple procedure. 21 These differences in narcotic use and occupational rehabilitation may be more reflective of psychological, socioeconomic, and cultural factors in our particular patient population than the specific results of a surgical procedure. 6,7,10
Patient selection is known to be critically important in the success of pancreatic resection for chronic abdominal pain. 11 Unfortunately, a standard system of stratification or subgrouping of patients with chronic pancreatitis by morphologic or functional criteria has never been accepted. This deficiency makes inclusion of patients in therapeutic trials inconsistent and interpretation of data between series problematic. 23 We chose to study patients with chronic small duct pancreatitis and an enlarged pancreatic head because they represent a relatively homogeneous group of patients, in contrast to patients with papillary stenosis, pancreas divisum, or minimal change pancreatitis. 1,22 In addition, both DPPHR and PPPD have been carefully studied previously in this defined patient population, providing a barometer for our assessment of safety and efficacy. 3–5 Because of our strict selection criteria, these results are specific to this particular subgroup of patients, and the ability to generalize these results outside this narrowly defined population is limited.
In summary, both DPPHR and PPPD are equally effective in providing long-term pain relief and a significant decrease in disease-specific hospital-based costs when used in carefully selected patients with chronic pancreatitis.
Footnotes
Correspondence: Thomas J. Howard, MD, EM #523, 545 Barnhill Dr., Indianapolis, IN 46202.
E-mail: tjhoward@iupui.edu
Accepted for publication January 24, 2001.
References
- 1.Warshaw AL, Banks PA, Fernàndez-del Castillo C. AGA technical review on treatment of pain in chronic pancreatitis. Gastroenterology 1998; 115: 765–776. [DOI] [PubMed] [Google Scholar]
- 2.Howard TJ, Selzer DJ. Operative management of chronic pancreatitis. Techniques Gastrointest Endosc 1999; 1: 186–191. [Google Scholar]
- 3.Izbicki JR, Bloechle C, Broering DC, et al. Extended drainage versus resection in surgery for chronic pancreatitis: a prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head excision with the pylorus-preserving pancreaticoduodenectomy. Ann Surg 1998; 228: 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izbicki JR, Bloechle C, Knoefel WT, et al. Duodenum-preserving resections of the head of the pancreas in chronic pancreatitis: a prospective randomized trial. Ann Surg 1995; 221: 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Büchler MW, Friess H, Müller MM, et al. Randomized trial of duodenum-preserving pancreatic head resection versus pylorus-preserving Whipple in chronic pancreatitis. Am J Surg 1995; 169: 65–70. [DOI] [PubMed] [Google Scholar]
- 6.Beger HG, Schlosser W, Friess HM, et al. Duodenum-preserving head resection in chronic pancreatitis changes the natural course of the disease: a single-center 26-year experience. Ann Surg 1999; 230: 512–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard JM, Zhang A. Pancreaticoduodenectomy (Whipple’s resection) in the treatment of chronic pancreatitis. World J Surg 1990; 14: 77–82. [DOI] [PubMed] [Google Scholar]
- 8.Eddes EH, Masclee AM, Gooszen HG, et al. Effect of duodenum-preserving resection of the head of the pancreas on endocrine and exocrine pancreatic function in patients with chronic pancreatitis. Am J Surg 1997; 174: 387–392. [DOI] [PubMed] [Google Scholar]
- 9.Brown M, Glick HA, Harrell F, et al. Integrating economic analysis into cancer clinical trials: the National Cancer Institute–American Society of Clinical Oncology Economics Workbook. J Natl Cancer Institute Monogr 1998; 24: 1–28. [PubMed] [Google Scholar]
- 10.Frey CF, Akimura K. Local resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy in the management of patients with chronic pancreatitis. Ann Surg 1994; 220: 492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez RE, Fernandez-del Castillo C, Rattner DW, et al. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg 2000; 231: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Power E, Eisneberg J. Are we ready to use cost effectiveness analysis in health care decision making? A health services research challenge for clinicians, patients, health care systems, and public policy. Med Care 1998; 36: MS10. [DOI] [PubMed] [Google Scholar]
- 13.Rice DP, Hodgson TA, Kopstein AN. The economic cost of illness: a replication and update. Health Care Finance Rev. 1985; 7: 61–80. [PMC free article] [PubMed] [Google Scholar]
- 14.Smits ME, Badiga SM, Rauws EAJ, et al. Long-term results of pancreatic stents in chronic pancreatitis. Gastrointest Endosc 1995; 42: 461–467. [DOI] [PubMed] [Google Scholar]
- 15.Ashby K, Lo SK. The role of pancreatic stenting in obstructive ductal disorders other than pancreas divisum. Gastrointest Endosc 1994; 40: 592–598. [DOI] [PubMed] [Google Scholar]
- 16.Grant JP, James S, Grabowski V, et al. Total parenteral nutrition in pancreatic disease. Ann Surg 1984; 200: 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gress F, Schmitt C, Sherman S, et al. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol 1999; 94: 900–905. [DOI] [PubMed] [Google Scholar]
- 18.Malfertheriner P, Mayer D, Büchler M, et al. Treatment of pain in chronic pancreatitis by inhibition of pancreatic secretion with octreotide. Gut 1995; 36: 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holbrook RF, Hargrave K, Traverso LW. A prospective cost analysis of pancreaticoduodenectomy. Am J Surg 1996; 171: 508–511. [DOI] [PubMed] [Google Scholar]
- 20.Bloechle C, Izbicki JR, Knoefel WQT, et al. Quality of life in chronic pancreatitis: results after duodenum-preserving resection of the head of the pancreas. Pancreas 1995; 11: 77–85. [DOI] [PubMed] [Google Scholar]
- 21.Traverso LW, Kozarek RA. The Whipple procedure for severe complication of chronic pancreatitis. Arch Surg 1993; 128: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 22.Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med 1995; 332: 1482–1489. [DOI] [PubMed] [Google Scholar]
- 23.Lankisch PG, Andren-Sandberg A. Standards for the diagnosis of chronic pancreatitis and for the evaluation of treatment. Int J Pancreatol 1993; 14: 205–212. [DOI] [PubMed] [Google Scholar]