Abstract
Objective
To compare portal and systemic venous drainage of pancreas transplants and demonstrate an immunologic and survival superiority of portal venous drainage.
Summary Background Data
Traditionally, solitary pancreas transplants have been performed using systemic venous and bladder drainage, but more recently, the advantages of enteric drainage have been well documented. Although physiologic benefits for portal venous drainage have been described, the impact of portal venous drainage, especially with solitary pancreas transplants, has yet to be determined.
Methods
Since August 1995, 280 pancreas transplants with enteric duct drainage were analyzed. One hundred and seventeen were simultaneous pancreas and kidney (SPK), 63 with systemic venous drainage (SV) and 54 with portal venous drainage (PV). The remainder were solitary transplants; 97 pancreas after kidney (PAK; 42 SV and 55 PV) and 66 transplants alone (PTA; 26 SV and 40 PV). Immunosuppressive therapy was equivalent for both groups.
Results
The groups were similar with respect to recipient characteristics and HLA matching. Thirty-six month graft survival for all transplants was 79% for PV and 65% for SV (P = .008). By category, SPK graft survival was 74% for PV and 76% for SV, PAK graft survival was 70% for PV and 56% for SV, and PTA graft survival was 84% for PV and 50% for SV. The rate of at least one rejection episode was also significantly higher in the SV group. At 36 months, for all pancreas transplants, the rejection rate was 21% for PV and 52% for SV (P < .0001). For SPK, rejection rates were 9% for PV and 45% for SV. For PAK, rejection rates were 16% for PV and 65% for SV, and for PTA 36% for PV and 51% for SV. The rejection rates for kidneys following SPK were also lower in the PV group (26% versus 43% for SV). Furthermore, the grades of rejection were milder in PV for all transplants (P = .017). By multivariate analysis, portal venous drainage was the only parameter that significantly affected rejection.
Conclusion
Graft survival and rejection is superior for PV. These clinical findings are consistent with published reports of experimentally induced portal tolerance and strongly argue that PV drainage should be the procedure of choice for pancreas transplantation.
During the past few years, we have witnessed a significant improvement in pancreas graft survival rates, resulting in a dramatic increase in the number of pancreas transplants performed. In 1998, more than 1,200 pancreas transplants were performed in the United States, a 15% increase from the previous year. 1 The technique of draining the pancreatic duct into the donor jejunum that was originally described 2 has historically been associated with a high incidence of intraabdominal infections. As a result, the modified technique of bladder drainage 3 gained wide acceptance as the method of choice for pancreatic duct drainage. Recently, enteric duct drainage has been readopted by many pancreas transplant centers primarily to avoid the well-known metabolic and urologic complications of bladder drainage. According to the International Pancreas Transplant Registry, 1 the proportion of enteric-drained procedures has continuously increased, concomitant with the number of centers using this technique. In 1995, 29% of all transplant centers performed at least one enteric-drained pancreas transplant, but by 1998, 98% of the transplant centers in the United States had at some point used enteric drainage. The graft survival and technical failure rates are comparable to those of bladder-drained pancreatic grafts. With recognition that more than 20% of bladder-drained grafts have to be converted to enteric drainage by 2 years, enteric drainage with systemic venous drainage has emerged as the procedure of choice.
Systemic venous drainage of pancreas transplants has been associated with hyperinsulinemia, 4–6 which itself has been associated with dyslipidemia. 7 This raised the concern that pancreas transplantation with systemic venous drainage might promote accelerated atherosclerosis independent from the dyslipidemic effects of immunosuppressive medication. 4,8 To circumvent this problem, in 1992 Rosenlof et al 9 described a more physiologic technique of draining the transplanted pancreas into the recipient’s portal circulation via the splenic vein. The technique was later modified by Gaber et al. to drain directly into the superior mesenteric vein. 10 This technique has resulted in significantly reduced plasma insulin and C-peptide levels 11 as well as improvements in lipoprotein composition. 12 However, the immunologic benefits, although alluded to in a small number of patients, 13 were not confirmed by appropriate comparisons of systemic- and portal-drained procedures in a larger series. Numerous experimental models have shown an immunomodulatory role of the liver after exposure of donor antigen into the portal vein. It is possible, therefore, that drainage of the transplanted pancreas into the portal vein mimics these experimental models and elicits a degree of immunomodulation. The purpose of this study was to assess whether an immunologic or survival advantage exists in portal venous versus systemic venous drainage in both solitary pancreas transplants and in combined kidney and pancreas transplants.
