Abstract
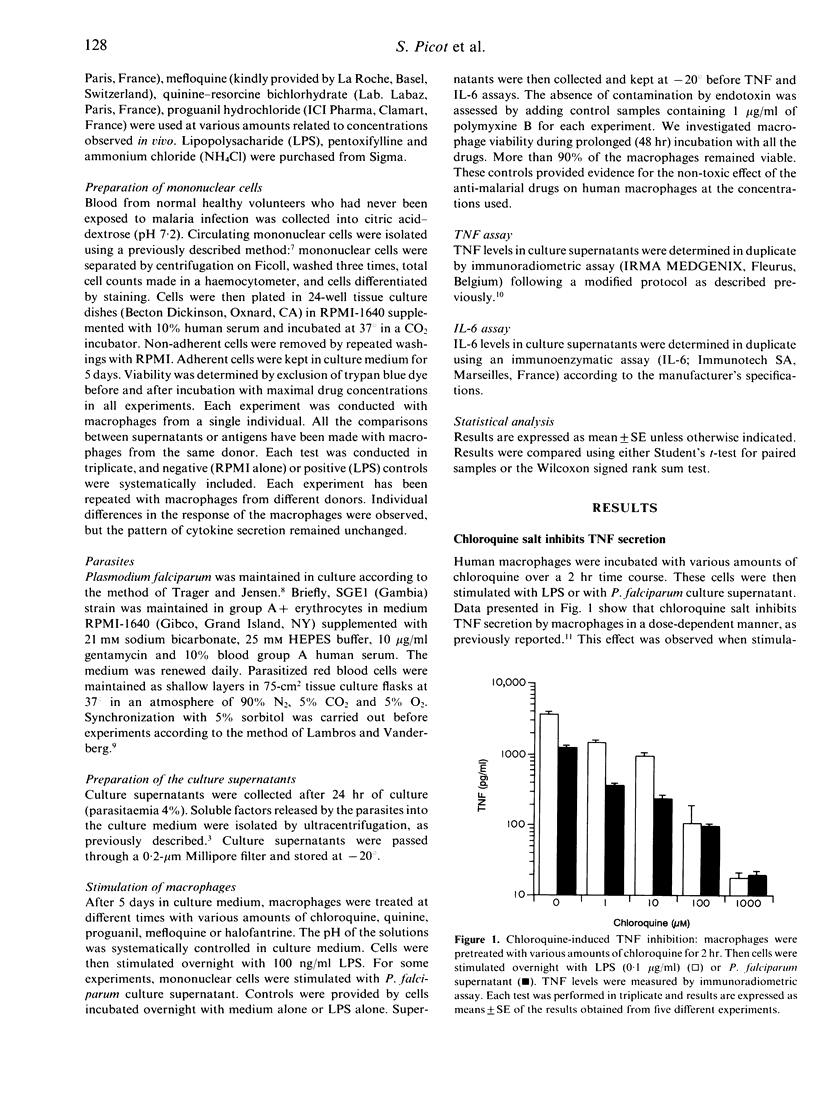
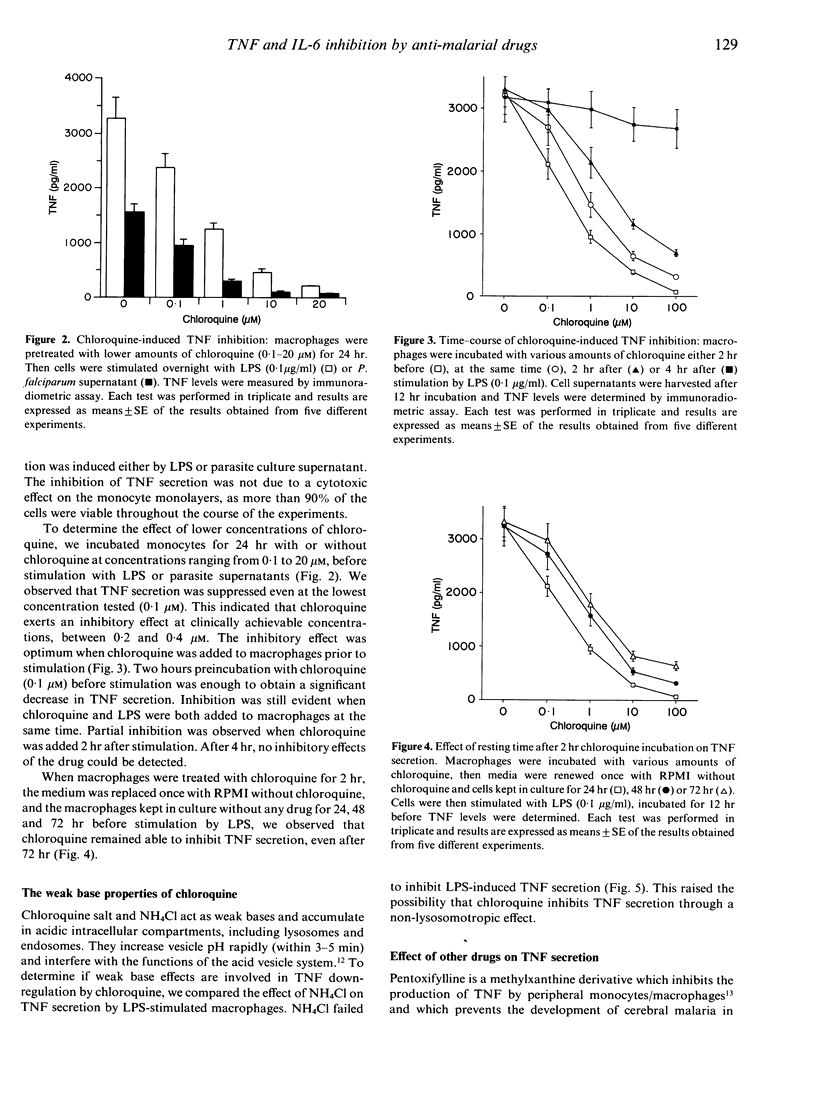
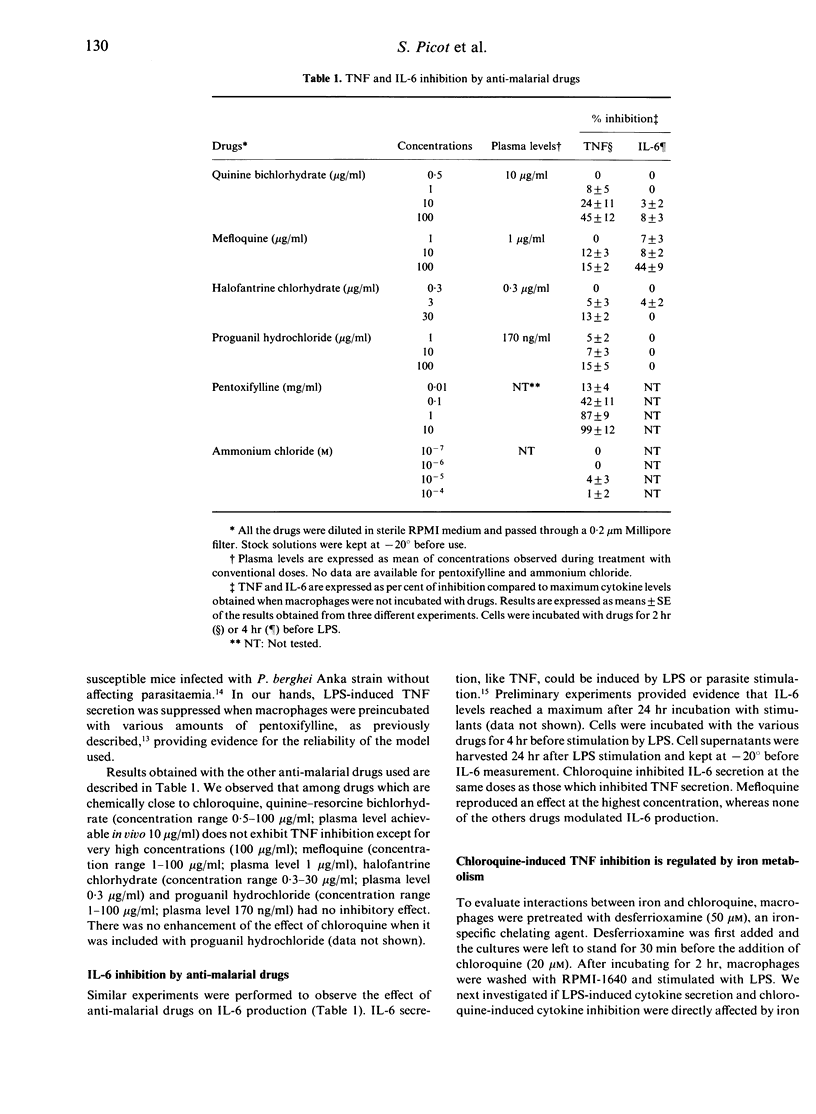
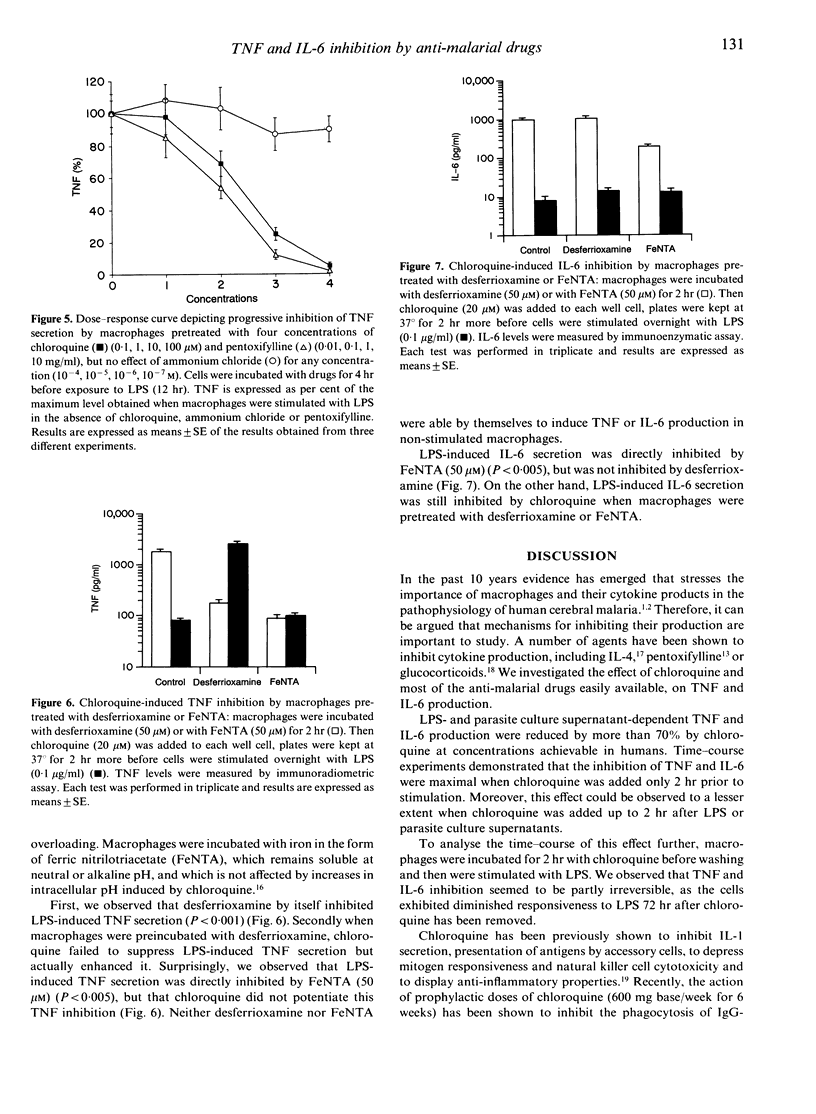
There is now considerable evidence that cerebral malaria may be related to the over-production of tumour necrosis factor (TNF). Nevertheless, our knowledge is very poor concerning the biological events which lead up to this TNF over-production. Furthermore, interleukin-6 (IL-6) is produced in large amounts during malaria infection and seems to have inhibitory action on TNF production. Anti-malarial drugs were investigated for their ability to interfere with TNF and IL-6 secretion by human non-immune macrophages stimulated by lipopolysaccharides (LPS) or Plasmodium falciparum culture supernatant. Macrophages were pretreated with chloroquine, quinine, proguanil, mefloquine or halofantrine before stimulation. TNF and IL-6 production were suppressed in a dose-dependent manner when macrophages were treated with chloroquine, but not with other anti-malarial drugs. Considering that chloroquine probably acts via lysosomotropic mechanisms, and that iron metabolism may interfere with the non-specific immune response, we focused our attention on these biochemical events in order to investigate the mechanisms by which chloroquine inhibits cytokine production. Our results demonstrated that chloroquine-induced inhibition of TNF and IL-6 production is not mediated through a lysosomotropic mechanism, and that chloroquine probably acts on TNF secretion by disrupting iron homeostasis. Inhibition of IL-6 production seems not to be mediated through these pathways. These observations suggest that chloroquine may help to prevent cerebral malaria