Abstract
Objective
To assess the influence of resection margins on survival for patients with resected pancreatic cancer treated within the context of the adjuvant European Study Group for Pancreatic Cancer-1 (ESPAC-1) study.
Summary Background Data
Pancreatic cancer is associated with a poor long-term survival rate of only 10% to 15% after resection. Patients with positive microscopic resection margins (R1) have a worse survival, but it is not known how they fare in adjuvant studies.
Methods
ESPAC-1, the largest randomized adjuvant study of resectable pancreatic cancer ever performed, set out to look at the roles of chemoradiation and chemotherapy. Randomization was stratified prospectively by resection margin status.
Results
Of 541 patients with a median follow-up of 10 months, 101 (19%) had R1 resections. Resection margin status was confirmed as an influential prognostic factor, with a median survival of 10.9 months for R1 versus 16.9 months months for patients with R0 margins. Resection margin status remained an independent factor in a Cox proportional hazards model only in the absence of tumor grade and nodal status. There was a survival benefit for chemotherapy but not chemoradiation, irrespective of R0/R1 status. The median survival was 19.7 months with chemotherapy versus 14.0 months without. For patients with R0 margins, chemotherapy produced longer survival compared with to no chemotherapy. This difference was less apparent for the smaller subgroup of R1 patients, but there was no significant heterogeneity between the R0 and R1 groups.
Conclusions
Resection margin-positive pancreatic tumors represent a biologically more aggressive cancer; these patients benefit from resection and adjuvant chemotherapy but not chemoradiation. The magnitude of benefit for chemotherapy treatment is reduced for patients with R1 margins versus those with R0 margins. Patients with R1 tumors should be included in future trials of adjuvant treatments and randomization and analysis should be stratified by this significant prognostic factor.
Pancreatic ductal adenocarcinoma is one of the top 10 causes of cancer death in the Western world and is responsible for approximately 40,000 deaths per year in Europe 1,2 and 28,000 deaths per year in the United States. 2,3 Great improvements in immediate surgical outcome have been shown to be directly related to greater centralization and increased volume case load in these centers. 4–6 Approximately 10% to 15% of patients undergo potentially curative surgery, but the median survival is only 10 to 18 months, with 5-year survival rates of 17% to 24%. 7–12 Survival beyond 5 years is rare. 7,13 Improvements in long-term outcome have not been observed with the use of procedures more radical than a standard Kausch-Whipple pancreatoduodenectomy. 14,15 There is, however, increasing interest in the potentially more promising use of various forms of adjuvant and neoadjuvant treatment. 10,16–26 To optimize the use of these additional forms of treatment, an understanding of prognostic variables is necessary.
The prognosis for patients who undergo pancreatic resection has been shown to be determined by both the pathologic and molecular characteristics of the resected tumor specimen. The best pathologic predictors of survival after surgery are the stage, grade, and size of tumor and the resection margin status. 7–13 Among the most notable molecular events that decrease survival are the overexpression of isoforms of the growth factor TGFβ, 27 increased activity of the apoptosis regulator Bcl-XL, 28 and certain mutation subtypes of the K-ras oncogene (GaT, cGT, and GcT at codon 12). 29 There are two other pivotal questions that will determine how prognostic variables will be used in the application of (yet to be proven) adjuvant therapies. First, what are the definitions of complete and incomplete pancreatic resection? Second, how should adjuvant therapy be used in these different categories?
Increasingly accepted in pancreatic cancer surgery is the R classification to define the extent of the resection. If there is no microscopic evidence of tumor at any of the edges of the resection specimen, even if there are lymph node metastases, it is referred to as an R0 resection. After a macroscopically curative resection, tumor cells may be observed by microscopy at one or more edges of the resected specimen. Commonly, these are at the posterior resection margin (fascia of Trietz) or beyond the pancreatic parenchyma anteriorly (base of the transverse colon or posterior peritoneum of the lesser sac). This is known as an R1 resection. The complex nature of the surgery required to remove a pancreatic cancer means that sometimes the “point of no return” has been reached but that not all tumor can be completely removed. A common example is tumor being left behind around the superior mesenteric vein/splenic vein/hepatic portal vein junction or along an edge of the superior mesenteric artery. This type of resection is called an R2 resection.
Should patients with an R1 resection be treated in the same way as patients with an R0 resection? Should patients with an R1 resection be considered to have advanced pancreatic cancer with an equally bad prognosis and offered the same treatment as other patients with advanced pancreatic cancer? Much of the data are based on retrospective studies and provide a very confusing picture, which may serve only to amplify prejudices. Early prospective adjuvant studies concentrated on patients with negative resection margins (R0), 16–18 but more recent trials have included patients with margin-positive (R1) resections. 19,23,25,26
The European Study Group for Pancreatic Cancer’s first trial (ESPAC-1) was a pragmatic study designed to assess the roles of adjuvant chemoradiation and chemotherapy; patients were stratified prospectively by resection margin status before randomization. 25,26 Hence, the trial was ideal for identifying prognostic factors for patients after resection of pancreatic ductal adenocarcinoma and exploring how resection margin status influenced outcome.
On behalf of the ESPAC group, we report on the influence of resection margin status on survival for patients with resected pancreatic cancer randomized between chemoradiation and chemotherapy treatments in the ESPAC-1 study, and explore treatment effects within these subgroups.
PATIENTS AND METHODS
Patients
Sixty-one cancer centers across 11 countries recruited 541 patients with histologically proven, macroscopically resected ductal adenocarcinoma of the pancreas with no evidence of local spread or distant metastases in the ESPAC-1 trial. 25,26 Most patients underwent a standard pancreatoduodenectomy resection, 30,31 either a classical Kausch-Whipple procedure (52%) or pylorus-preserving pancreatoduodenectomy (36%). The remainder (12%) underwent a standard left or total pancreatectomy 31 for tumors in the tail of the pancreas or large tumors in the body of the gland, respectively.