METHODS
Patient Population
An analysis was done on 280 patients with insulin-dependent diabetes mellitus type 1 who received pancreas transplants at the University of Maryland between August 1995 and June 2000. The indications for pancreatic transplantation included severe glycemic lability, evidence of progressive secondary diabetic complications, or end-stage renal disease. Three types of transplants were performed: simultaneous pancreas and kidney (SPK), pancreas after kidney (PAK), and pancreas transplant alone (PTA). One hundred seventeen SPK transplants were performed, 63 with systemic venous drainage and 54 with portal venous drainage. Ninety-seven PAK transplants (42 systemic venous and 55 portal venous) and 66 PTAs (26 systemic venous and 40 portal venous) were also performed. Recently, we have developed the technique of combining a cadaveric pancreas transplant with a living donor kidney in one operation with excellent results. 14 Forty-six of these procedures were performed, of which only two were drained systemically. These patients were not included in the analysis.
Immunosuppression
Postoperative antilymphocyte antibody (ATGAM to 38%, OKT3 to 54%) was given as immune induction for nearly all patients receiving pancreas transplants (8% received no induction as part of research studies). ATGAM doses were adjusted daily to achieve fewer than 50 CD3+ lymphocytes/mm3. OKT3 was administered at 5 mg/day with an increase to a maximum of 10 mg if the CD3+ cells exceeded 5% of the total lymphocyte count. Antilymphocyte antibody was discontinued between 10 and 14 days when satisfactory FK-506 levels were obtained. After April 1994, with the general availability of tacrolimus, we began a prospective trial of PTAs using tacrolimus-based immunosuppression. Based on these encouraging results, tacrolimus-based therapy became our standard in 1995 except in those few circumstances in which cyclosporine (Neoral) was used for tacrolimus intolerance (<5%). In August 1995, mycophenolate mofetil (MMF) replaced azathioprine. The dose of MMF was 1 to 1.5 g twice daily unless toxicity was a problem, in which case the dose was decreased. Postoperative prednisone was tapered from 2 mg/kg per day on the first postoperative day down to 0.3 mg/kg per day by day 15. Thereafter, prednisone was further tapered at the discretion of the clinic physician. Tacrolimus is dosed to achieve trough levels of 15 to 20 ng/mL during the first year and 12 to 15 ng/mL thereafter. Therefore, since August 1995, induction and maintenance immunosuppression has remained constant in our institution independent of surgical technique. To eliminate the effects of immunosuppression, our analysis began in August 1995 and ended in June 2000, a period during which all aspects of management except venous drainage were identical.
The mean follow-up for all pancreas transplants was 29.6 months (SPK, 29.6 months; PAK, 21.7 months; PTA, 23.9 months). The mean follow-up for systemic-drained pancreas transplants is 32.7 months and for portal-drained 19.6 months.
Surgical Procedure
The systemic-drained pancreas transplant is performed using previously published techniques. For systemic drainage, with combined kidney and pancreas transplants, the renal allograft is anastomosed to the left iliac vessels, and the pancreas to the right iliac vessels. The portal vein is anastomosed end to side to a completely mobilized right common and external iliac vein. A loop of jejunum is then used for a side-to-side anastomosis to the transplant duodenum without the use of a Roux-en-Y limb.
Portal venous drainage was first attempted in patients with previous pancreas transplants to decrease the technical difficulties encountered with previously dissected iliac veins. As portal venous drainage became more accepted and as the benefits became evident, it replaced systemic drainage as the procedure of choice, even in de novo patients. Currently, an attempt is made to drain all pancreas transplants to the portal circulation, but given anatomic limitations this cannot always be achieved. The anatomic limitations include significant recipient obesity, previous transplantation, and short donor iliac arterial Y grafts.
The technique of portal drainage has been previously described 10 but has been modified in our institution. The portal vein is anastomosed end to side to the superior mesenteric vein inferior to the transverse mesocolon. The donor iliac artery is passed through a small window made in the jejunal mesentery and anastomosed end to side to the right common iliac artery. Enteric drainage is then achieved via a side-to-side duodenojejunostomy without the use of a Roux-en-Y limb.
