Abstract
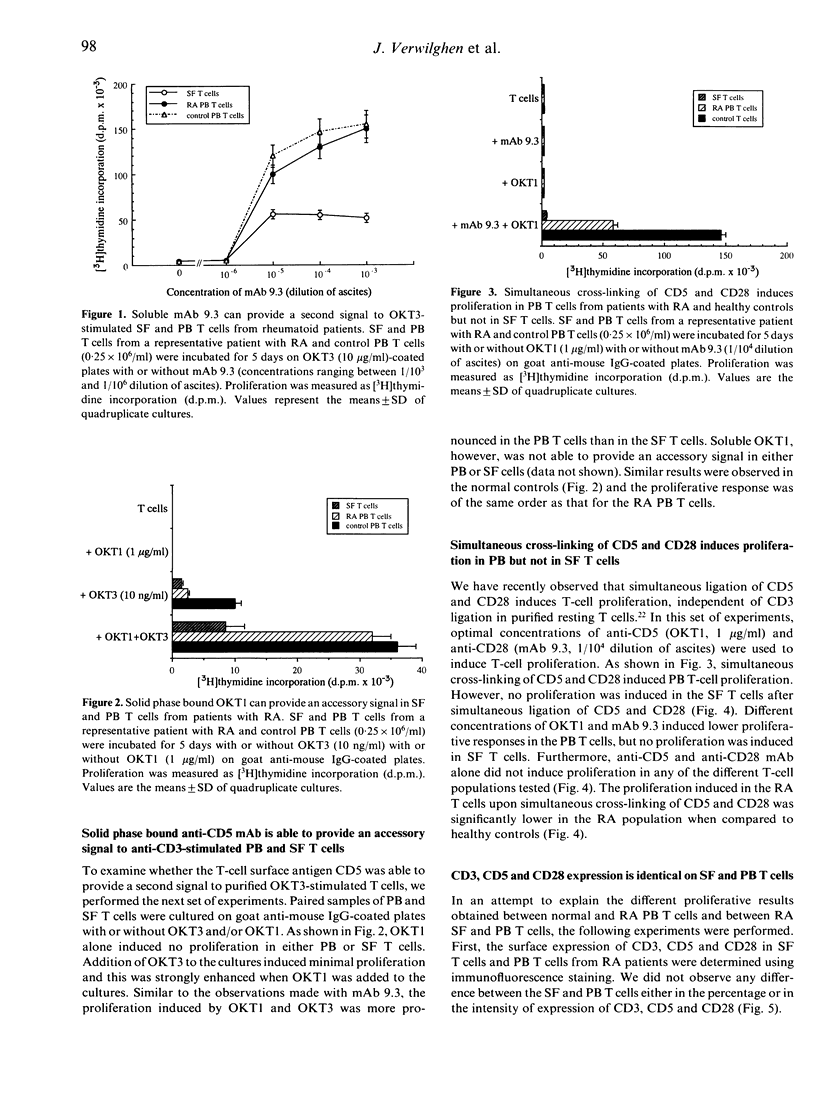
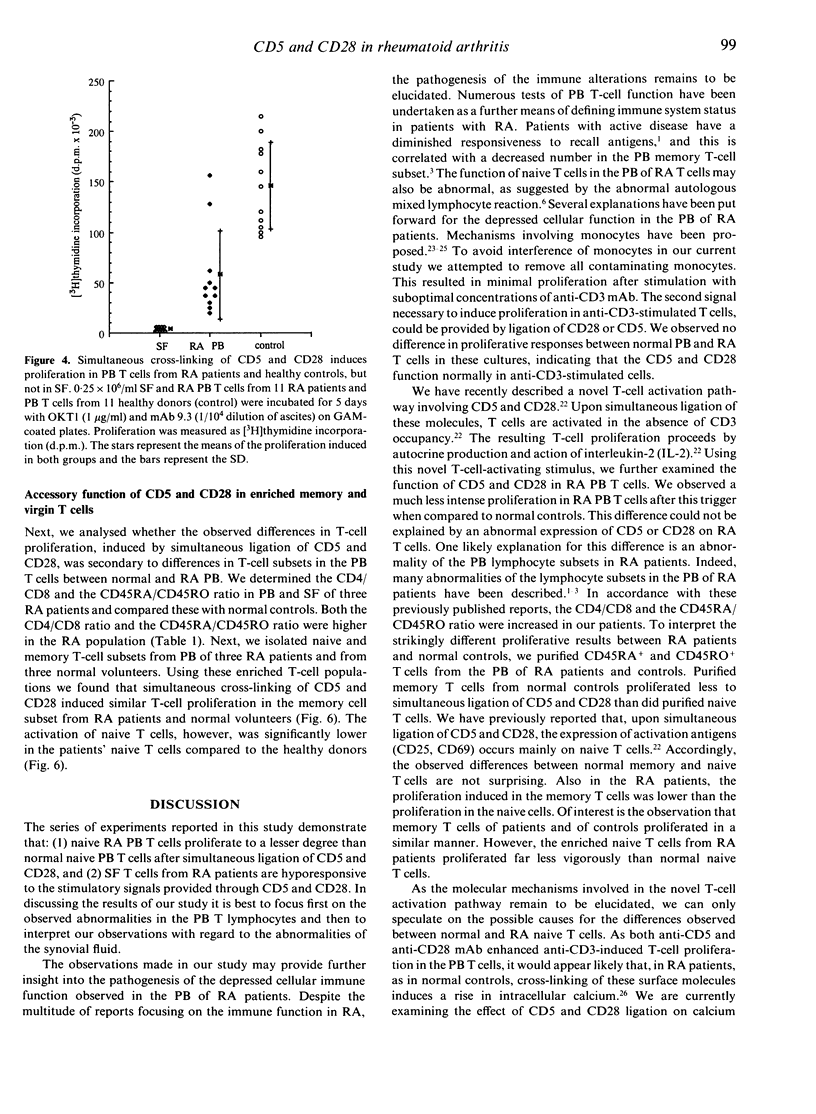
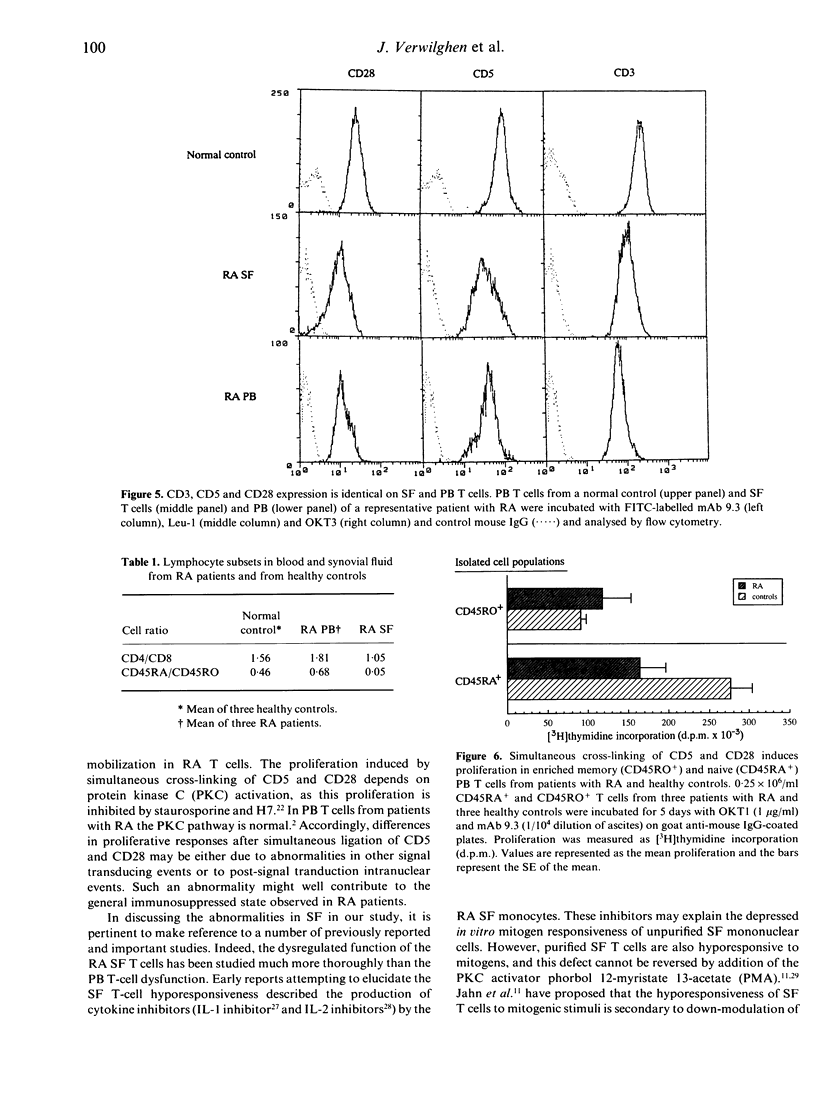
To assess the role of CD5 and CD28 in the pathogenesis of the decreased cellular immune function in patients with rheumatoid arthritis (RA) we analysed the expression and function of these T-cell surface molecules. The expression of CD5 as well as of CD28 in synovial and peripheral blood T cells was similar to that of control T cells. Monoclonal antibodies (mAb) directed at CD28 and CD5 were able to provide an accessory signal to anti-CD3 activated T cells both from the synovial fluid and from the peripheral blood. However, the proliferation induced by anti-CD3 mAb in conjunction with anti-CD5 or anti-CD28 mAb was always higher in peripheral blood (PB) T cells compared to the paired synovial fluid T cells. After simultaneous ligation of CD5 and CD28, proliferation was induced in the PB T cells. However, when compared to control PB T cells, this proliferation was significantly lower in the RA patients. Purified normal memory (CD45RO+) T cells proliferated less strongly than naive (CD45RA+) T cells, but no difference was observed between rheumatoid and normal memory T-cell proliferative responses. However, enriched PB CD45RA+ T cells from rheumatoid patients proliferated less vigorously to CD5 and CD28 ligation when compared to normal enriched CD45RA+ T cells. Synovial fluid (SF) T cells, which are mainly of the memory cell type, did not proliferate after simultaneous ligation of CD5 and CD28. This refractory state of synovial T cells could not be explained by a difference in the surface expression of CD5 or CD28. Our data suggest that the cellular immune dysfunction in the PB from rheumatoid patients may be due to a decreased responsiveness of the naive T-cell subset to accessory signals provided by CD5 and CD28. In addition, SF T cells appear hyporesponsive to stimulating signals provided through CD5 and CD28.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsen T. G., Frøland S. S., Natvig J. B. In vitro mitogen stimulation of synovial fluid lymphocytes from rheumatoid arthritis and juvenile rheumatoid arthritis patients: dissociation between the response to antigens and polyclonal mitogens. Scand J Immunol. 1978;7(1):81–90. doi: 10.1111/j.1365-3083.1978.