Treatment Allocation
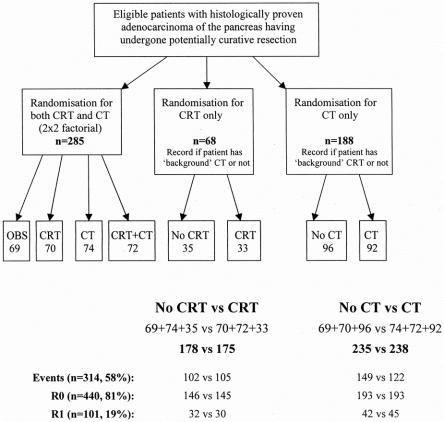
Randomization was stratified by randomization center and resection margin status (R0 or R1). The study aimed to randomize 280 patients for two treatments in a 2 × 2 factorial design to examine two issues: the role of adjuvant chemoradiation (40 Gy + 5-fluorouracil [5-FU] over 6 weeks, including a 2-week break) and the role for adjuvant chemotherapy (5-FU + folinic acid, 1 week in every 4 weeks for 6 months). 25,26 Although clinicians were encouraged to randomize into this 2 × 2 design, randomization was restricted, and so the trial design was expanded to be more pragmatic and to include single-arm randomization options for chemoradiation only and chemotherapy only (Fig. 1). This meant clinicians selected which randomization option they wished to pursue and provided details of any additional treatment they wished to give (i.e., background therapy) before patient entry. Patients were analyzed according to their randomized treatment and stratified by additional background therapy. All patients were invited to participate in quality of life assessments, and all were followed up at 3-month intervals until death.
Figure 1. Randomization procedure.
Number of Patients for Comparison
The two main comparisons were between patients randomized to receive chemoradiation versus no chemoradiation and between those randomized to receive chemotherapy versus no chemotherapy, combining patients from the 2 × 2 factorial design and the single-arm randomization options. Of the 541 patients, 285 patients were randomized for both chemoradiation and chemotherapy (in the 2 × 2 factorial design) and the other 256 were randomized either for chemoradiation only or for chemotherapy only (see Fig. 1). Overall, 353 patients were randomized for chemoradiation: 175 were randomized to receive chemoradiation and 178 were randomized to receive no chemoradiation. Overall, 473 patients were randomized for chemotherapy: 238 were randomized to receive chemotherapy and 235 were randomized to receive no chemotherapy. Among the 101 patients with positive resection margins, 48 were randomized for both chemoradiation and chemotherapy (in the 2 × 2 factorial design), and the other 53 patients were randomized via the single-arm randomization options for either chemoradiation or chemotherapy. Sixty-two of the 101 R1 patients were randomized for chemoradiation: 30 were randomized to receive chemoradiation and 32 to receive no chemoradiation. Eighty-seven of the 101 R1 patients were randomized for chemotherapy: 45 were randomized to receive chemotherapy and 42 to receive no chemotherapy.
Statistical Analysis
The study design assumed that approximately 80% of patients would be R0 (powered to detect an increase in 2-year survival rate from 20% to 40%) and that 20% of patients would be R1 (powered to detect an increase in 2-year survival rate from 1% to 20%). Survival was calculated from the date of resection until the date of death from any cause or censored at the latest follow-up. Chi-square tests were used for categorical data to compare proportions. Wilcoxon two-sample tests were used to compare groups of continuous measurements. Survival estimates were calculated by the method of Kaplan and Meier, 32 and the log-rank test 33 was used to assess the differences between groups. Cox proportional hazards modeling 34 was used to explore independent predictors of survival. Hazard ratios (HR) with 95% confidence intervals (CI) and P values are presented, and HR plots 35 by treatments were derived to display reduction of risk. All analyses were carried out using SAS Statistical Software (Cary, NC).
RESULTS
Patients
The median age was 60 years (interquartile range [IQR] 53–67), 60% of patients were men, and the median maximum tumor diameter was 3 cm (IQR 2.2—4.0). Four hundred forty patients (81%) had R0 and 101 (19%) patients had R1 resections. The complete table of characteristics by the two main treatment questions is given in the trial paper and showed that patients were balanced in terms of treatment group and prognostic factors. 26 Clinical and tumor characteristics generally appeared balanced between the R0 and R1 groups (Table 1). There was a slightly greater proportion of node-positive patients (67% vs. 50%;P ≤ .01) and patients with local invasion (35% vs. 15%;P ≤ .001) in the R1 group compared with the R0 group.
Table 1. CLINICAL AND TUMOR CHARACTERISTICS OF THE R1 AND R0 GROUPS
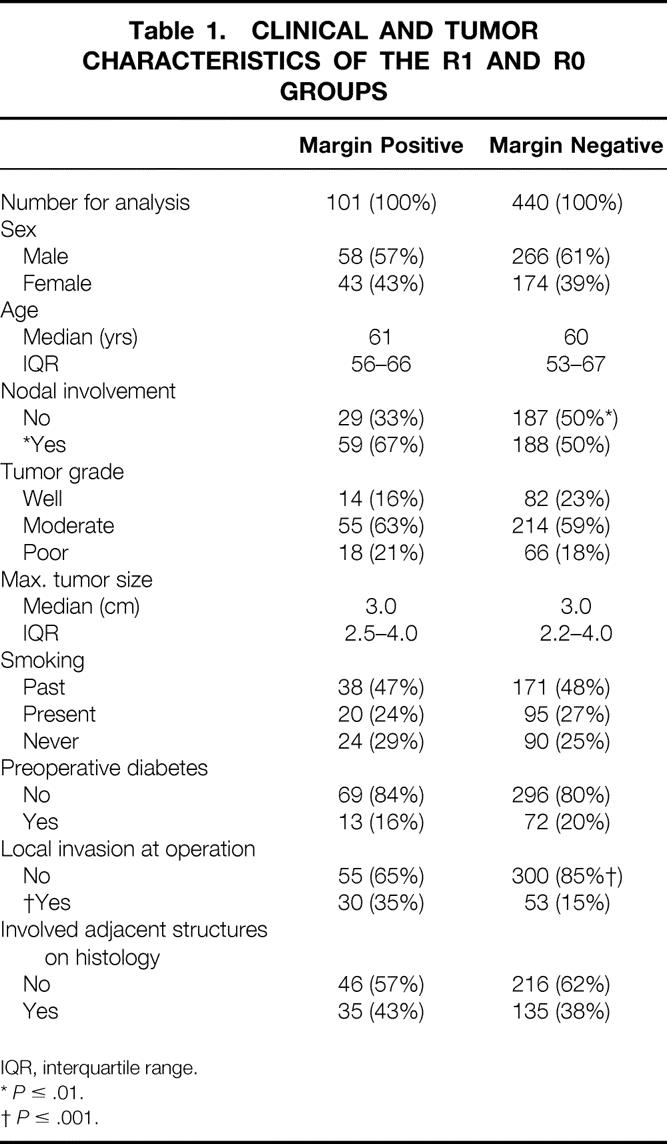
IQR, interquartile range.