Statistical Analysis
Graft survival is calculated using Kaplan-Meier curves and univariate analysis was done using the log-rank test. Graft survival was calculated based on grafts lost to technical failures, rejection, infections, or other reasons. Patients who died with functioning grafts are also included as grafts lost. In contrast to graft survival, rejection rates were calculated on technically successful transplants only. The cumulative rate of at least one rejection episode after transplantation was calculated using Kaplan-Meier curves. Rejection rate calculations were based on both biopsy-proven rejections and clinically suspected rejections that failed attempted biopsies. Univariate analysis was done using the log-rank test. Multivariate analysis using Cox regression with stepwise forward progression was performed on previously studied risk factors for pancreas allograft failure and rejection that include both donor and recipient characteristics. In the 1998 International Pancreas Transplant Registry, 1 the variables identified included the type of transplant, donor age, HLA matching between the donor and recipient, duct management, and immunosuppression, including the use of tacrolimus and MMF. Given that all our transplants involved enteric drainage and that immunosuppression did not vary between the groups studied, multivariate analysis of the following risk factors was performed: date of transplant, type of transplant, induction agent, donor age, HLA antigen match between donor and recipient, and venous drainage.
RESULTS
The patient demographics and clinical characteristics were similar in each group. There was no difference in mean age (38.1 for systemic venous drainage, 40.1 for portal venous drainage, P = .480), gender (57% male for systemic drainage, 63% for portal drainage, P = .070), or race (82% white for systemic drainage, 86% for portal drainage, P = .898). The study groups were also similar with respect to donor and recipient HLA matching. The mean HLA antigen mismatch for all pancreas transplants was 4.25 ± 1.44 for the systemic drainage group and 4.12 ± 1.52 for the portal drainage group (P = .441).
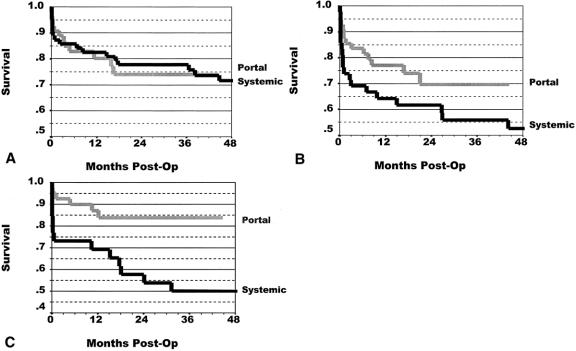
Kaplan-Meier 36-month patient survival rates were similar for both groups, 89% for portal drainage and 93% for systemic drainage (P = .24). Pancreas graft survival for all patients is shown in Figure 1. The overall graft survival rate for all categories of pancreas transplants was significantly greater (14%) in the portal drainage group (P = .008). The 36-month graft survival rate was 65% for the systemic drainage group and 79% for the portal drainage group. Figure 2 shows the graft survival rates for SPK, PAK, and PTA. For SPK, graft survival rates were equivalent for portal and systemic drainage. For PAK and PTA, however, graft survival rates were higher in the portal drainage groups, a difference more pronounced with PTA (P = .011).
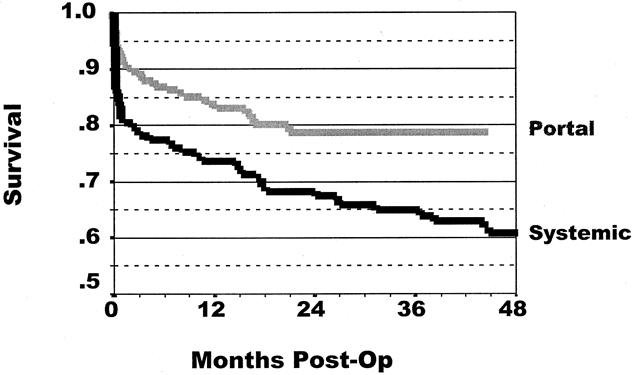
Figure 1. Overall graft survival rates after pancreas transplantation. The overall graft survival for all categories of pancreas transplants was significantly greater in the portal drainage group (P = .0008). The 36-month graft survival rate was 65% for systemic drainage and 79% for portal drainage.
Figure 2. Graft survival rates after (A) simultaneous pancreas and kidney transplant (SPK), (B) pancreas after kidney transplant (PAK), and (C) pancreas transplant alone (PTA). The 36-month graft survival rates were for SPK, 74% for portal drainage and 76% for systemic drainage (P = .880); for PAK, 70% for portal drainage and 56% for systemic drainage (P = .110); and for PTA, 84% for portal drainage and 50% for systemic drainage (P = .011).
