Abstract
Objective
To assess and compare the value of split-liver transplantation (SLT) and living-related liver transplantation (LRT).
Summary Background Data
The concept of SLT results from the development of reduced-size transplantation. A further development of SLT, the in situ split technique, is derived from LRT, which itself marks the optimized outcome in terms of postoperative graft function and survival. The combination of SLT and LRT has abolished deaths on the waiting list, thus raising the question whether living donor liver transplantation is still necessary.
Methods
Outcomes and postoperative liver function of 43 primary LRT patients were compared with those of 49 primary SLT patients (14 ex situ, 35 in situ) with known graft weight performed between April 1996 and December 2000. Survival rates were analyzed using the Kaplan-Meier method.
Results
After a median follow-up of 35 months, actual patient survival rates were 82% in the SLT group and 88% in the LRT group. Actual graft survival rates were 76% and 81%, respectively. The incidence of primary nonfunction was 12% in the SLT group and 2.3% in the LRT group. Liver function parameters (prothrombin time, factor V, bilirubin clearance) and surgical complication rates did not differ significantly. In the SLT group, mean cold ischemic time was longer than in the LRT group. Serum values of alanine aminotransferase during the first postoperative week were significantly higher in the SLT group. In the LRT group, there were more grafts with signs of fatty degeneration than in the SLT group.
Conclusions
The short- and long-term outcomes after LRT and SLT did not differ significantly. To avoid the risk for the donor in LRT, SLT represents the first-line therapy in pediatric liver transplantation in countries where cadaveric organs are available. LRT provides a solution for urgent cases in which a cadaveric graft cannot be found in time or if the choice of the optimal time point for transplantation is vital.
Living-related liver transplantation (LRT) and split-liver transplantation (SLT) are surgical strategies that have led to a reduction in the pretransplant death rate in children from 20% to nearly 0%. 1–5 LRT provides a graft of excellent quality by minimizing the cold ischemic time. Primary nonfunction (PNF) after LRT is rare. In addition, this procedure is elective and thus allows flexibility in choosing the optimal time for transplantation with regard to the recipient’s clinical status. Because of these advantages, worldwide long-term results of LRT are equal or even superior to those obtained with cadaveric full-size or reduced-size techniques. The actual 1-year graft and patient survival rate after LRT exceeds 80%. 6–10 The expansion of LRT for adult recipients reflects the great expectations of this procedure despite the higher risks for the donor associated with major hepatectomy.
Split-liver transplantation (SLT) is technically comparable to LRT. However, as in other cadaveric procedures, it is theoretically susceptible to potential negative effects resulting from logistical and clinical circumstances related to donor condition, organ transportation, and cold ischemic time. SLT is divided into the ex situ approach, which means back-table division of the flushed and cooled graft under preservation conditions, and the in situ split, which is performed by partitioning the liver within the heart-beating donor before flushing and explantation of the divided organ, thus saving the time needed for back-table partitioning under cold ischemic conditions. 11 Initial reports from split-liver programs from Europe and the United States indicate that SLT has acceptable results, 5,12 although initial results were not as encouraging as after the introduction of LRT. Growing surgical experience has resulted in improved outcomes, comparable to those of cadaveric liver trans-plantation. 11,13–17
Whether LRT should generally be preferred over SLT is a matter of debate. 18 LRT might be recommended as the method of choice in pediatric liver transplantation, arguing that it provides grafts of best quality. However, it carries potential risks for the donor that can be avoided by performing SLT. This led Azoulay et al 16 to state that “given the improving results of split-liver transplantation we believe that, whenever possible, this technique should be offered before living-related liver transplantation.”
This study aims at providing objective arguments for the choice of procedure in pediatric liver transplantation. Given this aim, the results after LRT and SLT, with regard to postoperative liver function parameters, complications, and survival rates are compared. This study was performed to answer the following questions: Does SLT provide results equal to LRT? Are there still indications in which the decision in favor of LRT over SLT is justified?
PATIENTS AND METHODS
Between April 1996 and December 2000, 159 pediatric liver transplants were performed at the University of Hamburg: 21 whole-organ transplantations, 19 reduced-size transplantations, 72 split-liver transplantations, and 47 living-related liver transplantations. The outcomes of 43 cases of primary LRT (all segments 2 and 3 according to Couinaud 19) were compared with 49 primary SLT (all segments 2 and 3), with known graft weights. Retransplantations and grafts of segments other than just 2 and 3 were excluded from this study.
Donor Selection
In the LRT group, the grafts were harvested after informed consent of the donors and close medical, laboratory, and psychological evaluation, according to our evaluation protocol described elsewhere. 20
The selection of suitable cadaveric donors for liver splitting was performed by an experienced transplant surgeon during surgical exploration of the donor liver according to the following criteria: amount of fatty degeneration, age of the donor, laboratory findings, cause of death, amount of catecholamines, length of stay in the intensive care unit, and macroscopic appearance of the liver.
Surgical Technique
Donor Operation
The procedure for obtaining split-liver grafts was entirely comparable to that for living donation. 11
The left hepatic artery is isolated and the arterial branch to segment 4 is identified. The segment 4 artery is preserved with the right graft, if possible, to leave as much perfusion as possible to segment 4. The portal branches to segment 4 are ligated on the right side of the round ligament. After isolating the left hepatic vein, parenchymal transsection is performed along the falciform ligament. The hilar plate, including the left bile duct(s), is sharply divided to minimize the injury to the parabiliary vascular plexus.
To increase the acceptance of liver splitting within the transplant community, the common trunks of the hepatic artery and portal vein remain to the right, which allows an unchanged implantation technique of the right graft. To minimize cold ischemic times, in situ splitting of the cadaveric donor organ was preferred in our center whenever possible.