*P ≤ .01.
†P ≤ .001.
Survival and Quality of Life
The interim trial results have been reported. 26 So far there have been 314 (58%) deaths at a median follow-up of all 227 (42%) alive patients of 10 months (range 0–62, IQR 1–25). Sixty-six of the 101 R1 patients (65%) had died compared with 248 (56%) of the 440 R0 patients. Median follow-up for the 35 R1 patients who were still alive was 7 months (range 0–62, IQR 1–21) compared with 10 months (range 0–62, IQR 1–25) for the 192 R0 patients. The majority of all deaths, 266 (85%), were disease-related. Of the remaining 48 deaths, there were only three treatment-related deaths; 12 were from causes unrelated to pancreatic cancer or the treatment given, and the causes of death in 33 other patients were not confirmed. Globally, there were no major differences in quality of life in any of the randomized groups. 26
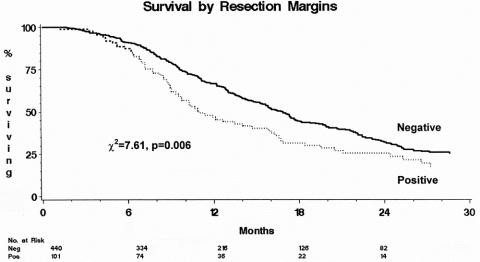
Resection margin involvement was confirmed to be an influential prognostic factor (Fig. 2; chi-square = 7.61, P = .006), with a median survival of 10.9 months (95% CI 9.3–15.8) for patients with R1 resections compared with 16.9 months (95% CI 15.3–17.9) for those with R0 resections (Table 2). The 1-year survival rate for R0 patients was 65.5% (95% CI 61.5–71.4%); it was 46.8% (95% CI 36.0–57.6%) for R1 patients. The 2-year survival rate for R0 patients was 32.0% (95% CI 26.7–37.4%); it was 25.6% (95% CI 15.6–35.6%) for R1 patients. Log-rank survival analysis also confirmed tumor grade (chi-square = 24.46, P < .001), tumor size (chi-square = 6.21, P = .013), and nodal involvement (chi-square = 13.12, P < .001) to be influential prognostic factors of survival.
Figure 2. Survival by resection margin status.
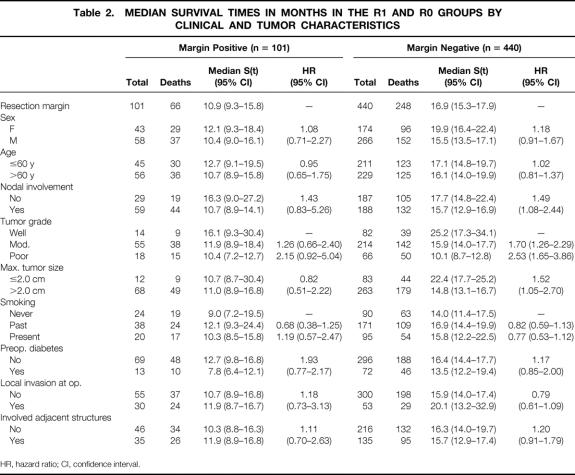
Table 2. MEDIAN SURVIVAL TIMES IN MONTHS IN THE R1 AND R0 GROUPS BY CLINICAL AND TUMOR CHARACTERISTICS
HR, hazard ratio; CI, confidence interval.
Within most of these subgroups, patients with R0 resections had an increased median survival compared with patients with an R1 resection (see Table 2), with the exception of patients with poorly differentiated tumors, in whom there was a very similar median survival rate: 10.1 months (95% CI 8.7–12.8) for R0 patients and 10.4 months (95% CI 7.2–12.7) for R1 patients.
Also, in R0 patients, larger tumors were associated with shorter survival: 14.8 months (95% CI 13.1–16.7) for tumors larger than 2 cm versus 22.4 months (95% CI 17.7–25.2) for tumors 2 cm or smaller. In contrast, the median survival was not affected by tumor size in the R1 group: for tumors larger than 2 cm, the median survival was 11.0 months (95% CI 8.9–16.8) versus 10.7 months (95% CI 8.7–30.4) for tumors 2 cm or smaller.
Cox Proportional Hazards Modeling of Prognostic Factors
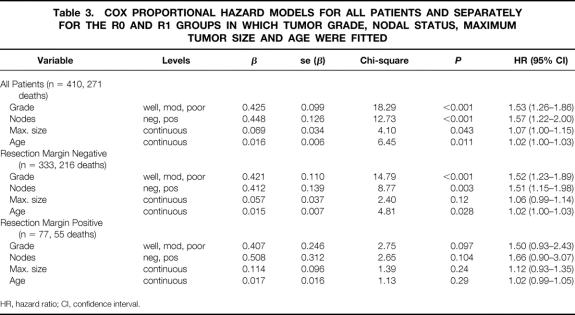
Cox proportional hazards modeling considered all possible prognostic factors for inclusion: resection margin status, tumor grade and size, lymph node status, smoking status, presence or absence of local invasion, presence or absence of preoperative diabetes, gender, and age. An initial model based on 410 patients (271 deaths) identified grade of disease, nodal status, maximum tumor size, and age as independent prognostic factors (Table 3). The presence of increasing tumor grade, lymph node metastases, increasing maximum tumor size, and increasing age were all associated with reduced survival.
Table 3. COX PROPORTIONAL HAZARD MODELS FOR ALL PATIENTS AND SEPARATELY FOR THE R0 AND R1 GROUPS IN WHICH TUMOR GRADE, NODAL STATUS, MAXIMUM TUMOR SIZE AND AGE WERE FITTED
HR, hazard ratio; CI, confidence interval.