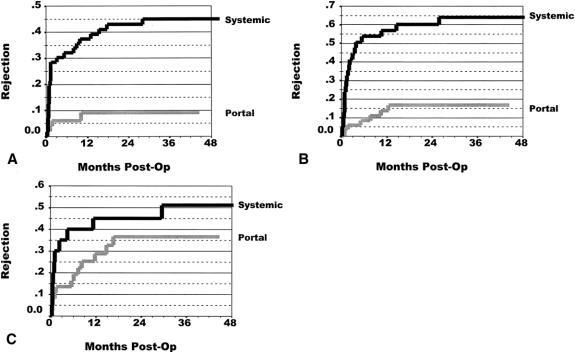
The rate of at least one rejection episode was calculated using Kaplan-Meier curves. Figure 3 depicts this rate of rejection in both groups. For all pancreas transplants, the portal drainage group had a significantly lower rejection rate than the systemic drainage group. At 36 months, the rejection rate was 52% for the systemic drainage group and 21% for the portal drainage group (P < .0001). Figure 4 displays a similar picture for each transplant category. For SPK, the rejection rate was 9% for the portal drainage group versus a 45% 36-month rejection rate in the systemic drainage group (P = .0002). For PAK, the 36-month pancreas rejection rate for the portal drainage group was 16% compared with 65% for the systemic drainage group (P = .0001). For PTA, pancreas rejection at 36 months was 36% for the portal drainage group, whereas systemic-drained transplants had a 51% rejection rate. Because of the smaller patient numbers, however, this did not reach statistical significance (P = .252).
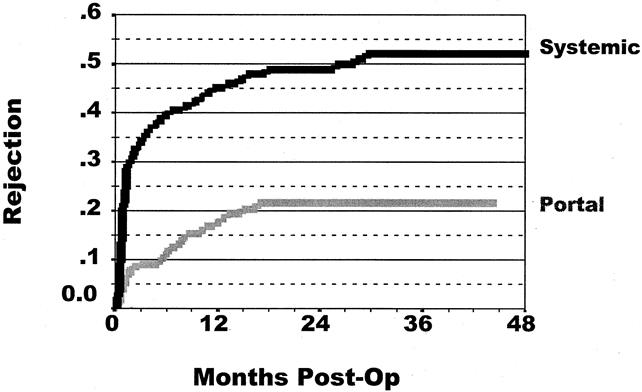
Figure 3. Cumulative rate of at least one rejection episode after pancreas transplantation. The overall rejection rate for all categories of pancreas transplants was significantly lower in the portal drainage group (P < .0001). The 36-month rejection rate was 21% for portal drainage and 52% for systemic drainage.
Figure 4. Rate of at least one rejection episode after (A) simultaneous pancreas and kidney transplant (SPK), (B) pancreas after kidney transplant (PAK), and (C) pancreas transplant alone (PTA). The 36-month rejection rates were for SPK, 9% for portal drainage and 45% for systemic drainage (P = .0002); for PAK, 16% for portal drainage and 65% for systemic drainage (P = .0001); and for PTA, 36% for portal drainage and 51% for systemic drainage (P = .252).
A similar difference in rejection rates was noted when kidneys were analyzed after SPK transplants. A 17% difference in rejection rates was seen: 43% for systemic drainage versus 26% for portal drainage (P = .002;Fig. 5).
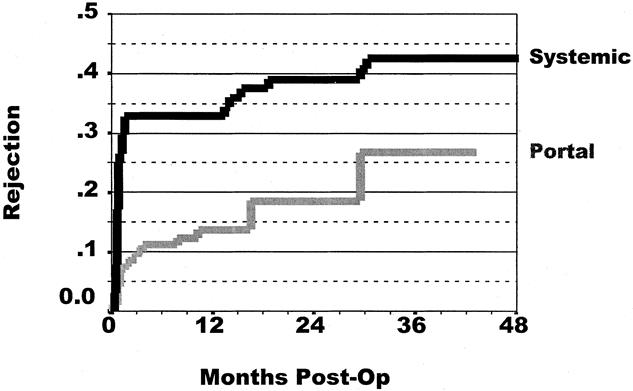
Figure 5. Cumulative rate of at least one rejection episode of kidneys after simultaneous pancreas and kidney transplantation. The rejection rate was 26% for portal drainage and 43% for systemic drainage (P = .002).