Recipient Operation
For implantation of the left lateral graft, the recipient hepatic vein confluence of left and middle hepatic vein is preserved and enlarged by a longitudinal ventral incision of the vena cava. Then, the left hepatic vein of the graft is anastomosed end-to-side to the caval vein. Portal vein and hepatic artery anastomoses are performed in an end-to-end fashion without interpositioning grafts. Biliary reconstruction is performed by Roux-en-Y hepaticojejunostomy without biliary stents.
Immunosuppression
All children received cyclosporine A (Neoral; Novartis, East Hanover, NJ) and prednisolone as primary immunosuppressive drugs. The desired trough levels of cyclosporine A were 180 to 200 μg/L (first month after transplant), 130 to 150 μg/L (months 2–6 after transplant), 100 to 130 μg/L (months 7–11 after transplant), and 80 to 100 μg/L (12–24 months after transplant). Prednisolone was started at 60 mg/m2 and then tapered down weekly to 0.1 mg/kg until it was stopped 12 months after the transplant. Mycophenolate mofetil or azathioprine was not used in our patients. Since January 1999 the monoclonal antibody basiliximab (Simulect, Novartis Pharmaceutical Corp., Basel, Switzerland) was given in two single doses on day 0 and day 4 in addition to standard immunosuppression. Other monoclonal antibodies, such as OKT3, were not used. Acute rejections were treated with a 3-day course of intravenous methylprednisolone bolus therapy (10 mg/kg). Four patients (two per group) were converted to tacrolimus because of steroid-resistant rejection between weeks 3 and 6 after the transplant; all other patients continued taking cyclosporine A.
Complications
Primary nonfunction was defined as retransplantation within 10 days after implantation or death resulting from a nonfunctioning graft. Rejection was defined when serum transaminase levels increased and signs of rejection were seen on biopsy. Biliary complications were defined as bile duct stenosis (early and late) or bile leakage requiring surgical intervention.
Fatty Degeneration and Reperfusion Injury
To evaluate liver damage induced by ischemia and reperfusion injury, the results of the liver biopsies taken before closure of the abdomen (postreperfusion biopsies) were compared between the groups. These biopsies were available for analysis in 39 LRT and 42 SLT patients. Routinely processed specimens were reviewed in a retrospective manner. Sections stained with hematoxylin and eosin were examined (50×–200× magnification) for the presence of histologic characteristics of reperfusion damage (cell ballooning, single- or multiple-cell necrosis, presence of inflammatory cells) and for type (microvacuolar, medium, and macrovacuolar) and degree of fatty change. The slides were scored as none (0), mild (1+), moderate (2+), and severe (3+) by two observers (H.S., E.G.A.) in a masked fashion. Grading of the reperfusion damage was performed using the following definitions: none, no morphologic signs of injury; mild, changes confined to fewer than 5% of cells; moderate, changes confined to 5% to 15% of cells; and severe, changes in more than 15% of cells. Each criterion (cell ballooning, single- or multiple-cell necrosis, presence of inflammatory cells) was individually scored and a final perfusion damage score was counted by adding the individual scores (none [0], mild [1–3], moderate [4–6], and severe [7–9]).
Grading of fatty changes was performed using the following definitions, as used by others 21,22 : none, no fatty change; mild, less than 30%; moderate, 30% to 60%; and severe, more than 60% of cells. The type of fat vacuoles was determined as microvacuolar (vacuoles smaller than the hepatocyte nucleus) and macrovacuolar (vacuoles displacing the hepatocyte nucleus or occupying the majority of the cytosol).
Postoperative liver function was assessed by measurements of factor V activity, prothrombin time, and bilirubin clearance. As markers of hepatocellular damage, levels of alanine aminotransferase, aspartate aminotransferase, and glutamate dehydrogenase were analyzed.
Statistical Analysis
Statistical analysis of patient and graft survival was performed according to the Kaplan-Meier method, calculated from the time of transplantation until May 2001. Comparison of patient survival in different groups was performed using the log-rank test. Categorical and continuous variables were compared using the Fisher exact test and the Mann-Whitney test, respectively. P < .05 was considered statistically significant.
RESULTS
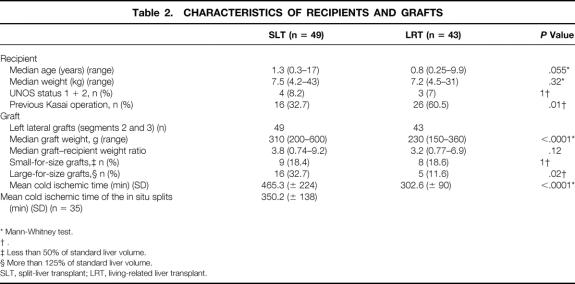
Forty-three pediatric recipients underwent a first LRT and 49 pediatric patients with end-stage liver disease underwent a first SLT (14 ex situ, 35 in situ). The indications for transplantation were equally distributed in both groups (Table 1). The leading indication in both groups was biliary atresia. Sixteen patients in the SLT group and 26 patients in the LRT group had undergone a previous Kasai operation (P = .01). The median recipient age was 0.8 years (range 0.25–9.9) in the LRT group and 1.3 years (0.3–17) in the SLT group (P = .055). Median weight of recipients was 7.2 kg (range 4.5–31) in the LRT group and 7.5 kg (4.2–43) in the SLT group (P = .32). In the LRT group, there were three patients (7%) with urgent status (United Network for Organ Sharing [UNOS] 1 + 2); in the SLT group, four patients (8.2%) had UNOS status 1 + 2 (Table 2).