Resection margin status was an independent factor in Cox regression modeling only in the absence of tumor grade and lymph node status. Resection margin status was substituted as a surrogate for grade of disease and nodal status in an alternative model based on 426 patients (281 deaths) consisting of maximum tumor size (chi-square = 4.54, P = .033) and resection margin status (chi-square = 3.51, P = .061). Nodal status and tumor grade were not independent factors in this alternative model and were influential only in the absence of resection margin status.
The original Cox model of tumor grade, nodal status, maximum tumor size, and age was then fitted to the R0 and R1 groups of patients separately to observe the influence of these prognostic factors within each group (see Table 3). The estimates for the R0 group were calculated using 333 patients (216 deaths) and confirmed the importance of grade of disease, nodal status, and age as influential factors in predicting long-term survival. The prognostic value of tumor size, however, was significantly reduced.
The estimates for the R1 group were calculated using a sample of 77 patients (55 deaths). At best, grade of disease and nodal status could be considered to be only of borderline significance in predicting long-term survival; any real significance was probably reduced because of the small number of patients. The prognostic value of tumor size and age was significantly reduced and could not be classified as influential in terms of predicting survival of this relatively small group of R1 patients.
Chemoradiation Treatment
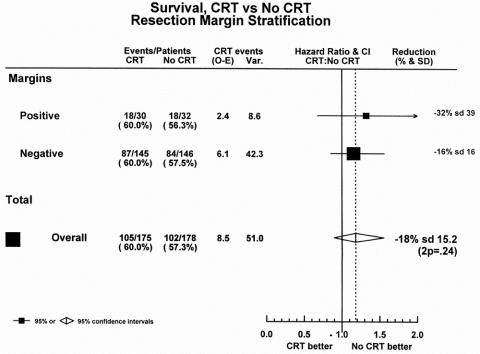
Preliminary results of the ESPAC-1 trial showed no evidence of a benefit for chemoradiation treatment irrespective of R0/R1 status. 26 The median survival for the 175 patients receiving chemoradiation was 15.5 months (95% CI 13.5–17.4); it was 16.1 months (95% CI 13.1–20.1) for the 178 patients allocated to no chemoradiation (chi-square = 1.4, P = .24), with an HR of 1.18 (95% CI 0.90–1.55).
This result was supported in the subgroups of R0 and R1 patients (Fig. 3). The 145 R0 patients allocated to chemoradiation had a median survival of 15.9 months (95% CI 13.8–19.4) compared with 16.9 months (95% CI 13.2–21.6) for the 146 patients allocated to no chemoradiation. Likewise, in R1 patients, the 30 patients allocated to chemoradiation had a median survival of 10.9 months (95% CI 8.8–20.5) compared with 12.1 months (95% CI 9.0–18.4) for the 32 patients randomized to no chemoradiation. The HR plot for the chemoradiation question showed that there was no overall benefit in favor of chemoradiation, and that this effect was similar across the resection margin subgroups.
Figure 3. Hazard ratio plot of chemoradiation treatment.
Chemotherapy Treatment
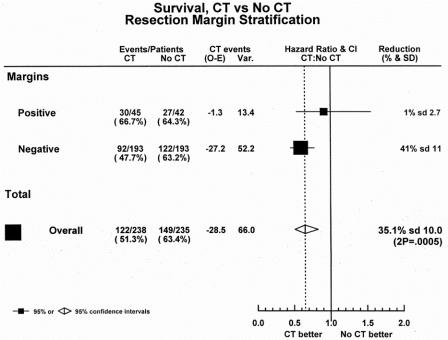
The preliminary results of ESPAC-1 showed overall a highly significant difference in survival in favor of chemotherapy. The median survival for the 238 patients receiving chemotherapy was 19.7 months (95% CI 16.4–22.4) compared with 14.0 months (95% CI 11.9–16.5) for the 235 patients allocated to no chemotherapy (chi-square = 12.3%P = .0005), with an HR of 0.66 (95% CI 0.52–0.83) in favor of chemotherapy.
Overall, in the R0 patients, the 193 patients allocated to chemotherapy had a survival advantage of more than 5 months compared with the 193 patients randomized to no chemotherapy: 20.7 months (95% CI 17.4–24.1) versus 15.3 months (95% CI 12.8–17.0), respectively. The effect was less apparent in the smaller subgroup of R1 patients: the median survival of the 45 patients allocated to chemotherapy was 11.0 months (95% CI 8.8–19.5) compared with 10.3 months (95% CI 8.5–16.3) for the 42 patients randomized to no chemotherapy.
The HR plot for the chemotherapy question showed an overall reduction in the hazard of death of approximately 35% (95% CI 17–48%) in favor of chemotherapy (Fig. 4). The beneficial effect of chemotherapy was apparent in both the R0 and R1 subgroups, but with wide confidence intervals for the R1 estimate because of the small number of patients in that subgroup. Although the magnitude of the benefit for adjuvant chemotherapy was reduced in the smaller subset of R1 patients, there was no significant heterogeneity between the R0 and R1 groups.
Figure 4. Hazard ratio plot of chemotherapy treatment.
DISCUSSION
The ESPAC-1 trial is the largest randomized trial ever conducted in resected pancreatic cancer. 25,26 Unlike previous smaller adjuvant trials that restricted recruitment to patients with negative resection margins, 16–18 the ESPAC-1 trial allowed all patients to enter the study, stratifying treatment allocation by resection margin status. It was anticipated that 20% of patients would have positive resection margins, and this was found to be the case. The inclusion of these patients allows the influence of resection margin status on survival to be explored in a prospective, randomized trial of patients with resected pancreatic cancer and to see whether prognostic factors were similar for patients with negative or positive microscopic resection margin involvement.
Several previous retrospective studies have examined resection margin status in the absence of any adjuvant treatment. Trede et al, 7 in a study of 133 resections, and Gall et al, 36 in a study of 138 resections, both reported significantly reduced survival outcomes in patients with resection margin-positive tumors. In a study of 75 resections using multivariate analysis, Millikan et al 37 reported that both blood transfusion and an R1 resection independently and significantly reduced long-term survival. On a similar note, Wenger et al, 38 in a study of 158 resections, reported that tumors larger than 2 cm in diameter and again R1 resections were associated independently with a significantly shorter survival.
