Abstract
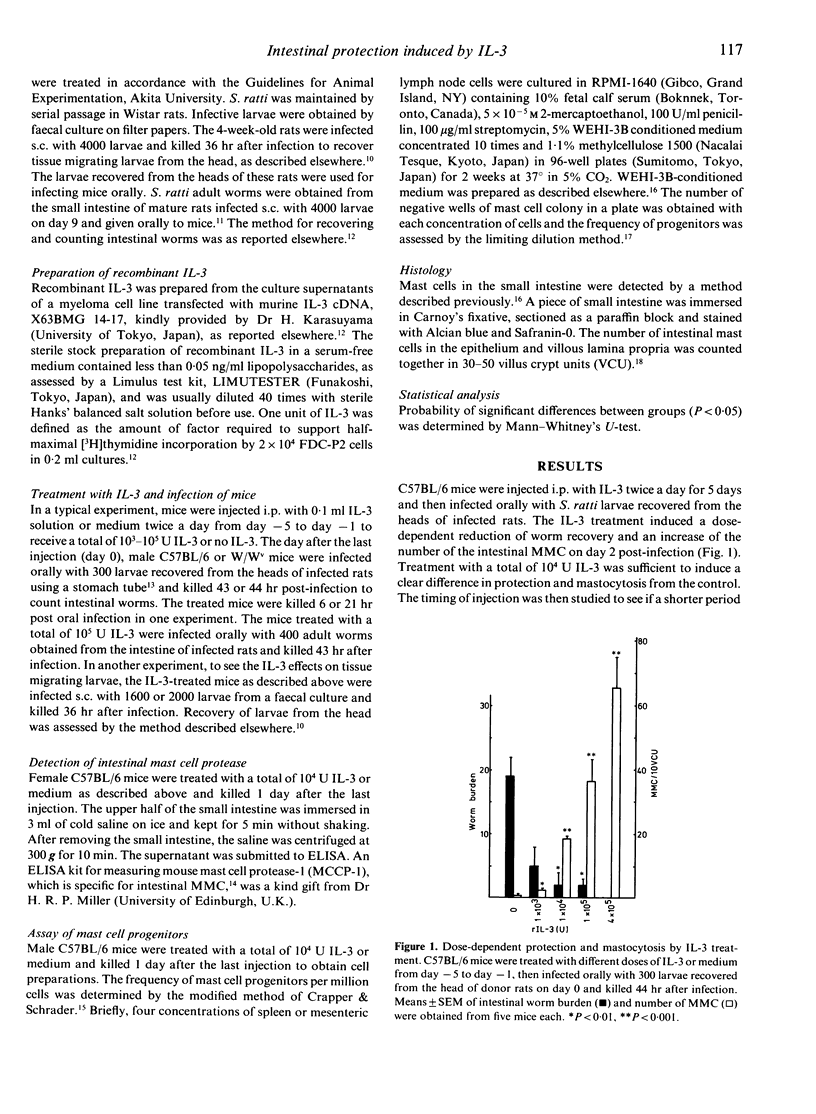
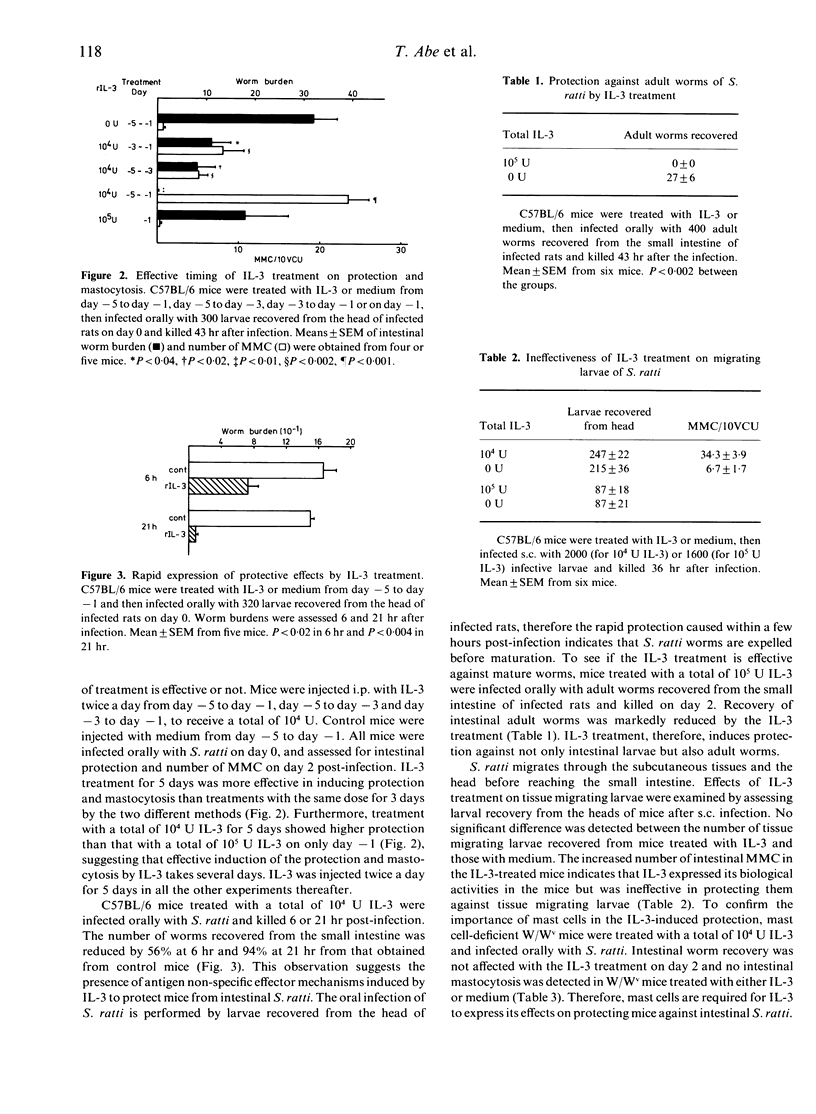
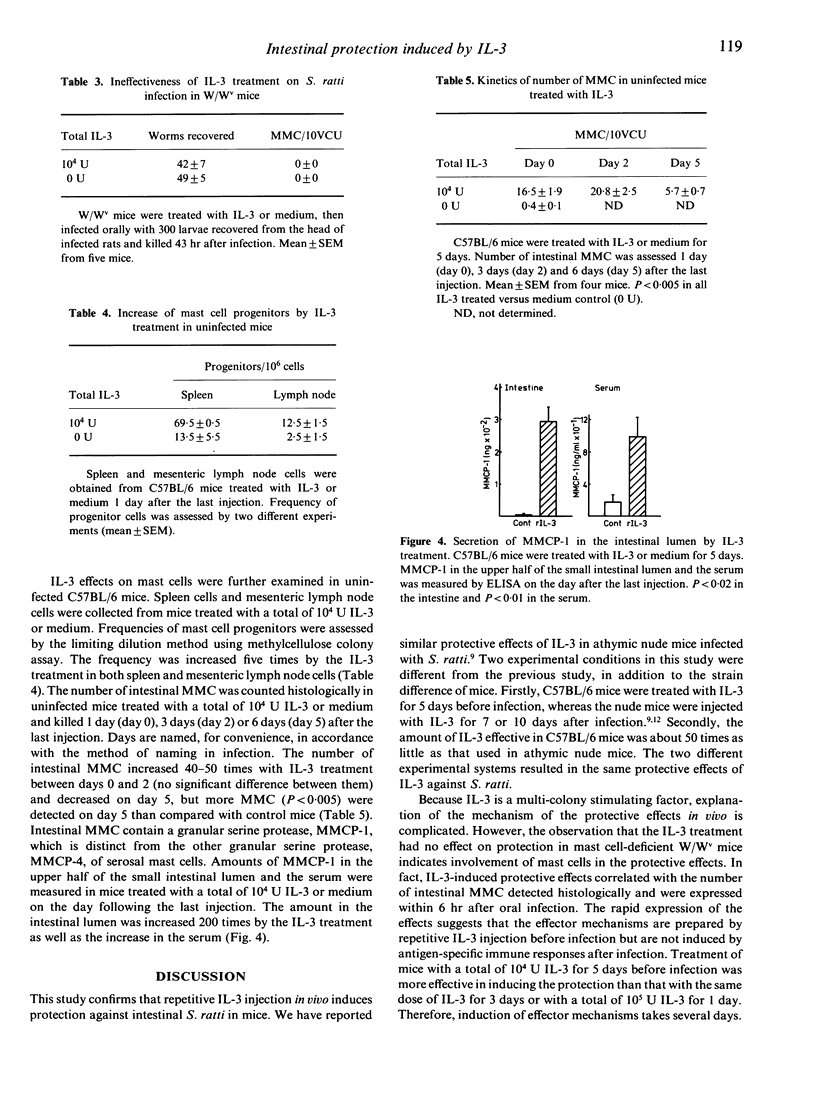
Information about interleukin-3 (IL-3) effects in vivo is limited compared with the in vitro effects. We found that a repetitive injection of a low dose of recombinant IL-3 induced protection against intestinal worms of Strongyloides ratti in C57BL/6 mice. When mice were injected i.p. with different doses of recombinant IL-3 twice a day from day -5 to day -1 and infected orally with larvae recovered from the head of infected rats on day 0, worm recovery from the small intestine was markedly reduced by a total of 10(4) U IL-3 or more on day 2 post-infection. The number of intestinal mucosal mast cells (MMC) was increased by the protective dose of IL-3. The IL-3 treatment, however, was ineffective in protecting mice against tissue migrating larvae, as assessed by recovery from the head. The protective effect of IL-3 on intestinal worms was observed within 6 hr post oral infection, suggesting little concern with antigen-specific immune responses. The effective dose of IL-3 treatment increased the number of MMC progenitors five times in the spleen and the mesenteric lymph nodes. An MMC-specific protease, MMCP-1, was secreted 200 times more than in controls in the intestinal lumen by the IL-3 treatment. The IL-3 treatment induced no protection or mastocytosis in mast cell-deficient W/Wv mice. These results suggest that the IL-3-induced intestinal protection against S. ratti is mediated by MMC.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Kiyota M., Nawa Y. Strongyloides ratti: increase in susceptibility to infection following blockade of the mononuclear phagocyte system in female mice. Aust J Exp Biol Med Sci. 1985 Dec;63(Pt 6):651–653. doi: 10.1038/icb.1985.68. [DOI] [PubMed] [Google Scholar]