Table 1. INDICATIONS FOR LIVER TRANSPLANTATION
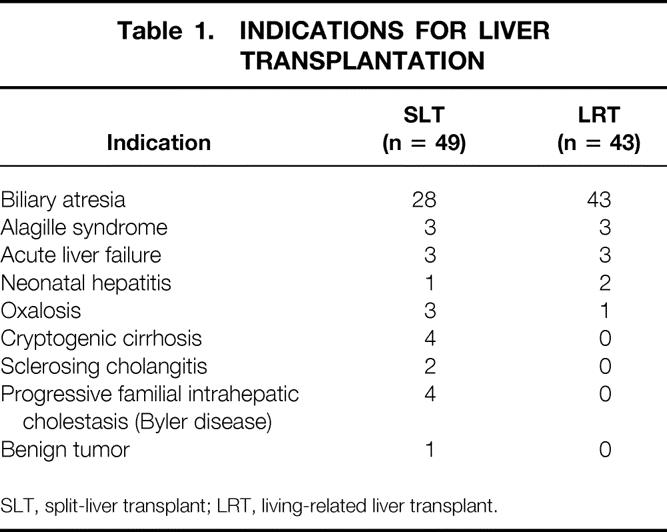
SLT, split-liver transplant; LRT, living-related liver transplant.
Table 2. CHARACTERISTICS OF RECIPIENTS AND GRAFTS
* Mann-Whitney test.
† .
‡ Less than 50% of standard liver volume.
§ More than 125% of standard liver volume.
SLT, split-liver transplant; LRT, living-related liver transplant.
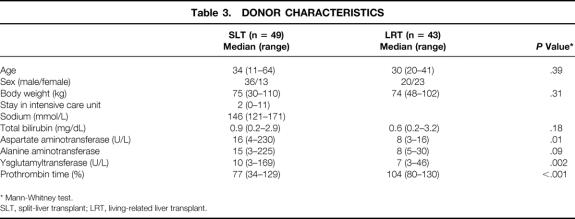
All grafts from living donors consisted of segments 2 and 3 (left lateral liver lobe) with a median graft weight of 230 g (range 150–360). The grafts in the SLT group comprised 49 left lateral lobes (segments 2 and 3) with a median weight of 310 g (range 200–600). Eighteen percent of the grafts in both groups were small-for-size grafts, whereas significantly more large-for-size grafts were used in the SLT group (see Table 2). The median graft–recipient weight ratio was comparable in both groups: SLT = 3.8 (range 0.74–9.2) and LRT = 3.2 (0.77–6.9) (P = .12). The mean cold ischemic time was 302 ± 90 minutes in the LRT group and 465 ± 224 minutes in the SLT group (P < .0001). The median age of the 43 living donors (23 female, 20 male) was 30 years (range 20–41); the median age of the 49 cadaveric donors (13 female, 36 male) was 34 years (range 11–64). Details of the living and cadaveric donors are listed in Table 3. Forty-seven cadaveric donors (96%) received hemodynamic support by catecholamines (dopamine or adrenaline). The median stay in the intensive care unit for the split donors was 2 days (range 0–11).
Table 3. DONOR CHARACTERISTICS
* Mann-Whitney test.
SLT, split-liver transplant; LRT, living-related liver transplant.
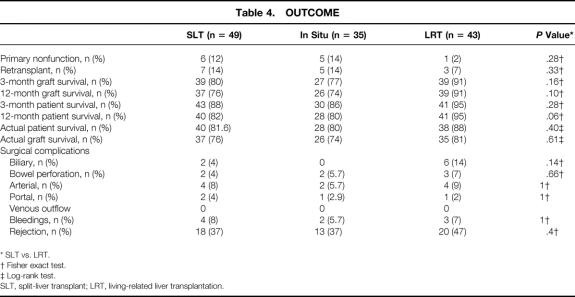
The median follow-up of the patients was 35 months (range 5–61). The 3- and 12-month patient survival rates after SLT were 88% and 82%; they were 95% and 95% after LRT (P = .28, P = .06). The 3- and 12-month graft survival rates were 80% and 76% after SLT and 91% and 91% after LRT (P = .16, P = .10). The actual patient survival rate was 88% for LRT and 82% for SLT (P = .29) (Fig. 1). Figure 2 shows the graft survival rates after LRT versus SLT. The actual graft survival rates were 81% and 76%, respectively (P = .39). The incidence of PNF was 2.3% (n = 1) in the LRT group and 12% (n = 6) in the SLT group (P = .28). Acute cellular rejection occurred in 47% after LRT and 37% after SLT (P = .4). The rate of retransplantation was 7% (n = 3) after LRT and 14% (n = 7) after SLT (P = .33) (Table 4).
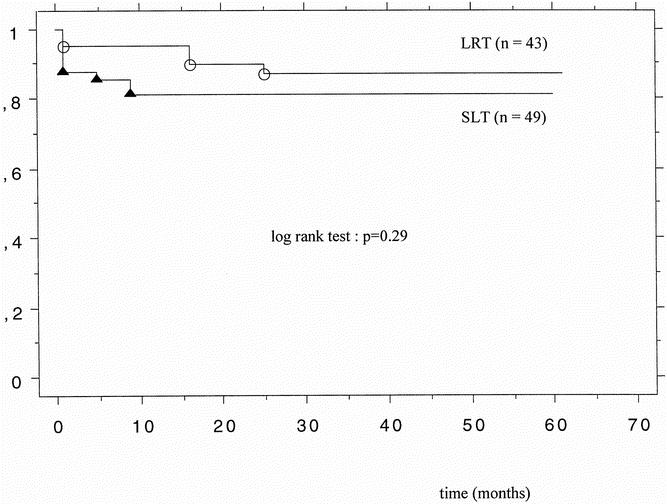
Figure 1. Patient survival after living-related liver transplantation (LRT, n = 43) and split-liver transplantation (SLT, n = 49).
