Abstract
Objective
To reappraise the results of auxiliary partial orthotopic liver transplantation (APOLT) compared with those of standard whole-liver transplantation (OLT) in terms of postoperative death and complications, including neurologic sequelae.
Summary Background Data
Compared with OLT, APOLT preserves the possibility for the native liver to recover, and to stop immunosuppression.
Methods
In a consecutive series of 49 patients transplanted for fulminant or subfulminant hepatitis, 37 received OLT and 12 received APOLT. APOLT was done when logistics allowed simultaneous performance of graft preparation and the native liver partial hepatectomy to revascularize the graft as soon as possible. Each patient undergoing APOLT (12 patients) was matched to two patients undergoing OLT (24 patients) according to age, grade of coma, etiology, and fulminant or subfulminant type of hepatitis. All grafts in the study population were retrieved from optimal donors.
Results
Before surgery, both groups were comparable in all aspects. In-hospital death occurred in 4 of 12 patients undergoing APOLT compared with 6 of 24 patients undergoing OLT. Patients receiving APOLT had 1 ± 1.3 technical complications compared with 0.3 ± 0.5 for OLT patients. Bacteriemia was significantly more frequent after APOLT than after OLT. The need for retransplantation was significantly higher in the APOLT patients (3/12 vs. 0/24). Brain death from brain edema or neurologic sequelae was significantly more frequent after APOLT (4/12 vs. 2/24). One-year patient survival was comparable in both groups (66% vs. 66%), and there was a trend toward lower 1-year retransplantation-free survival rates in the APOLT group (39% vs. 66%). Only 2 of 12 (17%) patients had full success with APOLT (i.e., patient survival, liver regeneration, withdrawal of immunosuppression, and graft removal). One of these two patients had neurologic sequelae.
Conclusions
Using optimal grafts, APOLT and OLT have similar patient survival rates. However, the complication rate is higher with APOLT. On an intent-to-treat basis, the efficacy of the APOLT procedure is low. This analysis suggests that the indications for an APOLT procedure should be reconsidered in the light of the risks of technical complications and neurologic sequelae.
Orthotopic liver transplantation (OLT) including removal of the whole native liver was a breakthrough in the treatment of fulminant and subfulminant hepatitis. 1 This was obtained at the price of lifelong immunosuppression and the associated long-term risks. The auxiliary liver transplantation procedure in acute liver failure preserves part of the native liver, leading to the possibility for the latter to regenerate, allowing graft removal or atrophy and cessation of immunosuppression. The first case of heterotopic liver transplantation for fulminant hepatitis was performed by Bismuth in 1980. 2 Although the liver function recovered, the patient died of intractable acute rejection 22 days after the transplant. The first case of auxiliary partial orthotopic liver transplantation (APOLT) was reported in 1985 by Bismuth et al 3; it was performed for chronic liver disease. Gubernatis et al 4 reported the first success of APOLT for fulminant hepatitis in 1991. The 33-year-old patient, who had hemolysis, elevated liver enzymes, and low platelet count syndrome, underwent a left lobectomy and the orthotopic transplantation of a left liver graft. Full native liver regeneration allowed the withdrawal of immunosuppression, and the patient returned to a normal life without sequelae. Several centers, including ours, have reported their experience of APOLT ranging from one to eight cases. 5–12
Outcomes of the APOLT procedure are reported as comparable to those of standard OLT. However, these comparisons are rare and focused on patient survival. In addition, these studies lack much data and are biased according to even their authors. 13
Although the concept of APOLT for fulminant and subfulminant hepatitis appears to be accepted and beyond the scope of the indications and contraindications of the procedure, several surgical issues remain to be addressed compared with OLT, including the price to pay in terms of death and complications. This was the goal of the current study. To improve the comparison, we performed a case-control study: each patient transplanted with APOLT was matched to two patients transplanted with OLT. Patients were matched on the basis of age, grade of coma at transplant, and the subtype (fulminant or subfulminant) of hepatitis. Complications and patient and retransplantation-free survival rates after APOLT were compared with those of matched controls.
PATIENTS AND METHODS
Study Population
From March 1993 to March 2000, 75 consecutive patients were referred to our center for fulminant or subfulminant hepatitis (defined as acute hepatitis complicated by acute liver failure and hepatic encephalopathy occurring less than 2 weeks and between 2 weeks and 12 weeks, respectively, after the onset of jaundice 14). Encephalopathy was classified into four stages according to Trey and Davidson’s 15 classification. Patients were admitted to the intensive care unit and managed according to a protocol detailed elsewhere. 16 The criteria for liver transplantation were the presence of encephalopathy (stage 3 or 4) associated either with a factor V level less than 20% of normal in a patient younger than 30 years of age, or with a factor V level less than 30% of normal in a patient older than 30. 16,17 As soon as the decision to transplant was made, patients were listed on the “super-emergency” list of the French Organ Sharing Organization, giving them absolute priority for available donor livers.
