Abstract
Objective
To analyze the feasibility, safety, complication and death rates, and early functional results of the transverse coloplasty pouch procedure after low anterior rectal resection and total mesorectal excision.
Summary Background Data
The authors previously developed a novel neorectal reservoir, the transverse coloplasty pouch, in an animal model; they report the first clinical data of a prospective phase 1 study.
Methods
Forty-one patients underwent low anterior rectal resection with total mesorectal excision for rectal cancer (n = 37) or benign pathology (n = 4). The continuity was restored with a transverse coloplasty pouch anastomosis, and the colon was defunctionalized for 3 months. Patients were followed up at 2-month intervals for functional outcome.
Results
Intraoperative complications occurred in three patients (7%), none related to the transverse coloplasty pouch. There were no hospital deaths and the total complication rate was 27% (11/41); an anastomotic leakage rate of 7% was recorded. The stool frequency was 3.4 per 24 hours at 2 months follow-up and gradually decreased to 2.1 per 24 hours at 8 months. Stool dysfunctions such as stool urgency, fragmentation, and incontinence grade 1 and 2 were regularly observed until 6 months; the incidence significantly decreased thereafter. None of the patients had difficulties in pouch evacuation.
Conclusions
The transverse coloplasty pouch is a small-volume reservoir that can safely be used for reconstruction after sphincter-preserving rectal resection. The early functional outcome is favorable and can be compared to other colonic reservoirs. The concept of reducing early dysfunction seen after straight coloanal anastomosis and avoiding long-term problems of pouch evacuation is supported by this study. Future trials will compare the transverse coloplasty pouch with other techniques of restorative resections of the rectum.
The formation of a colon pouch improves functional results after low anterior rectal resection. The colon J-pouch–anal and –low rectal anastomosis was developed by Lazorthes and Parc. 1,2 Its functional superiority over a straight coloanal anastomosis was shown in randomized controlled trials 3–6 and is rarely disputed. The long-term function of a large colon J-pouch (8–10 cm) can be complicated by defecatory dysfunctions that necessitate the use of medication, suppositories, and enemas in 25% to 37% of patients, 7–9 and at present surgeons tend to use smaller colon pouches with a limb length of 5 to 6 cm. 10 The improved functional results achieved with a smaller-volume colon J-pouch have been confirmed by randomized controlled trials. 11,12
With the transverse coloplasty pouch, we conceptually explored whether a very small colon pouch could decrease the early dysfunctions frequently seen after straight coloanal anastomosis and the late evacuation problems associated with large colonic neorectal reservoirs. The safety of the technique and its early outcome were initially tested and confirmed in an animal model and compared with the standard operations straight coloanal anastomosis and colon J-pouch. 13,14 The transverse coloplasty pouch in pigs was safe, with excellent early stool function; in contrast to the human situation, pigs with colon J-pouch already developed evacuation problems in the early follow-up.
The technique of the transverse coloplasty pouch anastomosis was then adapted for the use in humans. The aims of the study were to determine whether the inclusion of the transverse coloplasty pouch in the treatment strategy of patients undergoing restorative rectal resections was feasible and safe, and to analyze early functional results.
PATIENTS AND METHODS
We report the results of a prospective phase 1 study in patients who underwent rectal resection with transverse coloplasty pouch reconstruction from March 1999 to January 2001. The aim of the study was to determine the feasibility, safety, and complication and death rates of the procedure, as well as early functional results.
Forty-one patients with a mean age of 65 years (range 29–84) underwent low anterior rectal resection with total mesorectal excision for rectal cancer (37 patients) or a benign pathology (rectal adenoma, n = 2; endometriosis, n = 1; and reconstruction after Hartmann’s procedure, n = 1). The clinical characteristics are listed in Table 1. Patients with cancer of the middle and lower third of the rectum underwent preoperative endosonography, anorectal manometry, rigid rectoscopy by the operating surgeon, and abdominal and pelvic computed tomography scan (CT) or magnetic resonance imaging (MRI) for tumor staging. Seven patients (19%) with cancer underwent neoadjuvant chemoradiotherapy. Five of these patients were treated in a short-term protocol with a total dose of 25 Gy within 2 weeks, followed by the rectal resection and reconstruction within a few days. 15 This regimen was usually indicated in patients with endosonographically bulky T3 tumors, node-positive disease, or both. In two patients a radiation dose of 50.4 Gy was given over 5 weeks and the operation was performed 3 to 4 weeks after finishing chemoradiation. These two patients had large tumors adjacent to or with questionable invasion of the external sphincter muscle, and the aim of the neoadjuvant treatment was to downsize the tumor and achieve radical resection. Patients with UICC stage 3 disease underwent adjuvant chemotherapy for 4 to 6 months, and patients with stage 4 disease underwent palliative chemotherapy.
