Abstract
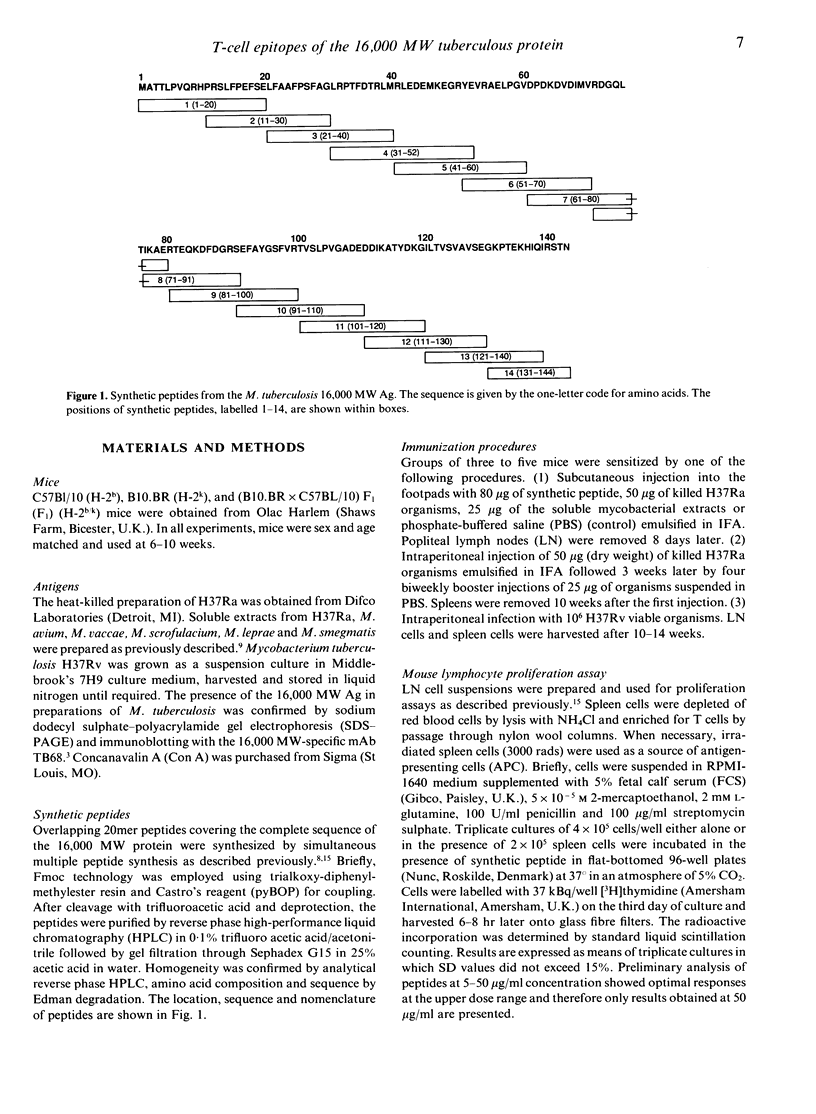
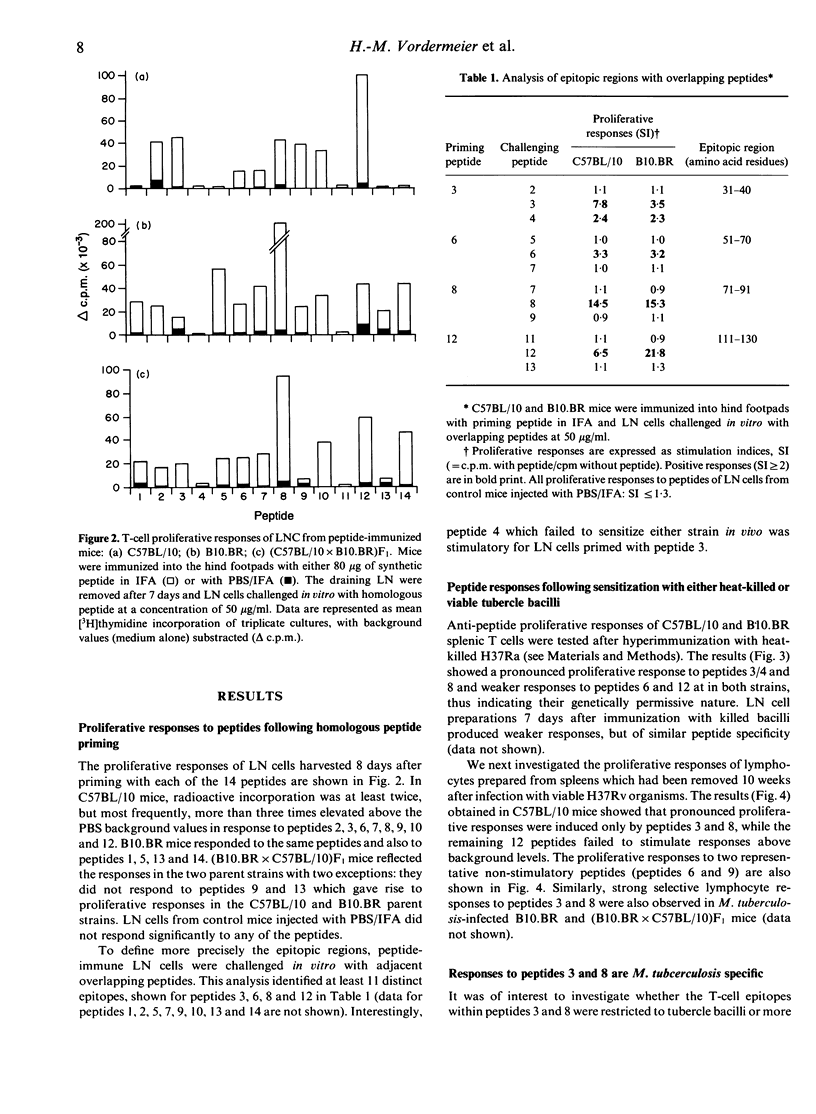
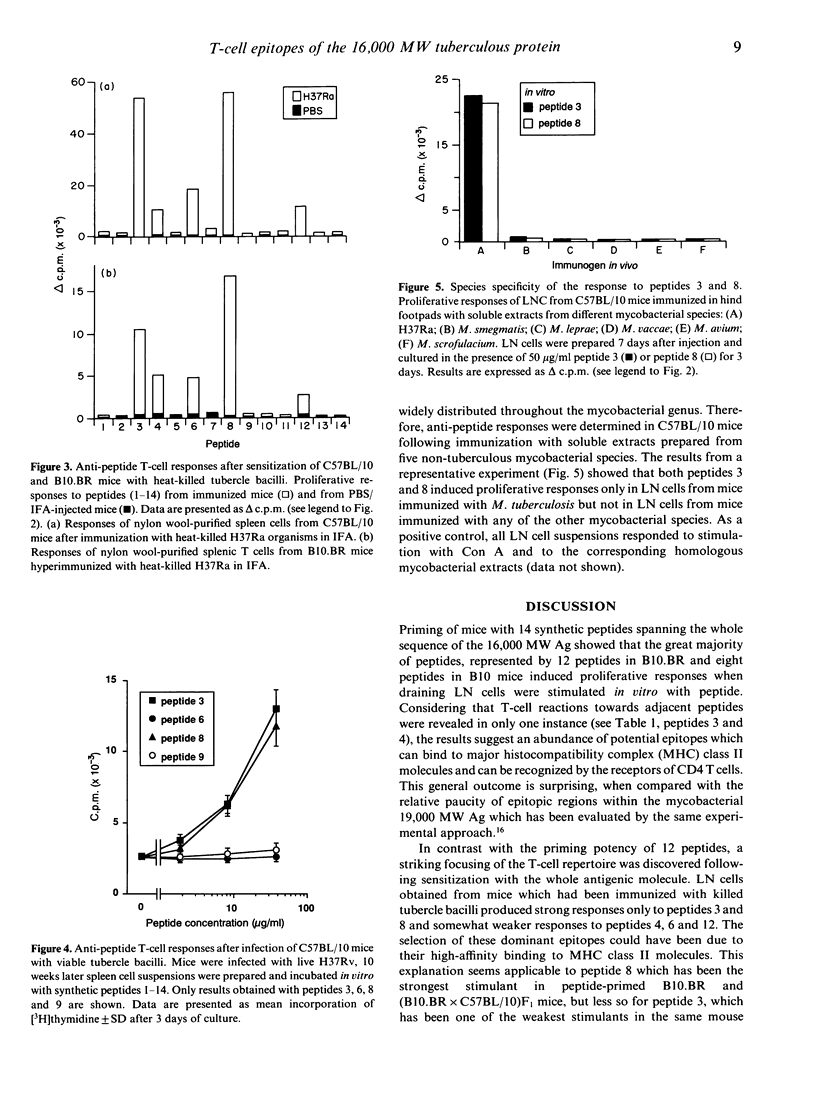
The T-cell repertoire to a prominent immunogen of Mycobacterium tuberculosis has been investigated on the assumption that differences in epitope specificity could influence the protective and pathogenic host reactions. Proliferative responses of lymph node and spleen cells to overlapping peptides, spanning the entire sequence of the 16,000 MW protein antigen were analysed in C57BL/10 and B10.BR mice. Following footpad priming and in vitro challenge with homologous peptide, 12 out of the 14 peptides tested were found to be immunogenic. However, only two peptides of residues 31-40 and 71-91 stimulated strong proliferative responses of T cells from mice which had been presensitized with either killed or live M. tuberculosis organisms; another three peptides were only weakly stimulatory. These epitopes have been immunodominant in both H-2b and H-2k mouse strains, indicating the genetically permissive nature of their recognition. Furthermore, both major immunodominant epitopes were found to be species specific for the M. tuberculosis complex and therefore potentially suitable for the early diagnosis of tuberculous infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Appella E., Doria G., Nagy Z. A. Mechanisms influencing the immunodominance of T cell determinants. J Exp Med. 1988 Dec 1;168(6):2091–2104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benichou G., Takizawa P. A., Olson C. A., McMillan M., Sercarz E. E. Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection. J Exp Med. 1992 Jan 1;175(1):305–308. doi: 10.1084/jem.175.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth R. J., Harris D. P., Love J. M., Watson J. D. Antigenic proteins of Mycobacterium leprae. Complete sequence of the gene for the 18-kDa protein. J Immunol. 1988 Jan 15;140(2):597–601. [PubMed] [Google Scholar]