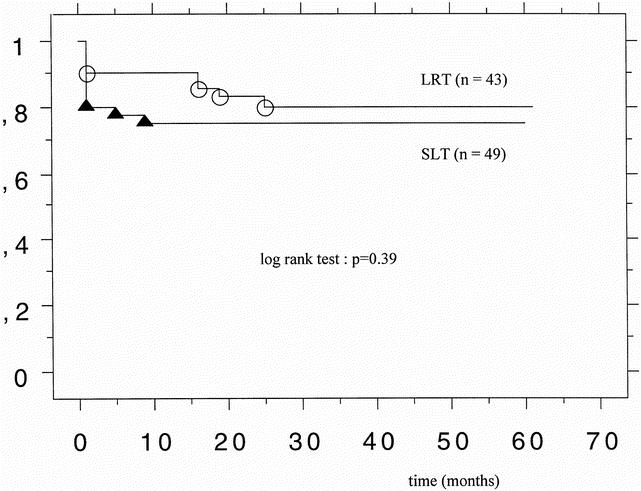
Figure 2. Graft survival after living-related liver transplantation (LRT, n = 43) and split-liver transplantation (SLT, n = 49).
Table 4. OUTCOME
* SLT vs. LRT.
† Fisher exact test.
‡ Log-rank test.
SLT, split-liver transplant; LRT, living-related liver transplantation.
Biliary complication rates were 14% after LRT and 4% after SLT (P = .14); arterial complications occurred in 9% and 8% (P = .66); intestinal perforations were observed in 7% and 4% (P = .66); and postoperative bleedings requiring reoperation occurred in 7% and 8% (see Table 4). Two (4%) portal vein complications led to surgical intervention (thrombectomy, repositioning) after SLT, and in one patient (2%) undergoing LRT, a portal vein complication developed (repositioning).
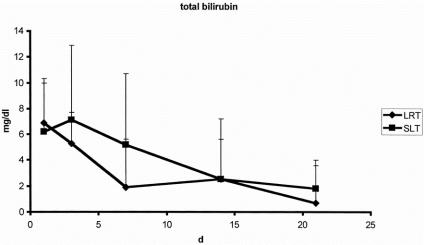
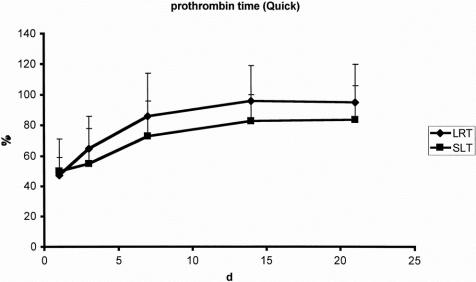
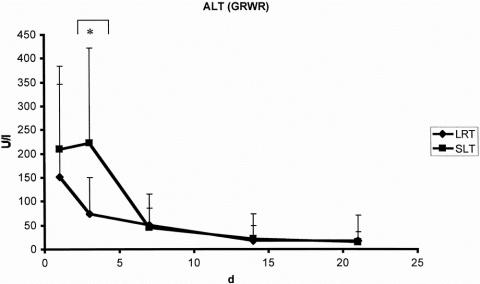
There were no significant differences with regard to laboratory markers of postoperative liver function. The curve of bilirubin clearance and prothrombin time showed a comparable pattern after both LRT and SLT (Figs. 3 and 4). Factor V activity showed no difference in the postoperative course after both procedures. The postoperative peak of alanine aminotransferase activity was significantly higher after SLT (Fig. 5). Similar results were found for aspartate aminotransferase and glutamine dehydrogenase.
Figure 3. Clearance of total bilirubin after split-liver transplantation (SLT, n = 49) and living-related liver transplantation (LRT, n = 43).
Figure 4. Prothrombin time after split-liver transplantation (SLT, n = 49) and living-related liver transplantation (LRT, n = 43).
Figure 5. Alanine aminotransferase (ALT) corrected by the graft–recipient weight ratio (GRWR) after split-liver transplantation (SLT, n = 49) and living-related liver transplantation (LRT, n = 43). *P < .0001 (Mann-Whitney test).
About one fourth (24%) of the split grafts were histologically proven absent of any fatty change (vs. 8% in LRT). Almost half (46%) of the LRT grafts were classified as having fatty change grade 1 (vs. one third [33%] in SLT). A microvacuolar fatty change was predominant in LRT (62%), whereas micro- or macrovacuolar changes were shown in approximately one third of SLT grafts (40% and 36%, respectively).
Most (67%) of the LRT grafts had moderate reperfusion damage; only 23% showed severe reperfusion damage. In contrast, 38% of SLT organs had severe reperfusion damage and 45% had moderate perfusion damage. LRT grafts with macrovacuolar steatosis tended to have moderate perfusion damage. In contrast, corresponding SLT grafts more often showed severe perfusion damage (Table 5).