Table 1. CLINICAL CHARACTERISTICS
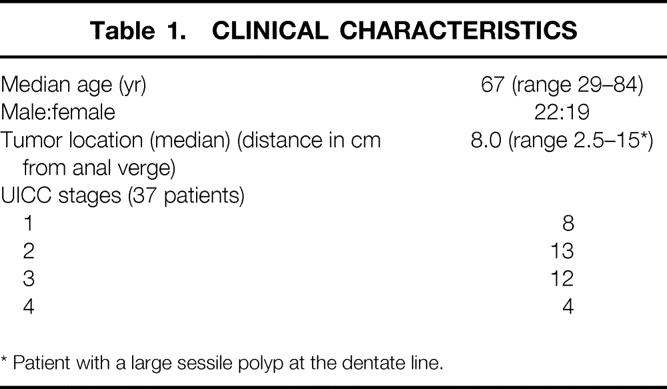
* Patient with a large sessile polyp at the dentate line.
Surgical Technique
A standardized rectal dissection, including a total mesorectal excision with special attention to autonomic nerve preservation according to Heald, was performed in all patients. 16 The total mesorectal excision determined the level of anastomosis (3–6 cm from the anal verge) in most patients. For reconstruction (Fig. 1), the left-sided colon was mobilized to the level of the middle colic vessels. A segment of the descending colon was used to construct the transverse coloplasty pouch as previously described. 17 Briefly, a purse-string suture (2-0 Prolene) is fitted to the cut end of the colon, the anvil of the stapler is inserted, and the purse-string is tied. Two centimeters proximal to the rim of the anvil, an 8-cm longitudinal colotomy is performed, which is placed between the two taenia. Lateral traction by stay sutures forms the reservoir and the colotomy is closed in two layers by transverse running sutures (5-0 PDS). An end-to-end double-stapled anastomosis is performed, usually with a 33-mm circular stapler (Proximate ILS, Ethicon Endosurgery, Johnson & Johnson, Cincinnati, OH). (In a single patient a transanally sutured colon pouch–anal anastomosis concluded the reconstruction.) After testing the anastomosis by air, a 28F catheter is inserted for luminal decompression until postoperative day 5. In all patients the colon was defunctionalized for at least 3 months by a loop ileostomy.
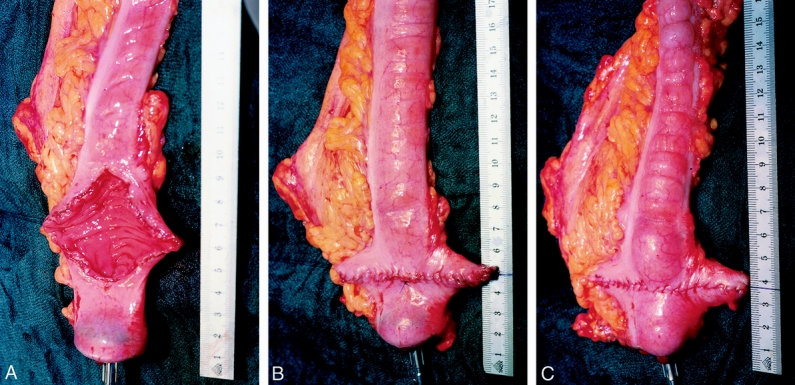
Figure 1. (A) The anvil of the stapler is inserted and secured by a 2-0 monofilament purse-string suture. The colon is opened longitudinally between the taenias beginning 2 cm proximal to the rim of the anvil. Lateral traction by stay sutures shows how the transverse coloplasty pouch is formed. (B) The colostomy is closed transversely by the first running suture (5-0 PDS). (C) The second running seromuscular suture line concludes the formation of the transverse coloplasty pouch, followed by the completion of the anastomosis. (A–C, copyright Karger AG, Basel, Switzerland: reference 17.)