- Abe T., Nawa Y. Kinetic study of mast-cell growth factor production by lymphocytes during the course of Strongyloides ratti infection in mice. Parasitol Res. 1988;74(5):484–488. doi: 10.1007/BF00535150. [DOI] [PubMed] [Google Scholar]
- Abe T., Nawa Y. Reconstitution of mucosal mast cells in W/WV mice by adoptive transfer of bone marrow-derived cultured mast cells and its effects on the protective capacity to Strongyloides ratti-infection. Parasite Immunol. 1987 Jan;9(1):31–38. doi: 10.1111/j.1365-3024.1987.tb00486.x. [DOI] [PubMed] [Google Scholar]
- Abe T., Nawa Y. Worm expulsion and mucosal mast cell response induced by repetitive IL-3 administration in Strongyloides ratti-infected nude mice. Immunology. 1988 Feb;63(2):181–185. [PMC free article] [PubMed] [Google Scholar]
- Abe T., Ochiai H., Minamishima Y., Nawa Y. Induction of intestinal mastocytosis in nude mice by repeated injection of interleukin-3. Int Arch Allergy Appl Immunol. 1988;86(3):356–358. doi: 10.1159/000234597. [DOI] [PubMed] [Google Scholar]
- Abe T., Yoshimura K., Nawa Y. Restoration of the defective natural defence of beige mice against tissue-migrating larvae of Strongyloides ratti by transfer with normal peritoneal cells. Int J Parasitol. 1992 Jul;22(4):545–547. doi: 10.1016/0020-7519(92)90159-i. [DOI] [PubMed] [Google Scholar]
- Chan W. L., Ziltener H. J., Liew F. Y. Interleukin-3 protects mice from acute herpes simplex virus infection. Immunology. 1990 Nov;71(3):358–363. [PMC free article] [PubMed] [Google Scholar]
- Crapper R. M., Schrader J. W. Frequency of mast cell precursors in normal tissues determined by an in vitro assay: antigen induces parallel increases in the frequency of P cell precursors and mast cells. J Immunol. 1983 Aug;131(2):923–928. [PubMed] [Google Scholar]