Other retrospective studies have examined the prognostic value of resection margin status in groups of patients among whom there were at least a reasonable number who had adjuvant treatment on a selective basis. Geer and Brennan, 8 in a study of 146 resections, found that tumor grade, tumor size, and lymph node status were powerful and independent predictors of survival. Neither resection margin status nor the use of adjuvant treatment had any bearing on long-term survival. 8 In the study by Nitecki et al, 9 no patient with an R2 resection survived beyond 18 months, and the use of adjuvant treatment also made no difference to survival outcome. Willet et al 20 reported no significant survival difference between patients who did or did not receive adjuvant chemoradiotherapy; survival was largely dictated by resection margin status, with no patient with a positive resection margin surviving much beyond 3 years. Yeo et al, 10 in a study of 174 resections, reported four factors to be of independent predictive power for prognosis: tumor size, intraoperative blood loss, adjuvant treatment (mostly chemoradiation followed by chemotherapy), and resection margin status.
From these studies, it might be concluded that the outcome in patients with a positive resection margin is poor and that perhaps even full adjuvant treatment is of no benefit. In contrast to the study by Willet et al, 20 the UKPACA study found surprisingly good survival in patients with R1 resections who had received a combination of chemoradiation followed by chemotherapy. 23 This finding seems to be supported by the first results from the ESPAC-1 trial, where patients with R1 resections appear to benefit from adjuvant chemotherapy treatment.
In this uniquely large prospective study of the 541 resections in the ESPAC-1 trial, we found that tumor grade and size, lymph node involvement, resection margin status, and age were significant survival predictors by log-rank analysis. In the Cox proportional hazards modeling, resection margin status ceased to be of independent prognostic significance in the presence of tumor grade and nodal status. It is interesting that resection margin status was a significant independent prognostic factor only when tumor grade and lymph node involvement were both absent from the model. There is an extremely important implication to this observation, which is entirely consistent with the findings of Geer and Brennan. 8 These data suggest that R1 status in resected pancreatic ductal adenocarcinoma is linked to the underlying biologic phenotype. In effect, this hypothesis says that R1 tumors behave as if they tend to be poorly differentiated and tend to have lymph node metastases. These findings would argue against R1 status being linked to other contingencies, such as surgical technique or that it is somehow a random occurrence. Whereas R2 resections might be related to tumor size, this study has clearly shown that is not the case for R1 resections.
The treatment effects were similar within the subgroups of patients with negative and positive resection margins for both the chemoradiation and chemotherapy questions. The statistical magnitude of the chemotherapy benefit was smaller for the resection margin-positive patients, probably because of the smaller numbers of patients in this group. Patients with positive resection margins in this prospective study had much better survival (to 2 years at least) than many retrospective studies would have suggested. 7,20,36–38 Although the magnitude of the survival effect was not as large as that seen in patients with resection margin-negative tumors, this is not surprising. Moreover, this would be consistent with our hypothesis that R1 status reflects the biologic state of the tumor.
For the overall ESPAC-1 study, there were two main findings. 25,26 First, adjuvant chemoradiotherapy (either alone or in combination with follow-on chemotherapy) did not improve 2-year survival rates. Second, there was strong evidence that adjuvant chemotherapy might prolong survival. We observed a confounding negative effect by chemoradiotherapy on the benefit of chemotherapy alone. Although the benefit of chemotherapy was observed in the larger overall analysis, this was not as clearly apparent in patients randomized to the 2 × 2 factorial design. Thus, we concluded that there was uncertainty as to the size of the chemotherapy effect, requiring further confirmatory controlled trials of chemotherapy versus surgery alone.
The results of the ESPAC-1 study are consistent with the prospective randomized controlled trial of Bakkevold et al, 18 which showed some survival benefit for adjuvant chemotherapy, and the EORTC trial, 19 which found no benefit for adjuvant chemoradiotherapy. The original GITSG trial, 16,17 which pioneered the concept of combined adjuvant chemoradiotherapy and follow-on chemotherapy, contained only 43 patients. Treated patients (n = 21), comprising only R0 cases, achieved a median survival of 20 months. In the ESPAC-1 study, patients with R0 tumors randomized to chemotherapy (n = 193) had a median survival of 20.7 months (95% CI 17.4–24.1).
Yeo et al 10 support combined adjuvant chemoradiotherapy and follow-on chemotherapy, but their conclusion is largely based on a retrospective biased study. Despite the selection bias, the median survival in their study of patients receiving combination chemoradiotherapy and follow-on chemotherapy was no better than that of patients randomized to chemotherapy in the ESPAC-1 study. 26 There might be criticism that neither the EORTC trial 19 nor the ESPAC-1 trial 26 used sufficient radiation doses for an optimum effect; however, this argument does not explain the apparently beneficial result of the same radiation dose given in the GITSG study. 16,17 The introduction of the conformal radiotherapy beam technique enables more radiation to be delivered to targeted areas in the abdomen. 24 However, even with intensive chemoradiotherapy (50.4–57.6 Gy to the pancreatic bed, 50.4–54.0 Gy to regional nodes, and 23.4–27 Gy to the liver) followed by protracted venous infusion with 5-FU and folinic acid, survival was not significantly better than in the no-treatment group. 10
A more recent report by the same group 24 of 23 patients with pancreatic cancer compared “low” with “high” radiation dosage in an intensive combination adjuvant regimen. All patients received a continuous infusion of 5-FU and folinic acid during radiation treatment 5 days per week and then 1 month later, four cycles of the same chemotherapy regimen for 2 weeks out of every 4. “Low” radiotherapy comprised 23.4 Gy to the whole liver, 50.4 Gy to regional nodes, and 50.4 Gy to the tumor bed. “High” radiotherapy comprised 27.0 Gy to the whole liver, 54.0 Gy to regional nodes, and 57.6 Gy to the tumor bed. The overall median survival was 15.9 months, with little difference in median survival between the low-dose and high-dose groups (14.4 vs. 16.9 months, respectively). An overall median survival of 15.9 months using this intensive combination adjuvant regimen contrasts with a median overall median survival of 19.7 months (95% CI 16.4–22.4) for a simpler chemotherapy regimen in the ESPAC-1 trial. The lower 95% CI actually exceeds the median survival of patients given the intensive combination regimen. Moreover, in the ESPAC-1 trial there was no detrimental effect on quality of life from chemotherapy.