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Kalden J. R., Peter H. H., Schedel I., Beck P., Wittenborg A. Immunological and functional characteristics of peripheral blood and synovial fluid lymphocytes from patients with rheumatoid arthritis. Scand J Immunol. 1978;7(5):405–417. doi: 10.1111/j.1365-3083.1978.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Ceuppens J. L., Baroja M. L. Monoclonal antibodies to the CD5 antigen can provide the necessary second signal for activation of isolated resting T cells by solid-phase-bound OKT3. J Immunol. 1986 Sep 15;137(6):1816–1821. [PubMed] [Google Scholar]
- Corrigall V., Panayi G. S., Laurent R. Lymphocyte studies in rheumatoid arthritis. III. A comparative study of the responses of peripheral blood and synovial fluid lymphocytes to phytomitogens. Scand J Rheumatol. 1979;8(1):10–16. [PubMed] [Google Scholar]
- DeFranco A. L. Between B cells and T cells. Nature. 1991 Jun 20;351(6328):603–604. doi: 10.1038/351603a0. [DOI] [PubMed] [Google Scholar]
- Emery P., Gentry K. C., Kelso A., Mackay I. R. Interleukin 2 inhibitor in synovial fluid. Clin Exp Immunol. 1988 Apr;72(1):60–66. [PMC free article] [PubMed] [Google Scholar]
- Hara T., Fu S. M., Hansen J. A. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J Exp Med. 1985 Jun 1;161(6):1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraoui B., Wilder R. L., Malone D. G., Allen J. B., Katona I. M., Wahl S. M. Immune function in severe, active rheumatoid arthritis: a relationship between peripheral blood mononuclear cell proliferation to soluble antigens and mononuclear cell subset profiles. J Immunol. 1984 Aug;133(2):697–701. [PubMed] [Google Scholar]
- Hovdenes J., Gaudernack G., Kvien T. K., Egeland T., Mellbye O. J. A functional study of purified CD4+ and CD8+ cells isolated from synovial fluid of patients with rheumatoid arthritis and other arthritides. Scand J Immunol. 1989 Jun;29(6):641–649. doi: 10.1111/j.1365-3083.1989.tb01168.x. [DOI] [PubMed] [Google Scholar]
- Jahn B., Burmester G. R., Stock P., Rohwer P., Kalden J. R. Functional and phenotypical characterization of activated T cells from intra-articular sites in inflammatory joint diseases. Possible modulation of the CD3 antigen. Scand J Immunol. 1987 Dec;26(6):745–754. doi: 10.1111/j.1365-3083.1987.tb02312.x. [DOI] [PubMed] [Google Scholar]