Of the 75 patients, 58 were listed for urgent transplantation and 17 did not meet the criteria for urgent transplantation. Of the latter patients, 12 improved spontaneously and 5 died. Of the 58 listed patients, 7 died of brain edema before a donor could be found and 2 improved spontaneously. Forty-nine patients were transplanted. Seven patients receiving a steatotic graft (n = 4) or transplanted for acute Wilson’s disease (n = 3) were transplanted with OLT because of the risk of a reduced graft and the absence of a chance of native liver regeneration, respectively. Forty-two patients were transplanted with a graft from an optimal donor (defined as aged younger than 50 years, hemodynamically stable, with normal liver function tests, and no macroscopic aspect of liver steatosis). These 42 patients underwent OLT or APOLT in 30 and 12 cases, respectively. APOLT was performed when logistics allowed us to perform simultaneously the graft preparation and the partial native liver hepatectomy in the recipient to revascularize the graft as soon as possible. Each patient undergoing APOLT was matched to two patients undergoing OLT according to age, grade of coma, etiology, and type (fulminant or subfulminant) of hepatitis. The patients were matched anonymously without knowledge of their postoperative course. The 12 patients transplanted with APOLT and the 24 matched patients transplanted with OLT represent the study population.
Surgical Procedures
We performed OLT according to the technique reported elsewhere. In brief, the native liver was totally removed with caval preservation 18 and temporary portacaval shunt. 19,20 The whole-liver graft was then implanted.
The APOLT technique has been reported in detail elsewhere. 12 In brief, a partial hepatectomy was performed on the native liver while a second team was simultaneously preparing the graft. The graft was a reduced-size graft in 10 patients, a split graft in 1 patient, and a graft from a living donor in 1 patient (Fig. 1). A right (segments 4–8) or left (segments 1–4) graft was prepared when a right hepatectomy (segment 5–8) or left lobectomy (segments 2 and 3) was performed, respectively, on the native liver. Eight patients received a right graft and four patients received a left graft. After our initial experience of two right and three left grafts, 12 we favored the use of a right graft to provide the largest graft possible.
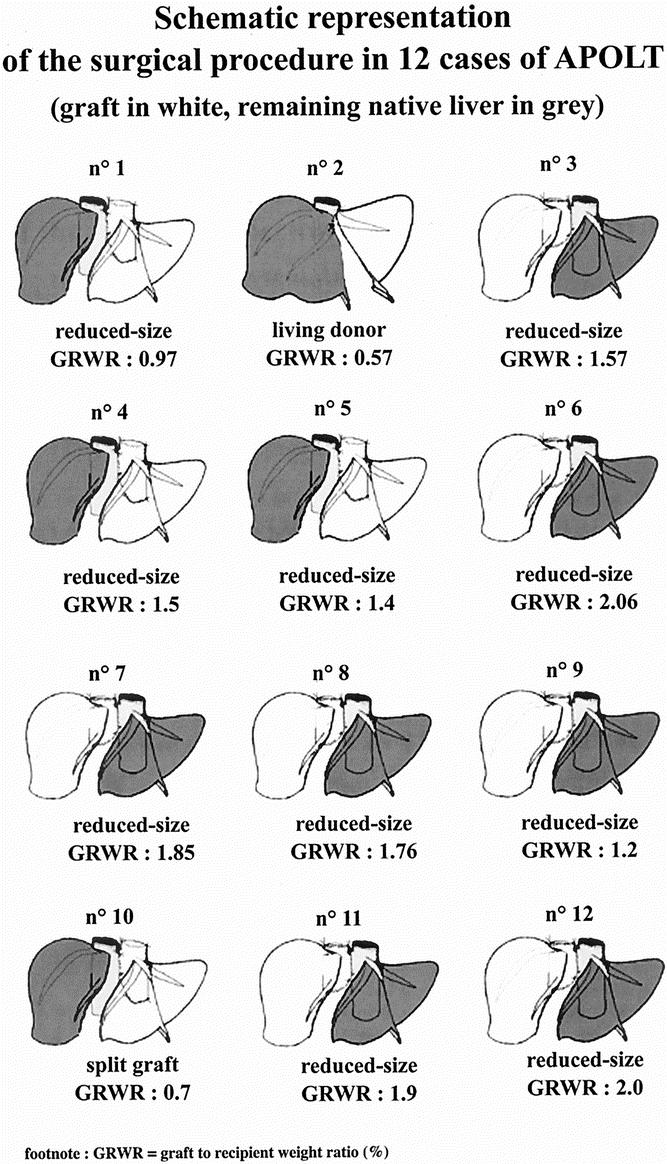
Figure 1. Schematic representation of the surgical procedure in 12 cases of APOLT (graft in white, remaining native liver in grey).
From the beginning of our experience, it was decided that in case of retransplantation, we would perform a standard OLT procedure.