Follow-Up and Functional Assessment
Pre- and postoperative data were collected in a standardized prospective fashion. Postoperative complications and in-hospital deaths were recorded. Anastomotic leakage was diagnosed if an anastomotic dehiscence with pelvic sepsis, transanal discharge of pus, or a fistula arising at the anastomosis during follow-up was present. The definition also included anastomotic leaks detected by contrast enema before closure of the ileostomy. An anastomotic stricture was deemed present when dilatation was needed, whether or not the treatment necessitated anesthesia. Before closure of the loop ileostomy, the anastomotic and transverse coloplasty pouch integrity was controlled by a contrast enema, a pelvic CT scan, or both with application of water-soluble contrast medium into the neorectal reservoir. The loop ileostomy was then closed by a segmental resection with an end-to-end hand-sutured anastomosis (5-0 PDS).
Patients were followed up at 2-month intervals. Anorectal function was evaluated clinically and by anorectal physiology measurements before surgery and 2 months after surgery. Frequency of bowel movements per 24 hours was recorded in patients with regular bowel movements, and the average frequency per 24 hours over a 7-day period was recorded in patients with irregular bowel activity. Fragmentation of stools was defined as the inability to defecate and empty the reservoir in one attempt. Fragmented stools were counted as multiple bowel movements. Urgency was recorded in patients who did not have the ability to defer defecation for more than 15 minutes. Incontinence was recorded as grade 1 (incontinence for gas, discriminate gas from stool), grade 2 (liquids), and grade 3 (solids). Incontinence grade 1 was defined as present if it occurred more than once per week. Patients were specifically asked about the use of soiling pads. Nocturnal dysfunction was defined as nocturnal incontinence or the use of soiling pads at night. Incontinence was retrospectively assessed by using a modified validated score with a range from 0 (perfect continence) to 18 (daily incontinence for solids), as previously described. 3
Anorectal physiology measurements were performed with a standardized technique using a water-perfused system, as described previously. 18 Valid data were available for 18 patients before surgery and for 10 patients 2 months after surgery. Resting and squeeze pressures were recorded in the upper and lower segment of the anal sphincter. First sensation, constant sensation/urge, and maximal tolerable volume were recorded using an inflatable balloon system.
Follow-up for tumor recurrence or progression was carried out according to a standardized protocol that included CT scan, neorectal endosonography and endoscopy for local recurrence, CT scan or abdominal ultrasound for liver metastasis, and conventional chest radiography or CT scan for pulmonary metastasis.
Statistical Analysis
Results are expressed as mean or median with range or interquartile range as indicated. Mann-Whitney rank-sum tests and Fisher exact tests were used where appropriate. Significance was defined at P < .05. SigmaStat 2.0 (Jandel Corp., San Rafael, CA) was used for statistical analysis.
RESULTS
Operative and postoperative results are shown in Tables 2 and 3. Intraoperative complications, recorded in 3 of the 41 patients (7%), were not associated with postoperative complications or related to anastomotic leakage. No technical problems during transverse coloplasty pouch formation or pouch–anal anastomosis were encountered. Treatment of the patients with anastomotic leakage consisted of a transabdominal reoperation in one patient and peri/transanal drainage of a perianastomotic abscess in two patients. Operative and postoperative complications were not related to neoadjuvant radiochemotherapy. Ileostomy closure, routinely done 3 months after the primary operation, was delayed in patients with anastomotic leakage for 6 to 8 months. At present, 28 of the 41 patients (68%) have undergone and 9 of the 41 (22%) are awaiting ileostomy closure. In four patients (10%), reversal of the ileostomy is not planned because of rapid tumor progression with hepatic metastasis (n = 1), incontinence not responding to biofeedback treatment (n = 1), death resulting from hypoglycemia in a diabetic patient (n = 1), or because the patient declined the operation (one patient with an anastomotic stricture).