- Janossy G., Panayi G., Duke O., Bofill M., Poulter L. W., Goldstein G. Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet. 1981 Oct 17;2(8251):839–842. doi: 10.1016/s0140-6736(81)91107-7. [DOI] [PubMed] [Google Scholar]
- Keystone E. C., Poplonski L., Miller R. G., Gorczynski R., Gladman D., Snow K. Reactivity of T-cells from patients with rheumatoid arthritis to anti-CD3 antibody. Clin Immunol Immunopathol. 1988 Sep;48(3):325–337. doi: 10.1016/0090-1229(88)90026-8. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Scheynius A., Kabelitz D., Wigzell H. Evidence in support of a self-perpetuating HLA-DR-dependent delayed-type cell reaction in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3632–3636. doi: 10.1073/pnas.79.11.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky H. P., Bauer K., Pope R. M. Increased helper inducer and decreased suppressor inducer phenotypes in the rheumatoid joint. Arthritis Rheum. 1988 Jan;31(1):52–59. doi: 10.1002/art.1780310108. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Grosmaire L. S., Rabinovitch P. S. Crosslinking of surface antigens causes mobilization of intracellular ionized calcium in T lymphocytes. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1384–1388. doi: 10.1073/pnas.84.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardopoulos S., Corrigall V., Panayi G. S. Activation of HLA-DR and interleukin-6 gene transcription in resting T cells via the CD2 molecule: relevance to chronic immune-mediated inflammation. Scand J Immunol. 1992 Sep;36(3):469–477. doi: 10.1111/j.1365-3083.1992.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Lotz M., Tsoukas C. D., Robinson C. A., Dinarello C. A., Carson D. A., Vaughan J. H. Basis for defective responses of rheumatoid arthritis synovial fluid lymphocytes to anti-CD3 (T3) antibodies. J Clin Invest. 1986 Sep;78(3):713–721. doi: 10.1172/JCI112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayi G. S. Response of rheumatoid-synovial-fluid lymphocytes to non-specific mitogens. Lancet. 1973 Sep 1;2(7827):512–513. doi: 10.1016/s0140-6736(73)92121-1. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Haskard D., Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988 Sep;18(9):1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Pope R. M., McChesney L., Talal N., Fischbach M. Characterization of the defective autologous mixed lymphocyte response in rheumatoid arthritis. Arthritis Rheum. 