Postoperative Management
After surgery, patients received a standard immunosuppression regimen of tacrolimus and methylprednisolone. In addition to standard graft function monitoring, the APOLT patients underwent serial computed tomography scan volumetry and hepato-iminodiacetic acid (HIDA) scintigraphy and native and graft histology as already reported. 12 Autopsy was performed in all patients who died in the hospital after transplantation.
Statistical Analysis
Continuous variables in recipients included age (years), pretransplant levels of factor V (% of normal level) and prothrombin (% of normal level), bilirubin (μmol/L), creatinine (μmol/L), alanine aminotransferase (IU/L), and aspartate aminotransferase (IU/L). Categorical variables included sex, stage of encephalopathy, grade of coma, and simplified acute physiologic score (SAPS II) according to Le Gall et al. 21 This physiologic score has been shown to be easier to perform and as accurate as the Acute Physiology and Chronic Health Evaluation score (APACHE III score). 22
For donors and grafts, continuous variables included age, preharvest levels of bilirubin, alanine aminotransferase, gamma glutamyl transpeptidase (IU/L), and the graft–recipient body weight ratio (%). Categorical variables included sex and type of transplant (OLT vs. APOLT). Other factors potentially associated with outcome were assessed, including cold ischemia time and duration of operation (minutes) and the number of units of packed red cells transfused during transplantation. Postoperative complications considered to be related to surgical technique, whatever the delay of onset, were classified into the following categories: postoperative hemorrhage needing reoperation, biliary, arterial, portal, or other. Infectious complications were classified as one or more episodes of bacteriemia, one or more episodes of fungemia, and cytomegalovirus infection.
Survival rates were calculated using the Kaplan-Meier method, and groups were compared with the log-rank test. P ≤ .05 was considered statistically significant. All statistical analyses were performed using the StatView 5.5 software (Abacus Concepts, Inc., Berkeley, CA).
Data from the European Liver Transplant Registry (ELTR) were retrieved from the ELTR. 23 Patient survival and retransplantation-free survival were calculated for 1,622 cases of OLT and 52 cases of APOLT performed for acute liver failure from January 1991 to January 2000.
RESULTS
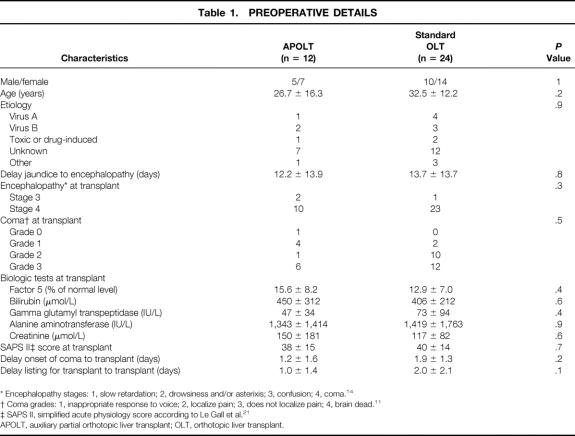
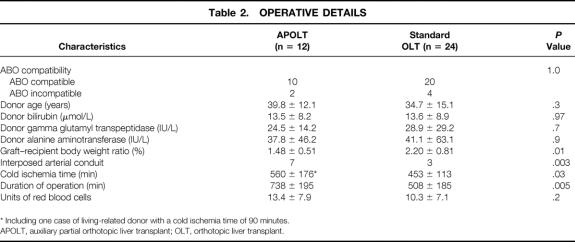
As shown in Table 1, recipients of APOLT or OLT were comparable at the time of transplant including the delay of jaundice to the onset of encephalopathy, the stage of encephalopathy, the grade of coma, the SAPS II score, the waiting time for the graft, and the values of liver and kidney function tests. Donors were comparable in both groups, including ABO blood group compatibility with the corresponding recipient, age, and liver function tests (Table 2).
Table 1. PREOPERATIVE DETAILS
* Encephalopathy stages: 1, slow retardation; 2, drowsiness and/or asterixis; 3, confusion; 4, coma. 14
† Coma grades: 1, inappropriate response to voice; 2, localize pain; 3, does not localize pain; 4, brain dead. 11
‡ SAPS II, simplified acute physiology score according to Le Gall et al. 21
APOLT, auxiliary partial orthotopic liver transplant; OLT, orthotopic liver transplant.
Table 2. OPERATIVE DETAILS
* Including one case of living-related donor with a cold ischemia time of 90 minutes.
APOLT, auxiliary partial orthotopic liver transplant; OLT, orthotopic liver transplant.