- Feng Z. Y., Louis J., Kindler V., Pedrazzini T., Eliason J. F., Behin R., Vassalli P. Aggravation of experimental cutaneous leishmaniasis in mice by administration of interleukin 3. Eur J Immunol. 1988 Aug;18(8):1245–1251. doi: 10.1002/eji.1830180815. [DOI] [PubMed] [Google Scholar]
- Ghildyal N., McNeil H. P., Stechschulte S., Austen K. F., Silberstein D., Gurish M. F., Somerville L. L., Stevens R. L. IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J Immunol. 1992 Sep 15;149(6):2123–2129. [PubMed] [Google Scholar]
- Grove D. I., Northern C. Oral transfer of Strongyloides ratti adult worms to mice. J Parasitol. 1987 Apr;73(2):424–425. [PubMed] [Google Scholar]
- Gurish M. F., Ghildyal N., McNeil H. P., Austen K. F., Gillis S., Stevens R. L. Differential expression of secretory granule proteases in mouse mast cells exposed to interleukin 3 and c-kit ligand. J Exp Med. 1992 Apr 1;175(4):1003–1012. doi: 10.1084/jem.175.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Dy M., Luffau G., Vassalli P. Gut mucosal mast cells. Origin, traffic, and differentiation. J Exp Med. 1984 Jul 1;160(1):12–28. doi: 10.1084/jem.160.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley J. F., Gooden C., Newlands G. F., Mackellar A., Lammas D. A., Wakelin D., Tuohy M., Woodbury R. G., Miller H. R. Distribution of intestinal mast cell proteinase in blood and tissues of normal and Trichinella-infected mice. Parasite Immunol. 1990 Jan;12(1):85–95. doi: 10.1111/j.1365-3024.1990.tb00938.x. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Weinstein Y. Immunological regulation of hematopoietic/lymphoid stem cell differentiation by interleukin 3. Adv Immunol. 1986;39:1–50. doi: 10.1016/s0065-2776(08)60347-8. [DOI] [PubMed] [Google Scholar]
- Kimoto M., Kindler V., Higaki M., Ody C., Izui S., Vassalli P. Recombinant murine IL-3 fails to stimulate T or B lymphopoiesis in vivo, but enhances immune responses to T cell-dependent antigens. J Immunol. 1988 Mar 15;140(6):1889–1894. [PubMed] [Google Scholar]