Table 5. DISTRIBUTION OF PERFUSION DAMAGE AS A FUNCTION OF FAT GRADE IN POSTREPERFUSION BIOPSIES
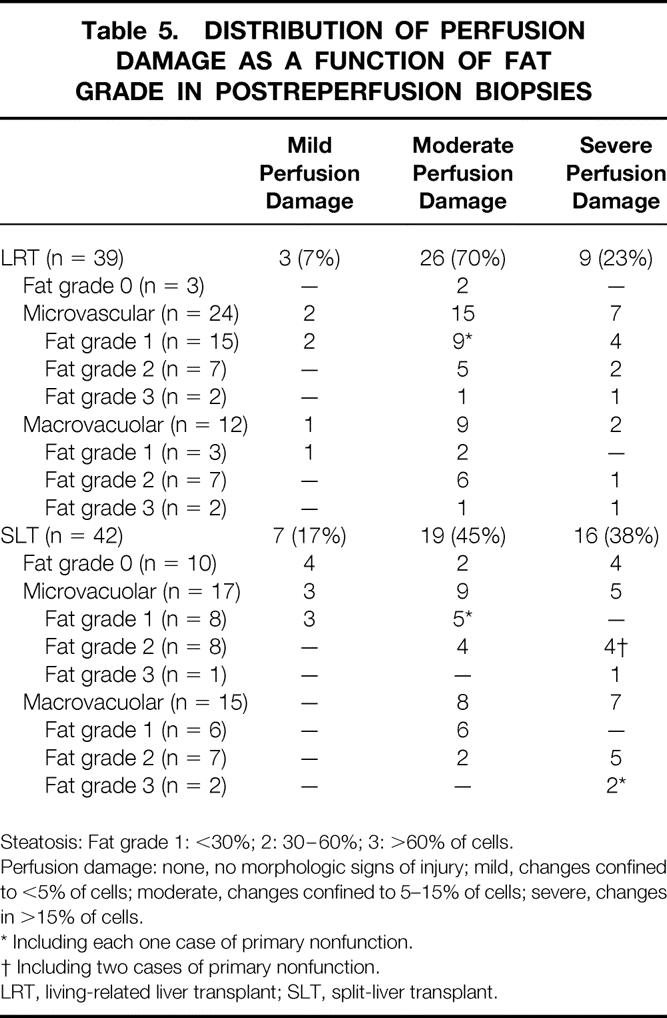
Steatosis: Fat grade 1: <30%; 2: 30–60%; 3: >60% of cells.
Perfusion damage: none, no morphologic signs of injury; mild, changes confined to <5% of cells; moderate, changes confined to 5–15% of cells; severe, changes in >15% of cells.
* Including each one case of primary nonfunction.
† Including two cases of primary nonfunction.
LRT, living-related liver transplant; SLT, split-liver transplant.
DISCUSSION
In the field of pediatric liver transplantation, LRT and SLT are important and widely accepted technical variants. In this study, we compared the outcomes of both techniques using comparable patient groups in terms of age, indications, and urgency of transplantation. For analysis of the underlying data, a time interval of 5 years was chosen, in which all discussed techniques were well established and routinely used.
Although only a small portion of the liver is harvested, LRT carries the disadvantage of the risk of death for the donor. 23 Grewal et al 24 performed a review of 100 cases of donor operations for LRT from 1989 to 1996. Left lateral segments were predominantly used for transplantation (91 cases vs. 9 donors of left lobes). They reported 14 major complications (7 biliary complications, 1 hepatic artery thrombosis, 1 intraabdominal abscess, 1 splenectomy, 1 perforated duodenal ulcer, 1 gastric outlet obstruction, and 2 cases of wound dehiscence). Minor complications were reported in 20% of patients and did not require surgical therapy. For this reason, for ethical reasons transplant surgeons must check all alternatives to provide appropriate grafts from cadaveric donors.
Our data show that both LRT and SLT provide adequate results. Because they make it possible to avoid any deaths on the waiting list, they should therefore reflect the standard repertoire in pediatric liver transplantation. Surgical complication rates do not differ significantly. Worldwide, outcomes of LRT and SLT in elective patients reach 1-year survival rates of 80%. 17,18,25–27 Several authors have concluded that the results of LRT are superior to those obtained by other techniques, especially in urgent situations 25,27; others state that the type of graft does not affect long-term survival. 6
From the presented data, LRT might have potential benefits for the recipient, especially with regard to the rate of PNF. This fact is underlined by a lower incidence of PNF (but not significantly different from that after SLT) and a tendency to higher graft survival rates during the first months and a lower peak of posttransplant transaminase levels. These aspects seem to be strongly dependent on the clinical status of the donor and the cold ischemic times and are more important in urgent situations. However, laboratory parameters of liver function such as bilirubin clearance and prothrombin time failed to identify functional superiority of grafts from living donors. The fact that there is a higher incidence of PNF after SLT (12% vs. 2.3% in the LRT group) clearly shows that this procedure involves disadvantages thus far unresolved with regard to the quality of the graft; it remains a challenge to identify the cadaveric donors who may present a higher risk of PNF.
It was recently shown that brain death itself with hemodynamic instability is accompanied by progressive organ dysfunction and unspecific upregulation of inflammatory activity. 28,29 Regarding the causes of death after SLT, in at least five patients fatal multiorgan failure was triggered by mild to moderate graft dysfunction and/or retransplantation. From this viewpoint, it is of utmost importance to choose the right donor for SLT and to keep in mind that hemodynamic stability and pretransplant clinical course in the intensive care unit are important predictors of later graft function and patient survival.
The outcome of pediatric liver transplantation is to a large extent dependent on the recipient’s pretransplant clinical status. 30,31 LRT makes it possible to plan the transplant and choose the best time for surgery. Therefore, LRT is the method of choice when transplanting children with an unstable course (e.g., children with intermittent cholangitis). This opportunity to plan the transplant before a significant worsening of the child’s clinical status takes place may contribute to the excellent results of LRT.