The graft–recipient body weight ratio was significantly lower for patients receiving APOLT versus those receiving OLT (1.48% ± 0.51% vs. 2.2% ± 0.8%, P = .01). Cold ischemia time (453 ± 113 vs. 560 ± 176 minutes, P = .03) and total duration of procedure (508 ± 185 vs. 738 ± 195 minutes, P = .005) were significantly longer in the APOLT group versus the OLT group. An interposed arterial conduit to the infrarenal aorta was significantly more frequently needed for the APOLT group (7/12 vs. 3/24, P = .003). The transfusion need tended to be higher in the APOLT group, but this did not reach statistical significance (13.4 ± 7.9 vs. 10.3 ± 7.1 blood units, P = .2). Indeed, 8 of the 12 (67%) APOLT patients received more than 10 blood units, whereas 7 of the 24 (29%) OLT patients received more than 10 blood units (P = .03).
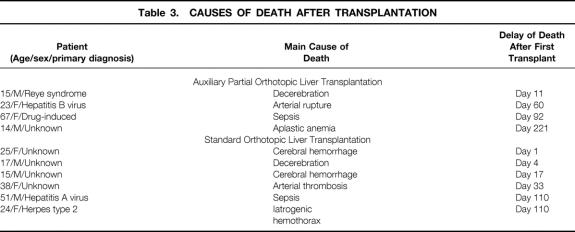
In-hospital death occurred in 4 of 12 patients (33%) with a median delay of 71 days (range 11–221) after APOLT versus 6 of 24 patients (25%, P = .6) with a median delay of 25 days (range 1–110) after OLT. Table 3 shows the main causes of death of these patients.
Table 3. CAUSES OF DEATH AFTER TRANSPLANTATION
Table 4 shows the postoperative complications that occurred in both groups. Seven technical complications occurred in 7 of the 24 patients in the OLT group; 12 technical complications occurred in 6 of the 12 patients in the APOLT group. The number of technical complications per patient was significantly higher in the APOLT group versus the OLT group (1.0 ± 1.3 vs. 0.3 ± 0.5, P = .02). This was mainly due to a higher incidence of biliary complications after APOLT (5 cases vs. 1 case for OLT patients, P = .004). In the OLT group, there was one instance of biliary leakage after biliary drain removal treated successfully by laparoscopy. In the APOLT group, there were two instances of biliary leak from the cut surface of the graft treated successfully by percutaneous drainage, one instance of stricture of the biliary–jejunal anastomosis treated by percutaneous drainage left in place until death from aplastic anemia, and two instances of intrahepatic cholangitis in the context of ABO-incompatible transplantation.
Table 4. POSTOPERATIVE COMPLICATIONS
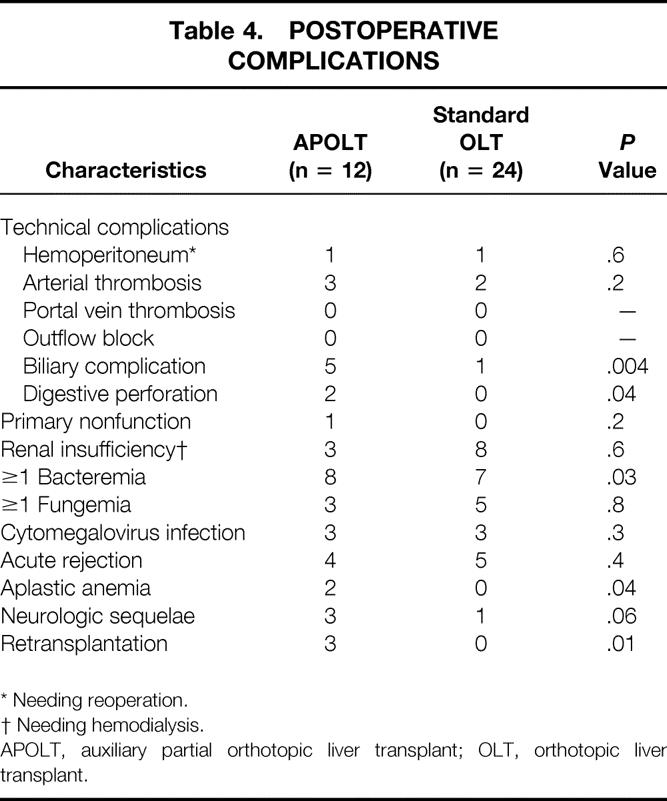
* Needing reoperation.
† Needing hemodialysis.
APOLT, auxiliary partial orthotopic liver transplant; OLT, orthotopic liver transplant.
No instance of primary nonfunction occurred in the OLT group; one instance occurred in the APOLT group (P = .2). The latter patient underwent urgent retransplantation and is alive and well 2.5 years later.
There was a significantly higher incidence of bacteria-positive blood cultures in the APOLT group (8/12 [67%] vs. 7/24 [29%] for the OLT group, P = .03). The incidence of fungemia and cytomegalovirus infection was comparable in both groups.