- Bothamley G. H., Beck J. S., Potts R. C., Grange J. M., Kardjito T., Ivanyi J. Specificity of antibodies and tuberculin response after occupational exposure to tuberculosis. J Infect Dis. 1992 Jul;166(1):182–186. doi: 10.1093/infdis/166.1.182. [DOI] [PubMed] [Google Scholar]
- Bothamley G., Udani P., Rudd R., Festenstein F., Ivanyi J. Humoral response to defined epitopes of tubercle bacilli in adult pulmonary and child tuberculosis. Eur J Clin Microbiol Infect Dis. 1988 Oct;7(5):639–645. doi: 10.1007/BF01964242. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Cease K. B., Berzofsky J. A. Influences of antigen processing on the expression of the T cell repertoire. Evidence for MHC-specific hindering structures on the products of processing. J Exp Med. 1988 Jul 1;168(1):357–373. doi: 10.1084/jem.168.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett S. J., Lamb J. R., Cox J. H., Rothbard J. B., Mehlert A., Ivanyi J. Differential pattern of T cell recognition of the 65-kDa mycobacterial antigen following immunization with the whole protein or peptides. Eur J Immunol. 1989 Jul;19(7):1303–1310. doi: 10.1002/eji.1830190723. [DOI] [PubMed] [Google Scholar]
- Chandramuki A., Bothamley G. H., Brennan P. J., Ivanyi J. Levels of antibody to defined antigens of Mycobacterium tuberculosis in tuberculous meningitis. J Clin Microbiol. 1989 May;27(5):821–825. doi: 10.1128/jcm.27.5.821-825.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. S., Lorenz R. G., Goldberg J., Allen P. M. Identification and characterization of a T cell-inducing epitope of bovine ribonuclease that can be restricted by multiple class II molecules. J Immunol. 1991 Dec 1;147(11):3672–3678. [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Feller D. C., de la Cruz V. F. Identifying antigenic T-cell sites. Nature. 1991 Feb 21;349(6311):720–721. doi: 10.1038/349720a0. [DOI] [PubMed] [Google Scholar]