The impact of the degree of graft steatosis on the incidence of primary dysfunction or PNF is debated. 32 This might be caused in part by the nonuniform classification systems for the degree of fatty change. Most of the authors used a four-category system that also was used here (none, mild [<30%], moderate [30–60%], severe [>60%]). Most authors agree that a graft with severe fatty change should either be omitted or used only with great caution because it poses a risk factor per se for primary dysfunction or PNF. 32 Our results show that even organs with moderate steatosis can be safely split. However, the risk of severe perfusion damage is especially evident in cadaveric split grafts with grade 2 or 3 fatty change. We and others 33 found no difference in graft and recipient survival for minimal and moderate fatty liver grafts compared with controls in LRT.
Our series also included organs with severe macrovacuolar fatty change (n = 12 [31%] in LRT, n = 15 [36%] in SLT). These most often showed moderate or severe perfusion damage, a finding reported by others. 34–36 According to Canelo et al, 37 the additional finding of necrosis in donor livers with severe fatty change could be an indicator for primary dysfunction or PNF. In our series PNF (n = 1 in LRT, n = 6 in SLT) was confined to grafts with moderate or severe perfusion damage. However, this was mostly (n = 4) observed in combination with a microvacuolar change, and only as an exception (n = 1) in combination with a macrovacuolar change. Despite those grafts with PNF, the successful use of grafts with even moderate or severe microvacuolar fatty change is shown here and has been emphasized by others as well. 22
Based on our experience, the SLT procedure itself carries no disadvantages to the recipient of the right graft. With our policy of maintaining the main vascular trunks with the right graft, the exchange of the right extended grafts between centers is readily feasible. Taking into account the demand for pediatric grafts and the pool of optimal cadaveric donors, it is therefore possible, from an epidemiologic point of view, to minimize the need for LRT.
In conclusion, pediatric liver transplantation represents 10% to 15% of all cases of liver transplantation in most countries; if it is confirmed that splitting can be applied to at least 20% of transplantation procedures, this technique could virtually abolish the graft shortage and waiting list deaths in children. Patient and graft survival rates after SLT and LRT do not differ significantly, and the rates of postoperative complications are comparable. This can be accomplished only by careful selection of the cadaveric donor for splitting and minimizing cold ischemic time. The comparable results shown in this study support the idea that in elective pediatric liver transplantation, there is no scientific reason to take any risk in using LRT, thereby alleviating the pressure on the potential living donor. LRT provides a solution for urgent situations in which a cadaveric graft cannot be found in time or if the choice of the optimal time point for transplantation is vital (“window of transplantation”).
To eliminate waiting list deaths, SLT and LRT are two solutions that go hand in hand. The choice of technique should be the result of a careful evaluation of the recipient’s condition, the donor’s potential risk factors, and the chances of obtaining an excellent cadaveric organ.
Acknowledgments
The work presented here is the result of the efforts of many people. Prof. Dr. C. E. Broelsch started the living donor program. The surgical procedures were performed by C. E. Broelsch, X. Rogiers, M. Malago, D. C. Broering, and M. Gundlach. The authors thank the nursing and anesthesiology staff of the University Hospital Eppendorf as well as the transplant coordinators, who play a vital role in the organization of split-liver transplantation.
Discussion
Prof. H. Bismuth: The title of your paper is really strong, but I agree with this idea that each time we can replace a living donor by a split cadaveric graft, it is a benefit. Even if the risk of mortality of a living donor is low, it cannot be lower – of course – than a cadaveric donor.
However, I must say that your data are not very convincing. Of course, it is not significant but in the split group, there are more PNF, more loss of graft, and even the curve of mortality is lower (but not significantly). Perhaps you may improve this comparison in favor of the split graft but looking at the reasons of the differences. It is clear, in your series, that for the split graft coming from a cadaveric donor, ischemic time is longer and reperfusion injury is greater, which surely impairs the results. If you consider in your series of split cadaveric grafts, the left lobe, harvested in situ, without important steatosis and without too long an ischemic time, you may improve the comparison between living and cadaveric graft results.
At that time, you will convince more people of the need to favor the split cadaver graft program. Currently, more and more living donor liver transplants are being performed, and it is a pity when we know that we may split 30% of the grafts. Which is much more than we need for children: in Europe, the number of pediatric liver transplants is about 10% of the total number of liver transplantations. Thus, we even have a margin to be more selective and to select the best organ for splitting; of course it is difficult to organize an effective organ-sharing program, but it is worth the effort if we can avoid the ethical issue of the living donor. Your paper is indeed very important to push into that direction.
For many years, we have thought of the liver anatomically and functionally as two livers. For transplantation purposes, we also have to admit that there are two livers. We have to consider the liver as a paired organ, like the kidneys, and if we succeed in convincing the transplant world that we have two livers, then we may consider that one cadaveric donor has a liver for two patients.
Prof. P. Neuhaus: I enjoyed the paper very much and agree with everything Prof. Bismuth has said. I would, however, like to point out the following. Prof. Broelsch, as part of your group, did living related pediatric transplantation a long time before you really started with ex situ and in situ liver splitting. I would like to know whether in your series there is a learning curve for split liver transplantation (for instance, regarding the bile duct complications). If the learning curve does not play a role, why, then, should bile duct complications occur more frequently in the living related group than in the split liver group?
I would appreciate it if you could provide more information on the high incidence of fatty changes in the liver. As we can select the most suitable donor of a big cadaveric organ pool for liver splitting, it would not be necessary to use compromised grafts. There would even be an advantage of cadaveric organs over a parent as living donor. Living donation with an overweight donor puts both mother and child at risk, which was obviously the case with your fatality in Hamburg. Shouldn’t it be our policy to use only excellent livers for pediatric cases, be it living or cadaveric donor?