Two patients transplanted with OLT had fatal intracerebral bleeding from misplacement of intracranial pressure monitoring probes (see Table 3). In addition, brain death from brain edema occurred in one patient per group. Neurologic sequelae persisted in 3 of the 12 patients in the APOLT group compared with 1 of the 24 patients in the OLT group (P = .06). Thus, brain death from brain edema or neurologic sequelae were more frequent after APOLT compared with OLT (4/12 vs. 2/24, P = .05).
The need for retransplantation was significantly greater in the APOLT group (3/12 vs. 0/24 for OLT patients, P = .01). Retransplantation was indicated for primary nonfunction (n = 1), arterial thrombosis (n = 1), and intrahepatic cholangitis after ABO-incompatible transplantation (n = 1).
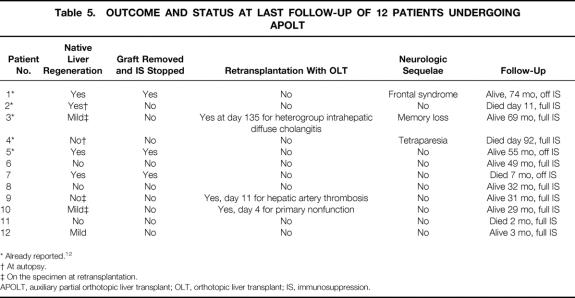
As shown in Table 5, native liver regeneration occurred in 7 of the 12 patients who received APOLT. However, this regeneration was found in one patient at autopsy and in two patients at retransplantation. One patient with mild regeneration is alive 9 months after APOLT with full immunosuppression. Only three of seven patients who had liver regeneration had withdrawal of immunosuppression and graft removal. One of the latter died of aplastic anemia and sepsis 1 month after detransplantation; two are alive and well 69 months and 68 months after graft removal. In summary, 2 of 12 patients had full success with APOLT (i.e., patient survival, liver regeneration, withdrawal of immunosuppression, and graft removal). One patient is doing well with full rehabilitation, whereas one is alive with normal liver function but with neurologic sequelae, requiring external support for some activities of daily living.
Table 5. OUTCOME AND STATUS AT LAST FOLLOW-UP OF 12 PATIENTS UNDERGOING APOLT
* Already reported. 12
† At autopsy.
‡ On the specimen at retransplantation.
APOLT, auxiliary partial orthotopic liver transplant; OLT, orthotopic liver transplant; IS, immunosuppression.
Only 1 of the 24 patients transplanted with OLT had neurologic sequelae.
The 1-year actuarial patient survival rate was 66% after OLT and 66% after APOLT (P = .9, log-rank). The 1-year actuarial retransplantation-free survival rate was lower after APOLT versus OLT (39% vs. 66%), but this difference did not reach statistical significance (P = .1, log-rank) (Fig 2).
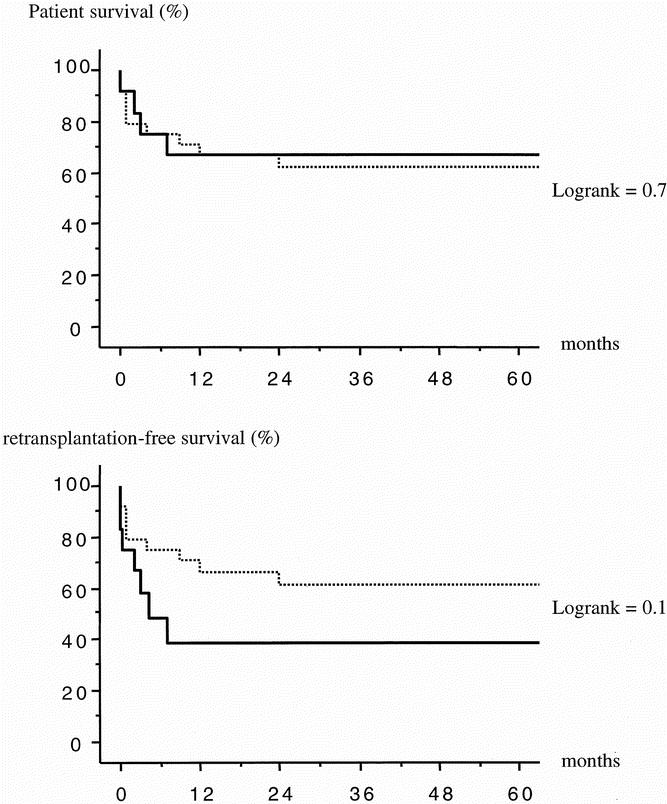
Figure 2. Actuarial patient survival (above) and retransplantation-free survival (below) of 36 patients transplanted with APOLT (12 cases, full line) or OLT (24 cases, interrupted line).
In the European Liver Transplant Registry, actuarial patient survival rates were significantly lower after APOLT versus OLT: 52%, 49%, and 49% versus 69%, 65%, and 63% at 1, 3, and 5 years, respectively (P = .03, log-rank). Actuarial retransplantation-free survival rates were also significantly lower after APOLT versus OLT: 42%, 42%, and 42% versus 62%, 58%, and 56% at 1, 3, and 5 years, respectively (P = .01, log-rank).