whatever the drug sensitivity of the parasite strain, and may provide new tools for an anti-disease therapy regardless of the emergence of parasite multi-drug resistance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Bates G. W., Wernicke J. The kinetics and mechanism of iron(3) exchange between chelates and transferrin. IV. The reaction of transferrin with iron(3) nitrilotriacetate. J Biol Chem. 1971 Jun 10;246(11):3679–3685. [PubMed] [Google Scholar]
- Bryan C. F., Leech S. H. The immunoregulatory nature of iron. I. Lymphocyte proliferation. Cell Immunol. 1983 Jan;75(1):71–79. doi: 10.1016/0008-8749(83)90306-4. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cohen M. S., Mao J., Rasmussen G. T., Serody J. S., Britigan B. E. Interaction of lactoferrin and lipopolysaccharide (LPS): effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation. J Infect Dis. 1992 Dec;166(6):1375–1378. doi: 10.1093/infdis/166.6.1375. [DOI] [PubMed] [Google Scholar]
- Crouch S. P., Slater K. J., Fletcher J. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood. 1992 Jul 1;80(1):235–240. [PubMed] [Google Scholar]
- Grau G. E., Frei K., Piguet P. F., Fontana A., Heremans H., Billiau A., Vassalli P., Lambert P. H. Interleukin 6 production in experimental cerebral malaria: modulation by anticytokine antibodies and possible role in hypergammaglobulinemia. J Exp Med. 1990 Nov 1;172(5):1505–1508. doi: 10.1084/jem.172.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Thompson P., Beutler B. Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J Exp Med. 1990 Jul 1;172(1):391–394. doi: 10.1084/jem.172.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremsner P. G., Grundmann H., Neifer S., Sliwa K., Sahlmüller G., Hegenscheid B., Bienzle U. Pentoxifylline prevents murine cerebral malaria. J Infect Dis. 1991 Sep;164(3):605–608. doi: 10.1093/infdis/164.3.605. [DOI] [PubMed] [Google Scholar]