How would you now inform the parents about living donation? Would you discourage them from living donation and tell them that the same results are possible with the use of cadaveric livers? Does the change in our allocation system influence at all your preference of cadaveric or living related transplants? In this regard, I do agree with Prof. Bismuth and with your conclusions that living donation for pediatric cases should really be indicated in the light of your excellent results with cadaveric split livers.
I congratulate you on this excellent and most important study.
Dr. D. C. Broering, Hamburg, Germany (closing) Thank you very much for your important comments and questions. I totally agree with Prof. Bismuth, that the main difference between living donor liver transplantation and split liver transplantation is the longer cold ischemic time.
Both groups consist only of left lateral grafts (segment s two and three according to C. Couinaud). Therefore, grafts in both groups are technically identical since the site of division of the hepatic artery, portal vein, hilar plate and hepatic vein are at the same level. The results of our in-situ split series show that the rate of primary non-function and graft and patient loss in the split group did not result from ex-situ splitting. Therefore, a comparison of only in-situ split and living donor liver transplantation would not significant ly change the conclusion.
Also, from our experience 30% of the cadaveric donor livers are suitable for splitting whereas we need only 10% to serve the whole pediatric population with split liver grafts. The remaining 20% of cadaveric livers could be split in full-right full-left grafts and transplanted in adult recipients, thus significantly enlarging the graft pool for adult recipients.
Prof. Neuhaus, whenever you start performing a new surgical technique you will pass through a learning curve: we experienced this in the first few years after the start of our living donor liver transplant program in 1991. The same was seen in the very early years of split liver transplantation. The routine split liver program at the University of Hamburg started in 1996 after adopting the surgical technique of harvesting the left lateral lobe from a living donor for left lateral splitting in a heart-beating donor. Therefore, for analysis of the underlying data a time interval of five years was chosen, in which all discussed techniques were well established and routinely used.
Biliary complications were more frequent in the living donor group compared with the split group. In statistical analysis this has shown not to be significantly different. Technically, there is also no reason why biliary complications should be more frequent after living donor liver transplantation.
The high percentage of fatty changes in the liver in both groups was also surprising for us. I absolutely agree that we should use only very good livers for pediatric recipients. In split liver transplantation we are faced with the problem that to date the donor liver can only be evaluated macroscopically by the splitting surgeon because of a lack of timely histological methods. Therefore, you cannot exclude underestimation of fatty changes in split liver transplantation.
In the living donor group nearly 50% of the grafts represent more than 30% fatty degeneration. This did not result in a disadvantage for the recipient since primary non-function was very rare in the LRT group. For left lateral lobe donation we do not perform routine liver biopsy but if the preoperative evaluation of the donor raises any signs of fatty changes in the liver, preoperative liver biopsy is recommended. A potential donor should be excluded from donation if the fatty degeneration is shown to be more than 30%.
All our parents are informed that we can achieve nearly the same results with a cadaveric graft as with grafts from living donors. The recommended option of transplantation depends very much on the time of referral to our institution. If the recipient’s condition is stable a split liver graft is recommended as the first choice to avoid the risk of the donor operation and living donation serves as a salvage therapy if the recipient’s status deteriorates. In consequence, the need for living donor liver transplantation depends very much on supply of appropriate cadaveric organs; the acceptance of split liver transplantation within the transplant community and, of course, the allocation system. The more livers allocated as potential split livers, the less need there is for living donor liver transplantation in children.
Footnotes
Correspondence: Dieter C. Broering, MD, University Hospital Eppendorf, Department of Hepatobiliary Surgery, Martinistr. 52, 20246 Hamburg, Germany.
E-mail: broering@uke.uni-hamburg.de
Accepted for publication April 2001.
References
- 1.Emond JC, Whitington PF, Thistlethwaite JR, et al. Reduced-size orthoptic liver transplantation: use in the management of children with chronic liver disease. Hepatology 1989; 5: 867–872. [DOI] [PubMed] [Google Scholar]
- 2.Rogiers X, Broering DC, Mueller L, et al. Living-donor liver transplantation in children. Langenbecks Arch Surg 1999; 384: 528–535. [DOI] [PubMed] [Google Scholar]
- 3.Malago M, Rogiers X, Broelsch CE. Reduced-size hepatic allografts. Ann Rev Med 1995; 46: 507–512. [DOI] [PubMed] [Google Scholar]
- 4.Gridelli B, Remuzzi G. Strategies for making more organs available for transplantation. N Engl J Med 2000; 343: 404–410. [DOI] [PubMed] [Google Scholar]
- 5.De Ville de Goyet J, Hausleithner V, Reding R, et al. Impact of innovative techniques on the waiting list and results in pediatric liver transplantation. Transplantation 1993; 56: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 6.Goss JA, Shackleton CR, McDiarmid SV, et al. Long-term results of pediatric liver transplantation: an analysis of 569 transplants. Ann Surg 1998; 228: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reding R, de Ville de Goyet J, Delbeke I, et al. Pediatric liver transplantation with cadaveric or living related donors: Comparative results in 90 elective recipients of primary grafts. J Pediatr 1999; 4: 280–286. [DOI] [PubMed] [Google Scholar]