DISCUSSION
Comparable patients transplanted with either APOLT or OLT have comparable patient survival rates. The retransplantation-free survival was lower after APOLT versus OLT (39% vs. 66%, P = .1, log-rank). The complication rate (1.0 ± 1.3 vs. 0.3 ± 0.5 technical complications per patient, respectively, P = .02) and the need for retransplantation (3/12 vs. 0/24 patients, respectively, P = .01) were higher after APOLT versus OLT. The graft could be removed and immunosuppression withdrawn in 3 of the 12 patients transplanted with APOLT.
Analysis of Survival Rates
The survival rates in our series are comparable to those of other limited series. 9,13 Sudan et al 9 reported a 1-year actuarial patient survival rate of 82% for 11 cases of OLT versus 57% for 7 cases of APOLT. This trend toward better survival after OLT did not reach statistical significance. The European Registry of Auxiliary Liver Transplantation compared 35 APOLT procedures performed in 12 centers to 384 OLT procedures performed in the region of Eurotransplant (including five countries) from 1986 to 1995. 13 Patient survival rates at 1 year were comparable in both groups: 61% for OLT versus 71% for APOLT. There was no significant difference in the retransplantation-free survival rate at 1 year: 52% for OLT versus 60% for APOLT. These comparable results from single-center and multicentric series might be due to small sample size. Indeed, the European Liver Transplant Registry, 23 providing samples of larger size, showed significantly lower patient and retransplantation-free survival rates for APOLT compared to OLT: 52% versus 69% (P = .03) and 42% versus 62% (P = .01) at 1 year, respectively.
Analysis of Complications
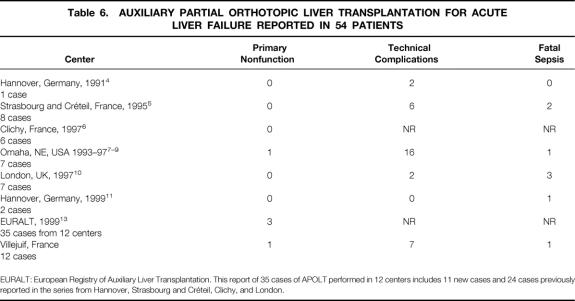
The incidence of primary nonfunction after APOLT seems higher compared with OLT. This occurred in 1 of 12 cases of APOLT and 0 of 24 cases of OLT in the present series. Even if this did not reach statistical significance, the incidence of primary nonfunction in the EURALT report was 8.5% after APOLT versus 5.5% after OLT 13 (Table 6).
Table 6. AUXILIARY PARTIAL ORTHOTOPIC LIVER TRANSPLANTATION FOR ACUTE LIVER FAILURE REPORTED IN 54 PATIENTS
EURALT: European Registry of Auxiliary Liver Transplantation. This report of 35 cases of APOLT performed in 12 centers includes 11 new cases and 24 cases previously reported in the series from Hannover, Strasbourg and Créteil, Clichy, and London.
Biliary complications occurred in 5 of 12 (42%) cases of APOLT versus 1 of 24 (4%) cases of OLT. This significantly higher incidence of biliary complications after APOLT is due to three causes: the use of ABO-incompatible grafts in some patients, biliary leak from the raw surfaces (native liver and/or graft), and biliary stenosis. In the series reported by Sudan et al, 9 biliary complications occurred in 4 of 7 cases of APOLT. This is more than for reduced-size liver (5–13% in reduced-size liver grafts 24–28) and split-liver transplantation (0–25% of recent series of split liver transplants. 29,30
In our series, the rates of vascular complications were comparable in both groups. However, the APOLT procedure is associated with a higher incidence of vascular complications. In the EURALT report, 13 the incidence of portal vein thrombosis was significantly higher after APOLT than after OLT (5/35 APOLT [14%] vs. 2/384 OLT [0.5%], P < .001). In the same report there was a trend toward a higher rate of arterial thrombosis after APOLT (2/35 APOLT procedures [6%] vs. 7/384 OLT procedures [2%], P = .2); however, this did not reach statistical significance.
The overall rate of infectious complication with APOLT or OLT seem comparable. 31 Our series showed a significant increase in bacterial complications after APOLT. This may be due to the smaller size of the graft compared with OLT and also to the longer duration of surgery. 32
Neurologic sequelae have rarely been reported after spontaneous recovery from acute liver failure before the era of liver transplantation. 33 Neurologic sequelae have been observed after OLT in patients with acute liver failure, 1,16 the main mechanism being the late recovery of the liver function by the graft at a time when cerebral lesions have occurred. The technique of transplantation itself may play a role. We observed significantly more neurologic sequelae after APOLT versus OLT. This phenomenon might be multifactorial, including the limited mass of graft compared with full-size liver, the longer ischemia time of the graft, and the hypothetic influence of the necrotic remaining native liver.