In an attempt to rule out an “era effect,” analysis was undertaken of all the pancreas transplants performed during a single year when portal venous drainage was first begun. Between September 1996 and September 1997, 69 pancreas transplants were performed, 26 with portal venous drainage and 43 with systemic venous drainage. The 3-year graft survival rate was superior for portal drainage, 67% versus 54% (P = .1120). Moreover, the incidence of at least one rejection episode was significantly lower in the portal drainage group, 14% versus 56% (P = .0006). Hence, the differences hold true even in this small cohort of patients, including many portal drainage patients who were receiving secondary or tertiary transplants.
Because portal venous drainage resulted in fewer rejection episodes, it would also be relevant to study the severity of rejection of patients who had rejection in each group. For all portal drainage transplants, the vast majority of rejections were borderline or mild (grade 1 or 2 based on the Maryland classification 15). In comparison, the grades of rejection for the systemic drainage transplants were more evenly distributed, with a larger percentage displaying moderate and severe (grades 3–5) rejection. The mean grade of rejection based on the Maryland classification was 1.83 for portal drainage versus 2.33 for systemic drainage (P = .017).
In the tacrolimus-based immunosuppression era, factors thought to influence the rejection rate after pancreas transplantation were analyzed by multivariate analysis. These included the year of transplant (era effect), induction therapy, the type of transplant, HLA matching (either 0 or 1 antigen mismatch, or a 5 or 6 antigen mismatch), and portal drainage. The results are shown in Table 1. The only parameter that affected rejection to a significant degree was the route of venous drainage (P < .0001). The relative risk of rejection for portal drainage was 0.25.
Table 1. MULTIVARIATE ANALYSIS OF VARIABLES THOUGHT TO AFFECT REJECTION
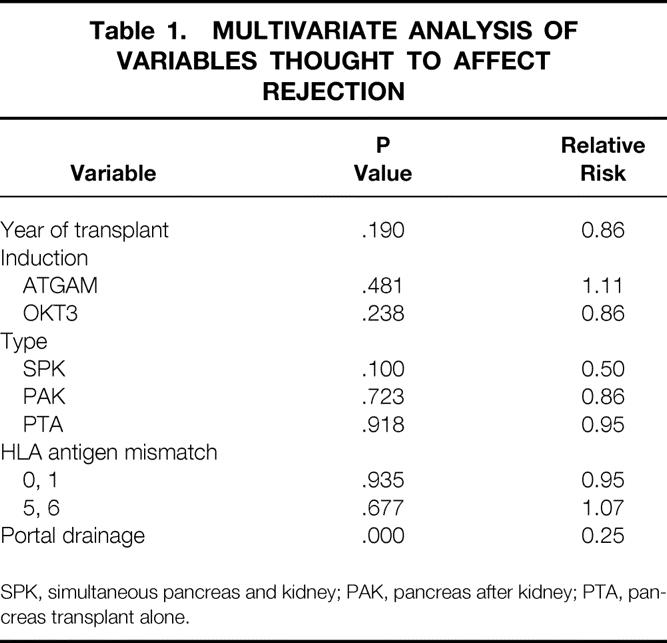
SPK, simultaneous pancreas and kidney; PAK, pancreas after kidney; PTA, pancreas transplant alone.
DISCUSSION
It has long been hypothesized that portal venous drainage of pancreas allografts should offer physiologic benefits. The prevention of hyperinsulinemia and improvements in the lipoprotein profiles in patients receiving portal-drained pancreas allografts have been well documented. 11,12 In this study we sought to determine whether portal venous drainage also conveys an immunologic advantage in addition to the physiologic benefits previously noted.
We have shown by both univariate and multivariate analysis that pancreas allografts drained into the portal vein, irrespective of whether a kidney was cotransplanted, had a significantly lower incidence of acute rejection than allografts that were drained systemically into the iliac vein. Several factors might influence these results. First, few would disagree that immunosuppression has improved significantly in the past decade. With the introduction of MMF and tacrolimus, allograft survival and rejection rates for both renal and pancreas transplants have significantly improved. In our institution, multivariate analysis of variables affecting graft survival and rejection after pancreas transplantation since 1991 revealed tacrolimus and MMF to be the only variable with a significant positive impact. HLA antigen matching has had no effect on either survival or rejection (manuscript in preparation). With these factors in mind, it was important to assess the immunologic impact of portal drainage during a period when the immunosuppressive protocol had not varied. Since August 1995, we have instituted the routine use of tacrolimus, MMF, and prednisone as maintenance immunosuppression after antilymphocyte induction therapy. Few patients required changing to cyclosporine (Neoral) as baseline immunosuppression as a result of intolerable toxicity to tacrolimus. Therefore, immunosuppression in itself could not account for the improved outcome with portal drainage of pancreas transplants.