Table 2. OPERATIVE CHARACTERISTICS
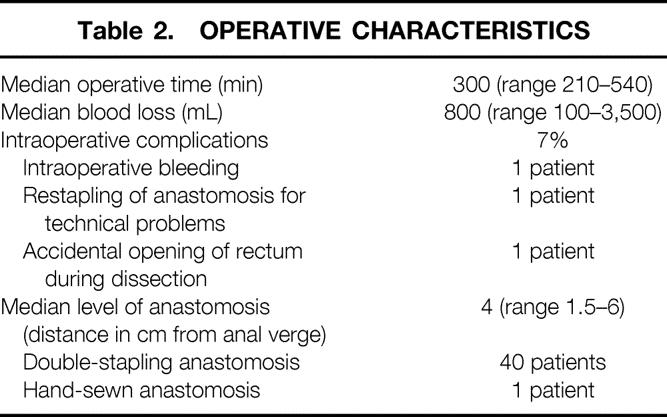
Table 3. POSTOPERATIVE COMPLICATIONS
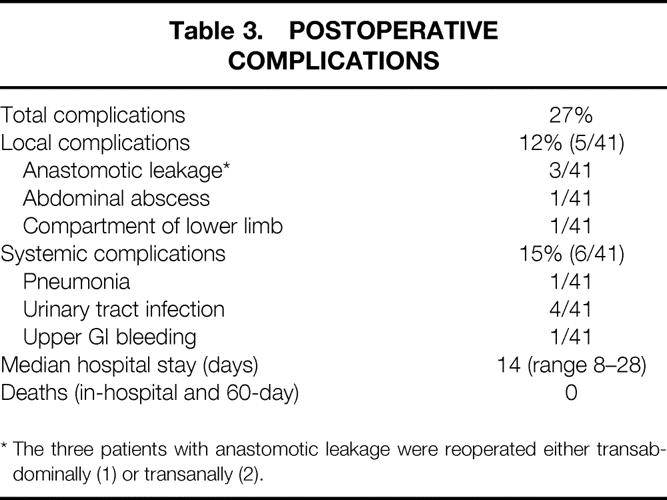
* The three patients with anastomotic leakage were reoperated either transabdominally (1) or transanally (2).
Anastomotic strictures occurred in 14% of patients (4/28) and required anastomotic dilatation in three patients. One of these patients had a postoperative symptomatic anastomotic leak necessitating reoperation and drainage of a perianastomotic abscess. Another patient underwent preoperative neoadjuvant chemoradiotherapy with a total radiation dose of 50.4 Gy for a bulky cancer of the lower third of the rectum with endosonographically potential involvement of the external anal sphincter.
Functional Results, Bowel Function
The preoperative frequency of bowel movements was 1.4 per 24 hours (range 0.5–3). Grade 1 incontinence was observed in 17% of patients before surgery (7/41). The median age of patients with preoperative incontinence was 67 years (interquartile range [IQR] 61–76.25) compared with 66.5 years (IQR 56–78) in patients with normal continence (P = .74). The distance from the anal verge to the distal tumor margin was less (6.0 cm; IQR 4.5–8.0) in patients with than in patients without incontinence (8.0 cm; IQR 6.0–10.0;P = .09). Patients who were incontinent before surgery had significantly higher average tumor diameters (median 5.5 cm [IQR 5.0–6.4] vs. 3.4 cm [IQR 2.5–4.5];P < .001) and largest tumor diameters (median 7.0 cm [IQR 5.0–8.5] vs. 3.8 cm [IQR 3.0–5.0];P < .001). The prevalence of preoperative urge was 12% (5/41) and was particularly seen in patients with bulky tumors of the lower third of the rectum. The presence of preoperative incontinence was not related to postoperative incontinence, but before surgery patients undergoing transverse coloplasty pouch anastomosis had clinical anal function and anorectal manometry measurements within normal limits . The median resting pressures in the upper (49 mm Hg [IQR 49–62]) and lower (43 mm Hg [IQR 39–61]) anal canal were not significantly different (P > .1) from results 2 months after surgery (upper anal canal, 50 mm Hg [IQR 28–56]; lower anal canal, 44 mm Hg [IQR 34–64]). The mean maximal tolerable volume in patients with transverse coloplasty pouch was 123 mL (IQR 120–145).