- Grimm B., Ish-Shalom D., Even D., Glaczinski H., Ottersbach P., Ohad I., Kloppstech K. The nuclear-coded chloroplast 22-kDa heat-shock protein of Chlamydomonas. Evidence for translocation into the organelle without a processing step. Eur J Biochem. 1989 Jul 1;182(3):539–546. doi: 10.1111/j.1432-1033.1989.tb14861.x. [DOI] [PubMed] [Google Scholar]
- Harris D. P., Bäckström B. T., Booth R. J., Love S. G., Harding D. R., Watson J. D. The mapping of epitopes of the 18-kDa protein of Mycobacterium leprae recognized by murine T cells in a proliferation assay. J Immunol. 1989 Sep 15;143(6):2006–2012. [PubMed] [Google Scholar]
- Harris D. P., Vordermeier H. M., Roman E., Lathigra R., Brett S. J., Moreno C., Ivanyi J. Murine T cell-stimulatory peptides from the 19-kDa antigen of Mycobacterium tuberculosis. Epitope-restricted homology with the 28-kDa protein of Mycobacterium leprae. J Immunol. 1991 Oct 15;147(8):2706–2712. [PubMed] [Google Scholar]
- Ivanyi J., Sharp K. Control by H-2 genes of murine antibody responses to protein antigens of Mycobacterium tuberculosis. Immunology. 1986 Nov;59(3):329–332. [PMC free article] [PubMed] [Google Scholar]
- Jackett P. S., Bothamley G. H., Batra H. V., Mistry A., Young D. B., Ivanyi J. Specificity of antibodies to immunodominant mycobacterial antigens in pulmonary tuberculosis. J Clin Microbiol. 1988 Nov;26(11):2313–2318. doi: 10.1128/jcm.26.11.2313-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston A. E., Salgame P. R., Mitchison N. A., Colston M. J. Immunological activity of a 14-kilodalton recombinant protein of Mycobacterium tuberculosis H37Rv. Infect Immun. 1987 Dec;55(12):3149–3154. doi: 10.1128/iai.55.12.3149-3154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Mouritsen S., Meldal M., Ruud-Hansen J., Werdelin O. T-helper-cell determinants in protein antigens are preferentially located in cysteine-rich antigen segments resistant to proteolytic cleavage by cathepsin B, L, and D. Scand J Immunol. 1991 Oct;34(4):421–431. doi: 10.1111/j.1365-3083.1991.tb01565.x. [DOI] [PubMed] [Google Scholar]