Although there has been much interest in other ways of delivering radiotherapy, any perceived advantages remain unconvincing. The use of intraoperative radiotherapy does not seem to have any survival advantage over conventional postoperative radiotherapy for resectable tumors. 21 Similarly, the use of neoadjuvant chemoradiation does not produce a survival benefit compared with postoperative chemoradiation, even with a comparison biased in favor of the neoadjuvant technique. 22
Thus, in our view, future studies of adjuvant treatment in pancreatic cancer should focus on chemotherapy regimens. We would not recommend further use of adjuvant radiotherapy, not only because of a lack of proven benefit but also because it may have a reduced survival effect in patients due to receive follow-on chemotherapy. 26 The lack of benefit of radiotherapy may simply be due to the delay in instituting effective chemotherapy, or some other reason. The ESPAC-2 trial is focusing on adjuvant regional chemotherapy. The ESPAC-3 trial is recruiting 990 patients into a three-arm trial comparing the chemotherapy regimen used in ESPAC-1 (bolus 5-FU and folinic acid) versus gemcitabine versus control (resection and observation only). The use of a control arm is essential to confirm the beneficial effect of chemotherapy and to obtain a true measure of its effect in the absence of the confounding influence of chemoradiation.
To improve outcome in pancreatic cancer, other large randomized controlled trials are required. In these studies we should investigate the behavior of R1 tumors in much more detail. For this reason, we would strongly advocate stratifying randomization and analysis by resection margin status in future prospective randomized trials.
In conclusion, this study has confirmed that R1 pancreatic tumors represent a biologically more aggressive cancer benefiting from resection and adjuvant chemotherapy. Although the magnitude of the response to adjuvant chemotherapy in patients with R1 tumors was less than those with R0 tumors, there was still a trend in favor of treatment. Patients with R1 tumors should be included in future trials of adjuvant treatments and randomization and analysis should be stratified by this significant prognostic factor.
Acknowledgments
ESPAC thanks the members of the Independent Data Monitoring Committee: Mr. R.C.G. Russell, Consultant Surgeon, The Middlesex Hospital, London; Dr. Sue O’Reilly, Consultant Clinical Oncologist, Clatterbridge Center for Clinical Oncology, Wirral; and Dr. Roger P. A’Hern, Statistician, Department of Computing and Information, The Royal Marsden, London.
MEMBERS OF ESPAC
Members of the ESPAC Working Party: surgery advisors, J.P. Neoptolemos (and ESPAC Co-secretary), H.G. Beger (and ESPAC Chairman), M.W. Büchler (and ESPAC Co-secretary); clinical oncology advisor, D. Spooner; medical oncology advisor, D.J. Kerr; pathology advisor, N. Lemoine (London, UK); Swiss center coordinator, H. Freiss; German center coordinator, K. Link; Italian center coordinators, C. Bassi, M. Falconi, P. Pederzoli; Spanish center coordinator, L. Fernandez-Cruz; French center coordinator, F. Lacaine; Greek center coordinator, C. Dervenis; senior ESPAC statistician, J.A. Dunn; ESPAC statistician, D.D. Moffitt; ESPAC senior trials coordinator, J. Almond.
The following specialists contributed to the treatment of patients in the ESPAC-1 trial. Austria: P. Steindorfer (Graz); Belgium: J.F. Gigot (Brussels); France (French Associations for Surgical Research): Ph. Segol (Caen), J. Chipponi (Clermont-Ferrand), J.M. Hay (Colombes), D. Cherqui, P.L. Fagniez, B. Malassagne (Creteil), M.S. Sbai-Idriasy (Eaubonne), L. Gambiez, P. Quandalle, J.P. Triboulet (Lille), A. Gainant (Limoges), B. Desousseaux (Lomme), B. Sastre (Marseille), S. Houry, H. Mosnier (Paris), M. Veyrieres (Pontoise), O. Bouche (Reims), G. Fourtanier (Toulouse); Germany: Dr. Kletha (Chemnitz), J. Schmidt (Heidelberg), J. Scheele (Jena), Dr. Waiandt (Lahr), Dr. Gerdes (Marburg), Dr. Weber (Trier); Greece: J.K. Poulantzas (Athens), J. Androulakis (Patras), G. Blantzas, D. Botsios, E. Hatzitheoklitos, C.H. Sbarouris (Thessaloniki); Hungary: L. Flautner, T. Tihanyi (Budapest), P. Sapy (Debrecen), A. Olah (Gyor), P. Horvath-Örs, D. Kelemen (Pècs), M. Wenczl (Szombathely); Italy: G. Marzoli (Bolzano), O. Pieramico (Meran), F. Meduri, S. Pedrazzoli (Padova), F. Dominioni (Varesel), G. Butturini (Verona); Spain: C. Pera (Cordoba); Sweden: I. Ihse, A. Andren-Sandberg (Lund); Switzerland: S. Hunziker (Aarau), K. Buser (Bern), S. Meier (Lausanne), J. Largiader (Luzern), G. Pichert, R. Trüb (Zürich); United Kingdom: J. Buckles, I. Fernando (Birmingham), D. Alderson, S. Falk (Bristol), P. Corrie (Cambridge), R. Carter, Hochhauser, C. Imrie (Glasgow), T. Leese (Lancaster), A. Crellin, D. Sebag-Montefiore, M. Seymour (Leeds), D. Lloyd, F. Madden (Leicester), M. Hartley, S. Myint, J. Slavin, D. Smith, R. Sutton (Liverpool/Wirral), P. Price (London), B. Davidson (London), T. Podd (Newcastle), F. Daniel, A. Kingsnorth (Plymouth), C. Baughan, C. Johnson (Southampton), F. Adab (Stoke on Trent), D. Cunningham (Surrey).