- Krogstad D. J., Schlesinger P. H. The basis of antimalarial action: non-weak base effects of chloroquine on acid vesicle pH. Am J Trop Med Hyg. 1987 Mar;36(2):213–220. doi: 10.4269/ajtmh.1987.36.213. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Hill A. V., Sambou I., Twumasi P., Castracane J., Manogue K. R., Cerami A., Brewster D. R., Greenwood B. M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990 Nov 17;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Liang Y. F., Peterson J. W., Jackson C. A., Reitmeyer J. C. Chloroquine inhibition of cholera toxin. FEBS Lett. 1990 Nov 26;275(1-2):143–145. doi: 10.1016/0014-5793(90)81459-2. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiya K., Horwitz D. A. Contrasting effects of lactoferrin on human lymphocyte and monocyte natural killer activity and antibody-dependent cell-mediated cytotoxicity. J Immunol. 1982 Dec;129(6):2519–2523. [PubMed] [Google Scholar]
- Osorio L. M., Fonte L., Finlay C. M. Inhibition of human monocyte function by prophylactic doses of chloroquine. Am J Trop Med Hyg. 1992 Feb;46(2):165–168. doi: 10.4269/ajtmh.1992.46.165. [DOI] [PubMed] [Google Scholar]
- Peyron F., Vuillez J. P., Barbe G., Boudin C., Picot S., Ambroise-Thomas P. Plasma levels of tumor necrosis factor during a longitudinal survey in an endemic area of malaria. Acta Trop. 1990 Jan;47(1):47–51. doi: 10.1016/0001-706x(90)90006-l. [DOI] [PubMed] [Google Scholar]