- Metcalf D., Begley C. G., Johnson G. R., Nicola N. A., Lopez A. F., Williamson D. J. Effects of purified bacterially synthesized murine multi-CSF (IL-3) on hematopoiesis in normal adult mice. Blood. 1986 Jul;68(1):46–57. [PubMed] [Google Scholar]
- Miller H. R., Jarrett W. F. Immune reactions in mucous membranes. I. Intestinal mast cell response during helminth expulsion in the rat. Immunology. 1971 Mar;20(3):277–288. [PMC free article] [PubMed] [Google Scholar]
- Moqbel R., Wakelin D., MacDonald A. J., King S. J., Grencis R. K., Kay A. B. Release of leukotrienes during rapid expulsion of Trichinella spiralis from immune rats. Immunology. 1987 Mar;60(3):425–430. [PMC free article] [PubMed] [Google Scholar]
- Reynolds D. S., Stevens R. L., Lane W. S., Carr M. H., Austen K. F., Serafin W. E. Different mouse mast cell populations express various combinations of at least six distinct mast cell serine proteases. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3230–3234. doi: 10.1073/pnas.87.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi T., Morita Y., Hirai K., Yamaguchi M., Yokota T., Arai K., Ito K., Miyamoto T. Mouse IL-3 induces histamine release from mouse peritoneal mast cells. Int Arch Allergy Immunol. 1992;98(3):205–210. doi: 10.1159/000236186. [DOI] [PubMed] [Google Scholar]
- Thompson-Snipes L., Dhar V., Bond M. W., Mosmann T. R., Moore K. W., Rennick D. M. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991 Feb 1;173(2):507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Miller H. R., Huntley J. F., Newlands G. F., Palliser A. C., Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. 1984 Nov 29-Dec 5Nature. 312(5993):450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]