A second factor that could affect rejection is the possibility of unrecognized rejection episodes because of the lower rate of successful percutaneous pancreas biopsies after portal drainage. Our center has adopted an ultrasound-guided percutaneous pancreas biopsy under local anesthesia that has historically yielded tissue in greater than 88% of the patients after systemic drainage. 16 However, these pancreas transplants were placed in the pelvis, where access for percutaneous biopsy is more successful than portal-drained pancreas transplants. Although this may influence outcome, missed rejection episodes should manifest as late graft loss, a finding we have not observed in patients with portal drainage. Further, biopsies were not essential in establishing a rejection episode. Therefore, suspected rejection events based on biochemical analysis that were not successfully biopsied were treated and therefore counted as rejection episodes in our analysis. Elevated serum amylase and lipase levels correlate with histologic rejection in greater than 85% of the patients based on previous reports from our institution. 17 Therefore, it can be estimated that 15% of the suspected rejections treated and therefore counted in our analysis were not truly rejections, thereby artificially increasing the rejection rate in the portal drainage group. Based on the above calculations, however, this difference would be small and probably insignificant.
Because the results of pancreas transplantation have improved nationwide during the past few years regardless of the drainage technique, it is theoretically possible that the survival and perhaps the rejection benefits observed were due to better techniques and better patient management, or perhaps more experience. Although confounding variables were addressed in the multivariate analysis, it was especially important to rule out an era effect. It was shown by multivariate analysis that the year of the transplant was not an independent prognostic factor and did not influence outcome. In a separate analysis of all the pancreas transplants performed during a single year when portal venous drainage was first begun, the differences in graft survival rates and especially rejection rates held true even in a small cohort of patients including many portal drainage patients who were receiving secondary or tertiary transplants. Therefore, the differences observed between the drainage techniques cannot be attributed to improvement in techniques or acquired patient management skills.
The impetus for this study was based on experimental models of portal tolerance that have been established for years. It has long been known that introduction of donor antigen into the portal vein can enhance allograft survival. In certain rodent strain models, cardiac 18–20 and renal 21 allografts have been prolonged by preimmunization with donor antigen specifically injected in the portal vein. Drainage of allografts themselves directly into the portal vein has also resulted in prolongation of heart, kidney, and small bowel allograft survival. 20–24 In a certain rat strain combination, Kamei et al 24 showed that indefinite survival of heart allografts can been achieved with a combination of portal preimmunization and portal drainage of the allograft. In that study, rats were preimmunized with UVB-treated donor splenocytes through the portal vein, systemic vein, or none at all, followed by transplantation of a heart allograft drained systemically or via the portal vein. Prolongation of graft survival was observed either by preimmunization or by draining the allograft through the portal vein. The most dramatic results were seen, however, when both preimmunization and allograft drainage were done via the portal vein: 80% of the grafts survived more than 120 days. This study clearly delineated the importance of constant stimulation of the hepatic environment to maintain permanent tolerance.
The liver is rich in migratory passenger leukocytes, including dendritic cells, widely regarded as the primary antigenic component of transplanted organs. The important interaction of the apoptotic cells (experimentally induced by UVB irradiation) presented in the portal vein with the resident hepatic dendritic cells could therefore prove extremely important. Albert et al 25 elegantly showed that dendritic cells acquire antigen from apoptotic (but not necrotic) cells and induce class 1 restricted CTL, also known as cross-presentation. Cross-presentation has also been shown to induce tolerance when nonprofessional antigen-presenting cells are recruited. 26 Liver dendritic cells are phenotypically and functionally immature and distinct from bone marrow-derived dendritic cells. 27 It is hypothesized that these dendritic cells lack coactivation molecules, therefore preferentially inducing anergy or tolerance through various mechanisms. Other mechanisms have also been proposed to support the hypothesis of the liver having an immunomodulatory role. Gorczynsky et al 28 have shown that after portal vein pretransplant immunization with splenocytes, hyporesponsiveness via a Th2 cytokine response is seen, and that activated γδ T cells, which are enriched in certain organs including the liver, appear essential in providing signals for αβ T cells to preferentially express a Th2 response. 29
The protective effect of the liver in combined kidney and liver transplantation has also been well established clinically. 30,31 Although this can be related to the mechanisms proposed above or to the presence of passenger leukocytes, the causal relationship has not been established. In our analysis of SPK transplants, kidney graft survival and rejection rates were improved with portal drainage of the pancreas transplant. The introduction of peripancreatic nodal tissue in the portal vein could theoretically induce an immunomodulatory response in the host liver, which can have a protective effect on both organs from the same donor. This is an interesting albeit unproven concept that is the basis of future investigation.