1984 Nov;27(11):1234–1244. doi: 10.1002/art.1780271105. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Seitz M., Napierski I., Kirchner H. Depressed PPD and tetanus toxoid presentation by monocytes to T lymphocytes in patients with rheumatoid arthritis: restoration by interferon gamma. Rheumatol Int. 1988;8(5):189–196. doi: 10.1007/BF00269194. [DOI] [PubMed] [Google Scholar]
- Silverman H. A., Johnson J. S., Vaughan J. H., McGlamory J. C. Altered lymphocyte reactivity in rheumatoid arthritis. Arthritis Rheum. 1976 May-Jun;19(3):509–515. doi: 10.1002/art.1780190301. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Talal N., Tovar Z., Dauphinee M. J., Flescher E., Dang H., Galarza D. Abnormalities of T cell activation in the rheumatoid synovium detected with monoclonal antibodies to CD3. Scand J Rheumatol Suppl. 1988;76:175–182. doi: 10.3109/03009748809102967. [DOI] [PubMed] [Google Scholar]
- Verwilghen J., Baroja M. L., Van Vaeck F., Van Damme J., Ceuppens J. L. Differences in the stimulating capacity of immobilized anti-CD3 monoclonal antibodies: variable dependence on interleukin-1 as a helper signal for T-cell activation. Immunology. 1991 Feb;72(2):269–276. [PMC free article] [PubMed] [Google Scholar]
- Verwilghen J., Kingsley G. H., Ceuppens J. L., Panayi G. S. Inhibition of synovial fluid T cell proliferation by anti-CD5 monoclonal antibodies. A potential mechanism for their immunotherapeutic action in vivo. Arthritis Rheum. 1992 Dec;35(12):1445–1451. doi: 10.1002/art.1780351207. [DOI] [PubMed] [Google Scholar]
- Verwilghen J., Vandenberghe P., Wallays G., de Boer M., Anthony N., Panayi G. S., Ceuppens J. L. Simultaneous ligation of CD5 and CD28 on resting T lymphocytes induces T cell activation in the absence of T cell receptor/CD3 occupancy. J Immunol. 1993 Feb 1;150(3):835–846. [PubMed] [Google Scholar]
- Verwilghen J., Vertessen S., Stevens E. A., Dequeker J., Ceuppens J. L. Depressed T-cell reactivity to recall antigens in rheumatoid arthritis. J Clin Immunol. 1990 Mar;10(2):90–98. doi: 10.1007/BF00918190. [DOI] [PubMed] [Google Scholar]
- Wolinsky S. I., Goodwin J. S., Messner R. P., Williams R. C., Jr Role of prostaglandin in the depressed cell-mediated immune response in rheumatoid arthritis. Clin Immunol Immunopathol. 1980 Sep;17(1):31–37. doi: 10.1016/0090-1229(80)90070-7. [DOI] [PubMed] [Google Scholar]