- O'Sullivan D., Sidney J., Appella E., Walker L., Phillips L., Colón S. M., Miles C., Chesnut R. W., Sette A. Characterization of the specificity of peptide binding to four DR haplotypes. J Immunol. 1990 Sep 15;145(6):1799–1808. [PubMed] [Google Scholar]
- Ozaki S., Berzofsky J. A. Antibody conjugates mimic specific B cell presentation of antigen: relationship between T and B cell specificity. J Immunol. 1987 Jun 15;138(12):4133–4142. [PubMed] [Google Scholar]
- Panina-Bordignon P., Tan A., Termijtelen A., Demotz S., Corradin G., Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989 Dec;19(12):2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Verbon A., Hartskeerl R. A., Schuitema A., Kolk A. H., Young D. B., Lathigra R. The 14,000-molecular-weight antigen of Mycobacterium tuberculosis is related to the alpha-crystallin family of low-molecular-weight heat shock proteins. J Bacteriol. 1992 Feb;174(4):1352–1359. doi: 10.1128/jb.174.4.1352-1359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vordemeier H. M., Harris D. P., Roman E., Lathigra R., Moreno C., Ivanyi J. Identification of T cell stimulatory peptides from the 38-kDa protein of Mycobacterium tuberculosis. J Immunol. 1991 Aug 1;147(3):1023–1029. [PubMed] [Google Scholar]
- Vordermeier H. M., Harris D. P., Friscia G., Román E., Surcel H. M., Moreno C., Pasvol G., Ivanyi J. T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38-kDa antigen in patients with active disease. Eur J Immunol. 1992 Oct;22(10):2631–2637. doi: 10.1002/eji.1830221024. [DOI] [PubMed] [Google Scholar]
- Wang Y., Smith J. A., Gefter M. L., Perkins D. L. Immunodominance: intermolecular competition between MHC class II molecules by covalently linked T cell epitopes. J Immunol. 1992 May 15;148(10):3034–3041. [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]