Chronic rejection is a poorly understood entity that probably involves multiple complex immunologic mechanisms. The correlation between the number or severity of acute rejections and late graft loss has been well documented in other organ transplants. Acute rejection has also predicted graft loss from chronic rejection in kidney transplants. 32 The potential advantages in terms of chronic rejection offered by portal venous drainage could conclusively be established only by long-term data and the collective experience of other centers’ data submitted to the registry. However, if the correlation between acute and chronic rejection exists in pancreas transplantation as it does in kidney transplantation, the immunomodulation offered by portal venous drainage would also protect the allografts from chronic rejection.
Footnotes
Correspondence: Benjamin Philosophe, MD, PhD, 29 S. Greene St., Suite 200, Baltimore, MD 21201.
E-mail: bphilosophe@smail.umaryland.edu
Accepted for publication February 5, 2001.
References
- 1.1998 Annual Report of the U.S. Scientific Registry of Transplant Recipients and the Organ Procurement and Transplantation Network. Transplant data, 1988–1998. UNOS, Richmond, VA and the Division of Transplantation, Bureau of Health Resources and Services Administration, U.S. Department of Health and Human Services, Rockville, MD; 1996.
- 2.Kelly WD, Lillehei RC, Merkel FK, et al. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery 1967; 61: 827. [PubMed] [Google Scholar]
- 3.Ngheim DD, Corry RJ. Transplantation with urinary drainage of pancreatic secretions. Am J Surg 1987; 153: 405. [DOI] [PubMed] [Google Scholar]
- 4.Diem P, Abid M, Redmon JB, et al. Systemic venous drainage of pancreas allografts as independent causes of hyperinsulinemia in type I diabetic recipients. Diabetes 1990; 39: 534. [DOI] [PubMed] [Google Scholar]
- 5.Luck R, Klempnauer J, Ehlerding G, et al. Significance of portal venous drainage after whole-organ pancreas transplantation for endocrine graft function and prevention of diabetic nephropathy. Transplantation 1990; 50: 394–398. [DOI] [PubMed] [Google Scholar]
- 6.Blackman JD, Polonsky KS, Jaspan JB, et al. Insulin secretory profiles and C-peptide clearance kinetics at 6 months and 2 years after kidney-pancreas transplantation. Diabetes 1992; 41: 1346–1354. [DOI] [PubMed] [Google Scholar]
- 7.Bagdade JD, Ritter MC, Kitabchi AE, et al. Differing effects of pancreas-kidney transplantation with systemic versus portal venous drainage on cholesteryl ester transfer in IDDM subjects. Diabetes Care 1996; 19: 1108–1112. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Ferranninni E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991; 14: 173. [DOI] [PubMed] [Google Scholar]
- 9.Rosenlof LK, Earnhardt RC, Pruett TL, et al. Pancreas transplantation. An initial experience with systemic and portal drainage of pancreatic allografts. Ann Surg 1992; 215: 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaber AO, Shokouh-Amiri H, Grewal HP, et al. A technique for portal pancreatic transplantation with enteric drainage. Surg Gynecol Obstet 1993; 177: 417. [PubMed] [Google Scholar]
- 11.Gaber AO, Shokouh-Amiri H, Hathaway DK, et al. Results of pancreas transplantation with portal venous and enteric drainage. Ann Surg 1995; 221: 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes TA, Gaber AO, Amiri HS, et al. Kidney-pancreas transplantation. The effect of portal versus systemic venous drainage of the pancreas on the lipoprotein composition. Transplantation 1995; 60: 1406. [PubMed] [Google Scholar]
- 13.Nymann T, Hathaway DK, Shokouh-Amiri MH, et al. Incidence of kidney and pancreas rejection following poral-enteric versus systemic-bladder pancreas-kidney transplantation. Transplant Proc 1997; 29: 640. [DOI] [PubMed] [Google Scholar]