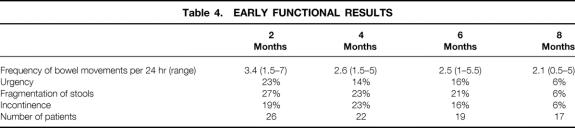
The mean follow-up was 10 months (range 1–22). Results of the early functional outcome are shown in Table 4. Frequency of bowel movement was not associated with the level of anastomosis. At all time points, the stool frequency in patients with an anastomosis at 4 cm or lower was not significantly different from that of patients with a higher anastomosis (4–6 cm;P = .07 to .7). Postoperative incontinence grade 1 and 2 improved over time in most patients, but grade 2 incontinence persisted in two patients without significant improvement until 6 months after closure of the ileostomy. Until 4 months of follow-up, incontinence was more prevalent in patients with an anastomosis at 4 cm or lower (2 months, 29%; 4 months, 36%) compared with patients with a pouch–anal anastomosis more than 4 cm from the anal verge (2 months, 8%; 4 months, 9%), but the difference was not significant (P > .17).
Table 4. EARLY FUNCTIONAL RESULTS
Nocturnal dysfunction was relatively rare at 2 months (12%), 4 months (5%), and 6 months (5%), and the regular use of soiling pads was noted in 15% of patients until 6 months after surgery. The retrospective analysis of incontinence data according to a validated score ranging from 0 (perfect continence) to 18 (complete incontinence) showed a mean of 3.4 (range 0–8) at 2 months, 3.0 (0–7) at 4 months, 2.8 (0–7) at 6 months, and 2.5 (0–7) at 8 months.
Follow-Up for Local Tumor Recurrence and Progression
In one patient (2.5%), local tumor recurrence and distant metastatic disease developed 24 months after tumor resection. One patient with hepatic metastasis at the time of the initial operation died at 22 months, and in two patients distant metastasis developed during the follow-up period. A diabetic patient died 3 months after the primary operation as a result of severe hypoglycemia, before the ileostomy was reversed.
DISCUSSION
A low anterior rectal resection with total mesorectal excision is the current standard treatment for rectal cancer. 19 The radicality of the operation is with few exceptions not compromised by the preservation of continence. The functional results after straight coloanal and low colorectal anastomosis can be improved by forming a pouch that increases the volume of the anastomosed colonic segment, and different pouch designs, including the ileocecal interposition pouch, have been clinically evaluated. 20 Pouch formation significantly decreases daily stool frequency compared with a straight coloanal anastomosis and improves the quality of life. The standard colon J-pouch achieves excellent early functional results, 3 and the trend toward the use of a small J-pouch significantly reduced the prevalence of evacuation problems from approximately 30% to 10% in the long-term follow-up. 11,12
The technically simpler transverse coloplasty pouch augments the neorectal volume by 40% compared with the straight coloanal anastomosis and has a significantly smaller capacity than a colon J-pouch. 13 The length of the colon after pouch formation was in all our patients sufficient to reach to the pelvic floor for a tension-free anastomosis to the low rectum, or the dentate line in patients undergoing intersphincteric resection. Optimal colonic perfusion is another decisive factor in anastomotic healing and has been an argument in favor of the colon J-pouch. 3,10,21 We experimentally showed with laser Doppler flow measurements that the antimesocolic transverse coloplasty does not impair perfusion proximally and distally to the suture line. 13 In addition, the left colon is completely mobilized toward the middle colic vessels and the pouch is placed as proximal as possible on the colon segment used for the anastomosis. In some cases the most proximal part of the descending colon can be used for pouch formation. This strategy improves perfusion at the level of the pouch and anastomosis and also avoids problems during pouch formation in patients with diverticular disease. The axial orientation of the transverse coloplasty pouch results in a better reach and may therefore be applicable in patients who do not have adequate bowel length to perform a colon J-pouch anastomosis and in case of a bulky mesentery or narrow pelvis. 17
The transverse coloplasty pouch was feasible and safe in this series, and intraoperative technical problems specifically related to pouch formation and anastomosis rarely occurred. The complications, with an anastomotic leakage rate of 7% and a 14% prevalence of anastomotic strictures, including three patients requiring dilatation, were similar to results after restorative rectal resection with total mesorectal excision. 22,23 All of the reported patients underwent radiologic control of the anastomosis in the meantime, and no further leaks were detected. A comparison between the transverse coloplasty pouch and the straight anastomosis and colon J-pouch procedures will have to be made in randomized controlled trials in the future, particularly because some reports indicate a reduction of the anastomotic leakage rate after colon J-pouch anastomosis. 3,10,24
The experience with the straight coloanal anastomosis procedure and the results observed with different sizes of the J-pouch indicate that function is related to the capacity of the neorectal reservoir. 8,25 The results obtained in our study support this hypothesis: the transverse coloplasty pouch reduced stool frequency compared with a straight coloanal reconstruction. The average frequency of 3.4 per 24 hours at 2 months of follow-up also seems higher than after a colonic J-pouch procedure. 3 In the 8 months of follow-up, the frequency of bowel movements further decreased to 2.1 per 24 hours, which is similar to the results after a colonic J-pouch procedure and less than after a straight coloanal procedure, but the range of daily bowel movements is larger after the transverse coloplasty pouch procedure. Interestingly, the frequency of bowel movements was not associated with the level of anastomosis from the anal verge. Further, the functional results may be negatively influenced by postoperative or preoperative irradiation, which aims at reducing the local recurrence rate or improving the chances for sphincter-saving procedures in very low rectal tumors. Anorectal physiology measurements were not significantly different before surgery and 2 months after surgery. The maximal tolerable volume of the transverse coloplasty pouch was almost identical to a recent analysis by Mantyh et al 26 that showed that the colon J-pouch and the transverse coloplasty pouch had similar functional outcome.