- 8.Cronin DC II, Alonso EM, Piper JB, et al. Biliary complications in living donor liver transplantation. Transplant Proc 1997; 29: 419–420. [DOI] [PubMed] [Google Scholar]
- 9.Inomata Y, Tanaka K, Uemoto S, et al. Living donor liver transplantation: An 8-year experience with 379 consecutive cases. Transplant Proc 1999; 31: 38. [DOI] [PubMed] [Google Scholar]
- 10.Drews D, Sturm E, Latta A, et al. Complications following living-related and cadaveric liver transplantation in 100 children. Transplant Proc 1997; 29: 421–423. [DOI] [PubMed] [Google Scholar]
- 11.Rogiers X, Malago M, Gawad K, et al. In situ splitting of cadaveric livers. The ultimate expansion of a limited donor pool. Ann Surg 1996; 22: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houssin D, Boillot O, Soubrane O, et al. Controlled liver splitting for transplantation in two recipients: technique, results and perspectives. Br J Surg 1993; 80: 75–80. [DOI] [PubMed] [Google Scholar]
- 13.Spada M, Gridelli B, Colledan M, et al. Extensive use of split liver for pediatric liver transplantation: a single-center experience. Liver Transpl 2000; 6: 415–428. [DOI] [PubMed] [Google Scholar]
- 14.Rela M, Vougas V, Muiesan P, et al. Split liver transplantation, King’s College experience. Ann Surg 1998; 227: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busuttil RW, Goss JA. Split liver transplantation. Ann Surg 1999; 229: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azoulay D, Astarcioglu I, Bismuth H, et al. Split liver transplantation: the Paul Brousse Policy. Ann Surg 1996; 224: 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyes J, Gerber D, Mazariegos GV, et al. Split-liver transplantation: a comparison of ex vivo and in situ techniques. J Pediatr Surg 2000; 35: 283–290. [DOI] [PubMed] [Google Scholar]
- 18.Otte J.B. The availability of all technical modalities for pediatric liver transplant programs. Pediatr Transplant 2001; 5: 1–4. [DOI] [PubMed] [Google Scholar]
- 19.Couinaud C. Le foie. In: Etudes anatomique et chirurgicales. Paris: Masson; 1957.
- 20.Sterneck MR, Fischer L, Nischwitz U, et al. Selection of the living donor. Transplantation 1995; 60: 667–671. [DOI] [PubMed] [Google Scholar]
- 21.D’Alessandro AM, Kalayoglu M, Sollinger HW, et al. The predictive value of donor biopsies for the development of primary nonfunction after orthotopic liver transplantation. Transplantation 1991; 51: 157–163. [DOI] [PubMed] [Google Scholar]
- 22.Urena MAG, Moreno Gonzalez E, Romero CJ, et al. An approach to the rational use of steatotic donor livers in liver transplantation. Hepato-Gastroenterology 1999; 46: 1164–1173. [PubMed] [Google Scholar]
- 23.Malago M, Rogiers X, Burdelski M, Broelsch C. Living related liver transplantation: 36 cases at the University of Hamburg. Transplant Proc 1994; 26: 3620–3621. [PubMed] [Google Scholar]
- 24.Grewal HP, Thistlethwaite JR, Loss GE, et al. Complications in 100 living-liver donors. Ann Surg 1998; 228: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chardot C, Brancherau S, de Dreuzy O, et al. Paediatric liver transplantation with a split graft: experience at Bicetre. Eur J Pediatr Surg 1999; 9: 146–152. [DOI] [PubMed] [Google Scholar]
- 26.Migliazza L, Lopez Santamaria M, Murcia J, et al. Long-term survival expectancy after liver transplantation in children. J Pediatr Surg 2000; 35: 5–8. [PubMed] [Google Scholar]
- 27.Sindhi R, Rosendale J, Mundy D, et al. Impact of segmental grafts on pediatric liver transplantation: a review of the United Network for Organ Sharing Scientific Registry data (1990–1996). J Pediatr Surg 1999; 34: 107–111. [DOI] [PubMed] [Google Scholar]
- 28.Herijgers P, Leunens V, Tjandra Maga TB, et al. Changes in organ perfusion after brain death in the rat and its relation to circulating catecholamines. Transplantation 1996; 62: 330–335. [DOI] [PubMed] [Google Scholar]
- 29.van der Hoeven JAB, Ter Horst GJ, Molema G, et al. Effects of brain death and hemodynamic status on function and immunologic activation of the potential donor liver in the rat. Ann Surg 2000; 232: 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghobrial RM, Yersiz H, Farmer DG, et al. Predictors of survival after in vivo split liver transplantation. Ann Surg 2000; 232: 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burdelski M, Nolkemper D, Ganschow R, et al. Liver transplantation in children: long-term outcome and quality of life. Eur J Pediatr 1999; 158: 34–42. [DOI] [PubMed] [Google Scholar]
- 32.Strasberg SM, Howard TK, Molmenti EP, et al. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology 1994; 20: 829–838. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi M, Kuji K, Uryuhara K, et al. Effects of fatty infiltration of the graft on the outcome of living related liver transplantation. Transplant Proc 1999; 31: 403. [DOI] [PubMed] [Google Scholar]
- 34.Adam R, Reynes M, Johann M, et al. The outcome of steatotic grafts in liver transplantation. Transplant Proc 1991; 23: 1538–1540. [PubMed] [Google Scholar]
- 35.Karayalcin K, Mirza DF, Harrison RF, et al. The role of dynamic and morphologic studies in the assessment of potential liver donors. Transplantation 1994; 57: 1325–1327. [DOI] [PubMed] [Google Scholar]
- 36.Kuo PC, Drachenberg CI, de la Torre A, et al. Apoptosis and hepatic allograft reperfusion injury. Clin Transplant 1998; 12: 219–223. [PubMed] [Google Scholar]
- 37.Canelo R, Braun F, Sattler B, et al. Is a fatty liver dangerous for transplantation? Transplant Proc 1999; 31: 414–415. [DOI] [PubMed] [Google Scholar]