Analysis of the Benefit of APOLT
Considering the double goal of APOLT for fulminant hepatic failure (i.e., full native liver regeneration and discontinuation of immunosuppression), the results of our series may be summarized as follows: only 3 of 12 (25%) APOLT patients had native liver regeneration allowing withdrawal of immunosuppression. One of the latter died 1 month after detransplantation, one is alive with neurologic sequelae, and only one is alive with full rehabilitation. This result was obtained at the price of a higher complication rate, including a higher rate of retransplantation. In the EURALT report, 13 at 1 year, 12 of 35 patients (34%) were alive without retransplantation and without immunosuppression (including 8 patients with graft removal), 10 (29%) were alive without retransplantation and with full immunosuppression, 3 (8%) were alive after retransplantation, 10 (29%) died without retransplantation (n = 8) or after retransplantation (n = 2).
The main advantage of APOLT over OLT—that is, the potential for withdrawal of immunosuppression—was already questioned when we reported our initial experience of APOLT in this journal. 34 It was already hypothesized that the development of new immunosuppressive drugs would make the rationale for APOLT obsolete. Indeed, since this report, several authors have reported the possibility of immunosuppression withdrawal in long-term survivors of OLT. 35–38
Unlike standard OLT, APOLT does not rule out the potential regeneration of the native liver after transplantation for fulminant hepatic failure. It therefore aims at reducing the incidence of drug-related side effects and neoplasia. Considering the present data, the goal of APOLT is rarely achieved. In addition, the price to pay in terms of complications is high. The correlation we found between neurologic complications and APOLT needs to be confirmed by further studies. Meanwhile, we consider that APOLT should have a limited place in the treatment of acute liver failure.
Footnotes
Correspondence: Daniel Azoulay, MD, Centre Hépato-Biliaire, Hôpital Paul Brousse, 94804, Villejuif, France.
E-mail: daniel.azoulay@pbr.ap-hop-paris.fr
Accepted for publication April 2001.
References
- 1.Bismuth H, Samuel D, Gugenheim J, et al. Emergency liver transplantation for fulminant hepatitis. Ann Intern Med 1987; 107: 337–341. [DOI] [PubMed] [Google Scholar]
- 2.Le Bihan G, Coquerel A, Houssin D, et al. Insuffisance hépatique aigue mortelle au cours d un traitement par le valproate de sodium. Gastroentérol Clin Biol 1982; 6: 477–481. [PubMed] [Google Scholar]
- 3.Bismuth H, Houssin D. Partial resection of liver grafts for orthotopic or heterotopic liver transplantation. Transplant Proc 1985; 17: 279–283. [Google Scholar]
- 4.Gubernatis G, Pichlmayr R, Kemnitz J, et al. Auxiliary partial orthotopic liver transplantation (APOLT) for fulminant hepatic failure: first successful case report. World J Surg 1991; 15: 660–666. [DOI] [PubMed] [Google Scholar]
- 5.Boudjema K, Cherqui D, Jaeck D, et al. Auxiliary liver transplantation for fulminant and subfulminant hepatic failure. Transplantation 1995; 59: 218–223. [PubMed] [Google Scholar]
- 6.Buyck D, Bonnin F, Bernuau J, et al. Auxiliary liver transplantation in patients with fulminant hepatic failure: hepatobiliary scintigraphic follow-up. Eur J Nucl Med 1997; 24: 138–142. [DOI] [PubMed] [Google Scholar]
- 7.Shaw BH Jr, Cattral M, Langnas AN, et al. Orthotopic auxiliary liver transplantation: the treatment of choice for acute liver failure? [abstract] Hepatology 1993; 18: 66A.8325623 [Google Scholar]
- 8.Shaw BW. Auxiliary liver transplantation for acute liver failure. Liver Transplant Surg 1995; 1: 194–200. [DOI] [PubMed] [Google Scholar]
- 9.Sudan DL, Shaw BW Jr, Fox IJ, et al. Long-term follow-up of auxiliary orthotopic liver transplantation for the treatment of fulminant hepatic failure. Arch Surg 1997; 122: 771–778. [DOI] [PubMed] [Google Scholar]
- 10.Pereira S, McCarthy M, Ellis AJ, et al. Auxiliary partial orthotopic liver transplantation for acute liver failure. J Hepatol 1997; 26: 1010–1017. [DOI] [PubMed] [Google Scholar]
- 11.Rodeck B, Kardorff R, Melter M, et al. Auxiliary partial orthotopic liver transplantation for acute liver failure in two children. Pediatr Transplant 1999; 3: 328–332. [DOI] [PubMed] [Google Scholar]
- 12.Bismuth H, Azoulay D, Samuel D, et al. Auxiliary partial orthotopic liver transplantation for fulminant hepatitis. The Paul Brousse experience. Ann Surg 1996; 224: 712–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Hoek B, De Boer J, Boudjema K, et al. Auxiliary versus orthotopic liver transplantation for acute liver failure. J Hepatol 1999; 30: 699–705. [DOI] [PubMed] [Google Scholar]