Discussion
Prof. H. Obertop: I want to congratulate John Neoptolemos and the members of the European Study Group for Pancreatic Cancer very much with this significant paper. I had the opportunity to read this paper and enjoyed it very much. This paper is part of the ESPAC-1 study. That is the largest randomized controlled study on the use of adjuvant chemoradiation and/or chemotherapy in pancreatic cancer. We have not seen the definitive paper; most of us look forward to this so that we can decide on good evidence about adjuvant therapy in pancreatic cancer. So far, on the basis of the same level 1 (two studies) and level 2 evidence, pancreatic surgeons have a different policy toward adjuvant therapy in Europe and in the U.S.A. But the present study is in fact an analysis of a subgroup with positive resection margins and negative resection margins. I think that the power calculation for the study (ESPAC-1) is based on the endpoint survival of the whole group, and my comment is whether the authors can make the conclusion, as they do, that postoperative chemotherapy is beneficial for this relatively small subgroup of margin-positive resection. It now shows in the data.
My first question deals with the definition of positive resection margins. Not only surgeons and surgical techniques were different in this study, but also the handling by the pathologists in the various institutions. Was the incidence of positivity (∼20% for the whole group) different in the various institutions? And my second question along this line is: what was meant by local invasion that was seen in 35% of the margin-positive resections and in 15% of margin-negative resections? Obviously, 15% of this local invasion could be resected radically. Were there any vascular resections in this group?
I thank you for the opportunity to discuss this paper and congratulate the ESPAC on this excellent study.
Prof. A. M. M. Eggermont: The essential question is whether or not there was stratification in the design of the randomized process was stratified for microscopic radical and nonradical tumors. If not, a subgroup analysis as performed in this study is only hypothesis-building at best.
Prof. J. P. Neoptolemos (closing): On behalf of all my colleagues from ESPAC throughout Europe, we greatly value the kind reception for this study by the European Surgical Association and the pertinent questions from Prof. Huug Obertop and Prof. Alex Eggermont.
The power calculations for ESPAC-1 were based on the overall group survival. Within the context of the 2 × 2 factorial design, we had originally planned to recruit 280 patients, of whom 20% would have a positive microscopic (R1) resection margin and the remainder would be entirely clear (R0). Patients were prospectively stratified by resection margin status (R0 vs. R1) and then randomized to treatment. The conduct of the trial was controlled by an Independent Data and Safety Monitoring Committee (IDSMC); members of the ESPAC-1 Working Party were never aware of the progress of the trial except in terms of numbers recruited. On reaching the accrual target of 280 patients, the IDSMC recommended continuation of the trial. There were several such reviews, each with the same recommendation. The trial was stopped after nearly 600 patients had been randomized in order to prevent further patients being given radiotherapy.
As a consequence, we have prospectively studied 541 patients with pancreatic ductal adenocarcinoma randomized to different adjuvant therapies and stratified by resection margin status, 101 with positive margins. Not only is this trial by far the largest ever randomized adjuvant trial, but it is also the largest to look at resection margins specifically.
The statistics clearly showed a similar effect of the treatments irrespective of the R0/R1 status. Chemoradiotherapy was of no value at all in any group! There was a benefit in all groups from chemotherapy.
Although the differences may seem small in absolute terms, the numbers of cases included along with the design makes ESPAC-1 an extremely powerful study with conclusions of colossal strength. There was no heterogeneity in the prevalence of resection margin status by country, center, or any other variable. “Local invasion” was that which was observed at operation, and “adjacent structure involvement” was that determined by histologic examination.
At the present time, we have not specifically analyzed outcomes in relation to vascular resections per se.
Why Americans from the U.S.A. behave differently from Europeans with regards to adjuvant treatment for pancreatic cancer is an interesting question. The importance of the ESPAC-1 trial results published in the Lancet is that we can achieve median survivals with chemotherapy alone that are at least as good as U.S.A. studies using incredibly high doses of hyperfractionated chemoradiotherapy (as well as chemotherapy), and we can do this with excellent quality of life. Indeed, what is worrying is that chemoradiotherapy may have an adverse effect on the benefits of chemotherapy, possibly by delaying its first use.
The hypothesis that resection margin status reflects the biologic nature of the tumor is novel, interesting, and exciting.
ESPAC are planning further randomized trials; indeed, ESPAC-3, which compares adjuvant 5-FU/folinic acid versus gemcitabine versus control, is already well underway in centers in Europe, Canada, Australia, and New Zealand.
All future adjuvant trials (such as ESPAC-3) must prospectively stratify by resection margin status; much will then be greatly learned from these single studies as well as from meta-analyses. In this respect some U.S.A. randomized controlled studies would be welcome.
Footnotes
This study is principally funded by the Cancer Research Campaign, United Kingdom, plus the Fonds de Recherche de la Société National Française de Gastroentérologie; the Consorzio Studi Universitari di Verona, Cariverona, and the Ministero Università e Ricerca Scientifica e Tecnologica (Cofin 9906195987), Rome, Italy; Associazione Italiana Ricerca Cancro (AIRC), Milan, Italy; and European Community grant BIOMED 2 CE-Contract No. BMH4-CT98-3805.
Correspondence: Prof. J. P. Neoptolemos, Department of Surgery, University of Liverpool, 5th Floor UCD Building, Daulby Street, Liverpool, L69 3GA, United Kingdom.
E-mail: j.p.neoptolemos@liverpool.ac.uk
Accepted for publication April 2001.
References
- 1.Parkin DM, Muir CS, Whelan SL, et al. Cancer incidence in five continents. Vol. VI. Lyon: International Agency for Research on Cancer, 1992 (IARC Scientific Publications No. 120) Oxford University Press.