Incontinence for gas and less frequently for stool can be regularly observed in patients after low anterior rectal resection. Some patients, however, already have preoperative incontinence despite clinically and manometrically normal sphincter function. A newly acquired incontinence for gas (17% in this series) does not usually preclude a restorative rectal resection, and the follow-up showed that preoperative and postoperative incontinence was not related. Preoperative incontinence is, rather, associated with local tumor characteristics, because these patients tended to have lower rectal tumors and significantly bulkier tumors than patients with normal continence before surgery. Stool dysfunctions—urge, fragmentation, and incontinence for gas or liquid stool—were relatively frequent in the early postoperative follow-up and improved with time. The prevalence of such stool dysfunctions is comparable with the outcome after large and small colon J-pouch procedures, 11,12 but the results on early incontinence after colon J-pouch reported by Hallbook et al 3 seem better than in this series of transverse coloplasty pouch procedures. Because patients have been followed for up to 18 months after ileostomy closure, the current analysis shows also that similar to a small colon J-pouch, evacuation problems may not appear in the late follow-up.
In conclusion, the transverse coloplasty pouch is a small-volume reservoir that can be safely used for reconstruction after sphincter-preserving rectal resection. The early functional outcome is favorable and can be compared to other colonic reservoirs. The concept of reducing the early dysfunctions seen after straight coloanal anastomosis and avoiding the long-term problems of pouch evacuation is supported by the current results. Further studies will be needed to compare this novel concept with other techniques of restorative resection of the rectum.
Discussion
Prof. Ch. Herfarth: It is a great pleasure for me to discuss the paper of the group of surgeons led by Markus Büchler in Bern describing a new surgical concept of rectal replacement after low anterior resection. We should comment on this presentation from two perspectives: first, the operative technique, and second the semantic definition, meaning the exact term for this operation.
The great advantage of this surgical procedure is its simplicity and logic. By longitudinal incision of about 7 cm and transverse closure at the antimesenteric wall of the transposed descending colon as a neorectum, the propulsive peristalsis is interrupted, and by widening the lumen of the colon, a type of pouch is constructed. Compared to the J-pouch of the colon for coloanal anastomosis, the reservoir capacity is smaller; on the other hand, it is proven that too large a pouch (i.e., >5 cm length of J-Pouch) may provoke coprostasis with a very inconvenient and annoying urgency and fragmentation. This manuscript is a very conclusive prospective phase 1 study of a new operative technique. In further publications, more anorectal physiology measurements should be added.
I have the following questions: Which anorectal physiologic measurements were performed in this trial? What is your strategy in the presence of severe diverticular disease? Did you use a validated score like that of Steve Wexner? Is there any influence of neoadjuvant chemo- and/or radiotherapy on the results?
Comment on the term “transverse coloplasty pouch”: In two publications in the Journal of Diseases of Colon and Rectum, Viktor Fazio used the definition of “colonic coloplasty.” What does it mean compared to transverse coloplasty pouch? Both definitions have the coloplasty as description of the longitudinal incision and transverse suturing. The technical term of transverse coloplasty pouch is caused by the fact that the propulsive peristalsis of the colon is interrupted. In this way a functional gain of capacity comparable to a small pouch is produced. Therefore, the term chosen by the Swiss group and published in 1997 and 1999 in the DDW abstract and the Journal of Digestive Surgery, respectively, should become standard.