- 14.Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: definition and causes. Semin Liv Dis 1986; 6: 97–106. [DOI] [PubMed] [Google Scholar]
- 15.Trey C, Davidson CS. The management of fulminant hepatic failure. In: Popper H, Shaffner F, eds. Progress in liver diseases: The management of fulminant hepatic failure. New York and London: Grune & Stratton; 1970: 282–298. [PubMed]
- 16.Bismuth H, Samuel D, Castaing D, et al. Orthotopic liver transplantation in fulminant and subfulminant hepatitis. The Paul Brousse experience. Ann Surg 1995; 222: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernuau J, Samuel D, Durand F, et al. Criteria for emergency liver transplantation in patients with acute viral hepatitis and factor V below 50% of normal: a prospective study. Hepatology 1991; 14: 49A. [Google Scholar]
- 18.Calne RY, William R. Transplantation in man. I Observations on technique and organization in five cases. Br Med J 1968; 4: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belghiti J, Panis Y, Sauvanet A, et al. A new technique of side to side caval anastomosis during orthotopic hepatic transplantation without inferior vena caval occlusion. Surg Gynecol Obstet 1992; 175: 271–272. [PubMed] [Google Scholar]
- 20.Cherqui D, Lauzet JY, Rotman N, et al. Orthotopic liver transplantation with preservation of the caval and portal flows. Technique and results in 62 cases. Transplantation 1994; 58: 793–796. [PubMed] [Google Scholar]
- 21.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on European/North American multicenter study. JAMA 1993; 270: 2957–2963. [DOI] [PubMed] [Google Scholar]
- 22.Lemeshow S, Le Gall JR. Modeling the severity of illness of ICU patients. A system update. JAMA 1994; 272: 1049–1055. [PubMed] [Google Scholar]
- 23.European Liver Transplant Registry: www.eltr.org
- 24.Busuttil RW, Seu PH, Millis JM, et al. Liver transplantation in children. Ann Surg 1991; 213: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houssin D, Soubrane O, Boillot O, et al. Orthotopic liver transplantation with a reduced-size graft: An ideal compromise in pediatrics? Surgery 1992; 111: 532–542. [PubMed] [Google Scholar]
- 26.Valayer J, Gauthier F, Yandza T, et al. Chirurgie de la transplantation hépatique chez l’enfant. Pédiatrie 1993; 48: 139–141. [PubMed] [Google Scholar]
- 27.De Ville De Goyet J, Hausleithner V, Reding R, et al. Impact of innovative techniques on the waiting list and results in pediatric liver transplantation. Transplantation 1993; 5: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 28.Greif F, Bronsther OL, Van Thiel DH, et al. The incidence, timing and management of biliary tract complications after orthotopic liver transplantation. Ann Surg 1994; 219: 40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azoulay D, Astarcioglu I, Bismuth H, et al. The split liver transplantation: the Paul Brousse policy. Ann Surg 1996; 224: 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bisuttil RW, Goss JA. Split liver transplantation. Review. Ann Surg 1999; 229: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolando N, Harvey F, Brams J, et al. Prospective study of bacterial infection in acute liver failure: an analysis of fifty patients. Hepatology 1990; 11: 49–53. [DOI] [PubMed] [Google Scholar]
- 32.Paya CV, Wiesner RH, Hermans PE, et al. Risk factors for cytomegalovirus and severe bacterial infections following liver transplantation: a prospective multivariate time-dependent analysis. J Hepatol 1993; 18: 185–195. [DOI] [PubMed] [Google Scholar]
- 33.Karvountzis GG, Redeker AG, Peters RL. Long-term follow-up studies of patients surviving fulminant viral hepatitis. Gastroenterology 1974; 67: 870–877. [PubMed] [Google Scholar]
- 34.Broelsch CH. Discussion of Bismuth H, Azoulay D, Samuel D, et al. Auxiliary partial orthotopic liver transplantation for fulminant hepatitis. The Paul Brousse experience. Ann Surg 1996; 224: 712–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazariegos GV, Reyes J, Marino IR, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation 1997; 63: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delvin J, Doherty D, Thomson L, et al. Defining the outcome of immunosuppression withdrawal after liver transplantation. Hepatology 1998; 27: 926–933. [DOI] [PubMed] [Google Scholar]
- 37.Riordan SM, Williams R. Tolerance after liver transplantation: does it exist and can immunosuppression be withdrawn ? J Hepatol 1999; 31: 1106–1119. [DOI] [PubMed] [Google Scholar]
- 38.Jain A, Reyes J, Kashyap R, et al. Long-term survival after liver transplantation in 4000 consecutive patients at a single center. Ann Surg 2000; 232: 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]