- 2.Bramhall SR, Allum WH, Jones AG, et al. Incidence, treatment and survival in 13,560 patients with pancreatic cancer: an epidemiological study in the West Midlands. Br J Surg 1995; 82: 111–115. [DOI] [PubMed] [Google Scholar]
- 3.Boring CC, Squires TS, Tong T, et al. Cancer statistics. CA Cancer J Clin 1994; 44: 7–26. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Russell RCG, Bramhall SR, et al. Low mortality following resection for pancreatic and periampullary tumors in 1026 patients: UK survey of specialist pancreatic units. Br J Surg 1997; 84: 1370–1376. [PubMed] [Google Scholar]
- 5.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998; 280: 1747–1751. [DOI] [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Warshaw AL, Finlayson STG, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery 1999; 126: 178–183. [PubMed] [Google Scholar]
- 7.Trede M, Schwall G, Saeger H-D. Survival after pancreatoduodenectomy: 118 consecutive resections without an operative mortality. Ann Surg 1990; 211: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geer RJ, Brennan MF. Prognostic indicators of survival after resection of pancreatic adenocarcinoma. Am J Surg 1993; 165: 68–72. [DOI] [PubMed] [Google Scholar]
- 9.Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 1995; 221: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeo C, Abrams R, Grochow L, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival: a prospective, single institution experience. Ann Surg 1997; 225: 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosca F, Giulianotti PC, Balestracci T, et al. Long-term survival in pancreatic cancer: pylorus-preserving vs. Whipple pancreatoduodenectomy. Surgery 1997; 122: 553–566. [DOI] [PubMed] [Google Scholar]
- 12.Allema JH, Reinders ME, Vangulik TM, et al. Prognostic factors for survival after pancreaticoduodenectomy for patients with carcinoma of the pancreatic head region. Cancer 1995; 75: 2069–2076. [DOI] [PubMed] [Google Scholar]
- 13.Allison DC, Piantadosi S, Hruban RH, et al. DNA content and other factors associated with ten-year survival after resection of pancreatic carcinoma. J Surg Oncol 1998; 67: 151–159. [DOI] [PubMed] [Google Scholar]
- 14.Van Heerden JA, McIlrath DC, Ilstrup DM, et al. Total pancreatectomy for ductal adenocarcinoma of the pancreas: an update. World J Surg 1988; 12: 658–662. [DOI] [PubMed] [Google Scholar]
- 15.Pedrazzoli S, Di Carlo V, Dionigi R, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a Multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg 1998; 228: 508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalser MH, Ellenberg SS. Pancreatic cancer: Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985; 120: 899–903. [DOI] [PubMed] [Google Scholar]
- 17.Douglass HO. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer 1987; 59: 2006–2010. [DOI] [PubMed] [Google Scholar]
- 18.Bakkevold KE, Arnesjo B, Dahl O, et al. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater: results of a controlled, prospective, randomised multicenter study. Eur J Cancer 1993; 5: 698–703. [DOI] [PubMed] [Google Scholar]
- 19.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region. Phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg 1999; 230: 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willett CG, Lewandrowski K, Warshaw AL, et al. Resection margins in carcinoma of the head of the pancreas. Implications for radiation therapy. Ann Surg 1993; 217: 144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Carlo V, Zerbi A, Balzano G, et al. Intraoperative and postoperative radiotherapy in pancreatic cancer. Int J Pancreatol 1997; 21: 53–8. [DOI] [PubMed] [Google Scholar]
- 22.Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol 1997; 15: 928–937. [DOI] [PubMed] [Google Scholar]
- 23.Neoptolemos JP, Baker P, Johnson CD, et al. Adjuvant radiotherapy and follow-on chemotherapy in patients with pancreatic cancer. Results of the UK Pancreatic Cancer Group study (UKPACA-1). Gastrointest Cancer 1998; 2: 235–245. [Google Scholar]
- 24.Abrams RA, Grochow LB, Chakravarthy A, et al. Intensified adjuvant therapy for pancreatic and periampullary adenocarcinoma: Survival results and observations regarding patterns of failure, radiotherapy dose and CA 19-9 levels. Int J Radiat Oncol Biol Phys 1999; 44: 1039–1046. [DOI] [PubMed] [Google Scholar]
- 25.Neoptolemos JP, Kerr DJ, Beger HG, et al. ESPAC-1 Trial Progress Report: The European randomised adjuvant study comparing radiochemotherapy, six months chemotherapy and combination therapy versus observation in resected pancreatic cancer. Digestion 1997; 58: 570–577. [DOI] [PubMed] [Google Scholar]
- 26.Neoptolemos JP, Dunn JA, Stocken DD, et al. ESPAC-1: A European, randomised controlled study of adjuvant chemoradiation and chemotherapy in resectable pancreatic cancer. Lancet (in press). [DOI] [PubMed]
- 27.Friess H, Yamanaka Y, Büchler M, et al. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology 1993; 105: 1846–1856. [DOI] [PubMed] [Google Scholar]
- 28.Friess H, Lu Z, Andrén-Sandberg Å, et al. Moderate activation of the apoptosis inhibitor bcl-xL worsens the prognosis in pancreatic cancer. Ann Surg 1998; 228: 780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawesha A, Ghaneh P, Evans JD, et al. K-ras oncogene subtype mutations are associated with survival but not the expression of p53, p16INK4a, p21WAF-1, Cyclin D1, erb-B2 and erb-B3 in resected pancreatic adenocarcinoma. Int J Cancer 2000; 89: 469–474. [DOI] [PubMed] [Google Scholar]
- 30.Jones L, Russell C, Mosca F, et al. Standard Kausch-Whipple pancreatoduodenectomy. Dig Surg 1999; 16: 297–304. [DOI] [PubMed] [Google Scholar]
- 31.Pedrazzoli S, Beger H, Obertop H, et al. A surgical and pathological based classification of resection of pancreatic cancer: summary of an international workshop on surgical procedures in pancreatic cancer. Dig Surg 1999; 16: 337–345. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 33.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomised clinical trials requiring prolonged observation of each patient II. Analysis and example. Br J Cancer 1977; 35: 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cox DR. Regression models and life-tables. J Roy Stat Soc [B] 1972; 34: 187–220. [Google Scholar]
- 35.Early Breast Cancer Trialists’ Collaborative Group. Introduction and methods sections, reproduced from Treatment of Early Breast Cancer. Vol 1. Worldwide Evidence 1985–1990. Oxford University Press, 1990.
- 36.Gall FP, Kessler H, Hermanek P. Surgical treatment of ductal pancreatic carcinoma. Eur J Surg Oncol 1991; 17: 173–181. [PubMed] [Google Scholar]
- 37.Millikan KW, Deziel DJ, Silverstein JC, et al. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg 1999; 65: 618–624. [PubMed] [Google Scholar]
- 38.Wenger FA, Peter F, Zieren J, et al. Prognosis factors in carcinoma of the head of the pancreas. Dig Surg 2000; 17: 29–35. [DOI] [PubMed] [Google Scholar]