- Picot S., Peyron F., Vuillez J. P., Barbe G., Marsh K., Ambroise-Thomas P. Tumor necrosis factor production by human macrophages stimulated in vitro by Plasmodium falciparum. Infect Immun. 1990 Jan;58(1):214–216. doi: 10.1128/iai.58.1.214-216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picot S., Peyron F., Vuillez J. P., Polack B., Ambroise-Thomas P. Chloroquine inhibits tumor necrosis factor production by human macrophages in vitro. J Infect Dis. 1991 Oct;164(4):830–830. doi: 10.1093/infdis/164.4.830. [DOI] [PubMed] [Google Scholar]
- Salmeron G., Lipsky P. E. Immunosuppressive potential of antimalarials. Am J Med. 1983 Jul 18;75(1A):19–24. doi: 10.1016/0002-9343(83)91266-4. [DOI] [PubMed] [Google Scholar]
- Schindler R., Mancilla J., Endres S., Ghorbani R., Clark S. C., Dinarello C. A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990 Jan 1;75(1):40–47. [PubMed] [Google Scholar]
- Taverne J., Bate C. A., Kwiatkowski D., Jakobsen P. H., Playfair J. H. Two soluble antigens of Plasmodium falciparum induce tumor necrosis factor release from macrophages. Infect Immun. 1990 Sep;58(9):2923–2928. doi: 10.1128/iai.58.9.2923-2928.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz P. S., Yamazaki K., LaRusso N. F. Time-dependent effects of chloroquine on pH of hepatocyte lysosomes. Biochem Pharmacol. 1990 Sep 15;40(6):1419–1421. doi: 10.1016/0006-2952(90)90414-g. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Wellems T. E. Malaria. How chloroquine works. Nature. 1992 Jan 9;355(6356):108–109. doi: 10.1038/355108a0. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde A. A., Klomp J. P., Yard B. A., de Vries J. E., Figdor C. G. Modulation of phenotypic and functional properties of human peripheral blood monocytes by IL-4. J Immunol. 1988 Mar 1;140(5):1548–1554. [PubMed] [Google Scholar]