- 14.Farney AC, Cho E, Schweitzer EJ, et al. Simultaneous cadaver pancreas living donor kidney transplantation (SPLK): a new approach for the type I diabetic uremic patient. Ann Surg (in press). [DOI] [PMC free article] [PubMed]
- 15.Drachenberg CB, Papadimetriou JC, Klassen DK, et al. Evaluation of pancreas transplant needle biopsy. Transplantation 1997; 63: 1579–1586. [DOI] [PubMed] [Google Scholar]
- 16.Klassen DK, Hoehn-Saric EW, Weir MR, et al. Isolated pancreas rejection in combined kidney–pancreas transplantation. Transplantation 1996; 61: 974–977. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett ST, Schweitzer EJ, Johnson LB, et al. Equivalent success of simultaneous pancreas kidney and solitary pancreas transplantation. Ann Surg 1996; 224: 440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao VK, Burris DE, Gruel SM, et al. Evidence that donor spleen cells administered through the portal vein prolong the survival of cardiac allografts in rats. Tranplantation 1988; 45: 1145. [DOI] [PubMed] [Google Scholar]
- 19.Lowry RP, Kenick S, Lisbona R. Speculation on the pathogenesis of prolonged cardiac allograft survival following portal venous inoculation of allogeneic cells. Tranplant Proc 1987; 19: 3451. [PubMed] [Google Scholar]
- 20.Holman JM, Todd R. Enhanced survival of heterotopic rat heart allografts with portal venous drainage. Transplantation 1990; 48: 229. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura N, Matsui S, Hamashima T, et al. The effects of perioperative portal venous inoculation with donor lymphocytes on renal allograft survival in the rat. I. Specific prolongation of donor grafts and suppressor factor in the serum. Transplantation 1990; 49: 167. [DOI] [PubMed] [Google Scholar]
- 22.Sakai A. Role of the liver in kidney allograft rejection in the rat. Transplantation 1970; 9: 333. [Google Scholar]
- 23.Gorczynski RM, Chan Z, Chung S, et al. Prolongation of rat small bowel or renal allograft survival by pretransplant transfusion and/or by varying the route of allograft venous drainage. Transplantation 1994; 58: 816. [PubMed] [Google Scholar]
- 24.Kamei T, Callery MP, Flye MW. Pretransplant portal venous administration of donor antigen and portal venous allograft drainage synergistically prolong rat cardiac allograft survival. Surgery 1990; 108: 415–422. [PubMed] [Google Scholar]
- 25.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 1998; 392: 86–89. [DOI] [PubMed] [Google Scholar]
- 26.Carbone FR, Kurts C, Bennett SR, et al. Cross-presentation. A general mechanism for CTL immunity and tolerance. Immunol Today 1998; 19: 368–373. [DOI] [PubMed] [Google Scholar]
- 27.Thomson AW, Lu L. Are dendritic cells the key to liver transplant tolerance? Immunol Today 1999; 20: 27–32. [DOI] [PubMed] [Google Scholar]
- 28.Gorczynski RM, Wojcik D. A role of nonspecific (cyclosporin A) or specific (monoclonal antibodies to ICAM-1, LFA-1, and IL-10) immunomodulation in the prolongation of skin allografts after antigen-specific pretransplant immunization or transfusion. J Immunol 1994; 152: 2011–2019. [PubMed] [Google Scholar]
- 29.Gorczynski RM, Cohen Z, Levy G, et al. A role for γδ TCR+ cells in regulation of rejection of small intestinal allografts in rats. Transplantation 1996; 62: 1–8. [DOI] [PubMed] [Google Scholar]
- 30.Gonwa TA, Nery JR, Husberg BS, et al. Simultaneous liver and renal transplantation in man. Transplantation 1988; 46: 690–693. [DOI] [PubMed] [Google Scholar]
- 31.Flye MW, Duffy BF, Phelan DL, et al. Protective effects of liver transplantation on a simultaneously transplanted kidney in a highly sensitised patient. Transplantation 1990; 50: 1051–1054. [PubMed] [Google Scholar]
- 32.Matas A. Chronic rejection in renal transplant recipients–risk factors and correlates. Clin Transplant 1994; 8: 332–335. [PubMed] [Google Scholar]