The final proof of the validity of the new therapy will be acquired by a multicenter prospective trial. For this very interesting operative procedure, the proof of principle is very encouraging, and I congratulate the authors on this very original and convincing presentation.
Prof. P. R. Hawley: I enjoyed your paper. I take issue with you on your title, as I do not think this is a pouch. A pouch has to be a loop of bowel stitched to another loop. You do not describe a pyloroplasty as an antral pouch nor a small bowel stricturoplasty as a small bowel pouch. I also wonder if this is new. I know you take issue with Victor Fazio about who described the technique first. It has been talked about in colorectal circles for a number of years. Fazio had a poster on it in 1999 where he described some patients and has published a paper this year, and I understand that Seow-Chung, from Singapore, has already done a prospective trial which is about to be published. I wonder who was first with a clinical paper, and I am uncertain as to why you had carried out animal experiments, which you published, if you wished your name in the literature as being the first to describe this procedure in patients. I actually think that the animal experiments are a total waste of time in this context, as they give you no knowledge of human function. It is a very straightforward procedure that is being done in other parts of the colon, so you could have performed it in patients from the outset, and had you done so 2 or 3 years ago, nobody would doubt that you had been first.
The results of coloanal pouch anastomosis compared with a straight coloanal anastomosis are variable, but it is generally agreed that bowel action is decreased in the early follow-up. However, some patients have difficulty in emptying the pouch and fragmented stools. The advantage of the coloanal pouch gradually diminishes with time, and long-term follow-up fails to find any benefit. Perhaps your procedure will help to decrease early frequency of defecation while preventing difficulties in evacuation.
I would like to ask if it is correct that some of your patients had carcinomas at the top of the rectum. On your slide the highest tumor was at 15 cm and you could easily do a straight anastomosis. The one advantage of your operation is that it is simple, and I would have thought this could have been done without a covering stoma. If this has to be carried out in all patients, you have lost some of the advantage. Some of your anastomoses were in the lower rectum rather than a true coloanal anastomosis, and many surgeons would carry out such an anastomosis without a defunctioning stoma.
Prof. P. R. F. Bell: Vascular surgeons have already been put in their place earlier in the discussion. We were told we could not do cholangiocystectomy, so you will excuse this question: I am, being a nonrectal surgeon, uncertain about the actual mechanism of evacuation following these procedures. Is it to do with pouch size? Is it that you have to have a certain volume of pouch or “rectum,” or is it to do with the fact that the patient gets used to having a smaller “rectum” and accommodates to it? If this is not the case, is it the nerve supply or the pelvic muscles that are important? It strikes me that the piece of colon that you have enlarged has not been enlarged very much. What is the increase in volume that you have achieved; is it 10% or 50%?
Prof. M. W. Büchler: Thank you, Mr. Chairman, I just want to clear the political issue. There were two people mentioning this issue with Victor Fazio, and therefore I would like to explain this to the members of ESA.
After Victor Fazio and his colleagues had published in the Journal of Diseases of Colon and Rectum last year and this year again a clinical series about the pouch, we have sent a letter to the editor of this journal where we explained the originality of this technique. Victor Fazio has just replied to me, and he will publish his reply in this journal, that this technique has been developed in Bern and that he has not seen our primary publication about this technique in humans. He admits that there is no longer a conflict of who has developed this technique, namely the Bern team.
Dr. K. Z’graggen (closing): Thank you for your questions and thank you, Professor Büchler, for clarifying the political issue. The first question from Prof. Herfarth concerned stool function. Were the patients with ileostomy included or not? We assessed the function from the time of ileostomy closure and did physiology measurements preoperatively, 2 months and 12 months postoperatively. We have not presented validated incontinence scores but presented our data according to the definitions you’ll find in the paper and assessed incontinence according to the most commonly used incontinence score. In response to Dr. Hawley’s question, we always do protective loop ileostomy because an anastomotic leak without ileostomy will have a higher risk of pelvic sepsis than an anastomotic leak protected by an ileostomy. In response to the question about adjuvant therapy and function, I would like to add the data on our seven patients that underwent neoadjuvant chemo- or radiotherapy. Five of these patients (bulky T3 and node-positive tumors) had a short radiation regimen with 25 Gy over 5 days, followed by the operation; two of these patients (T4 tumors) received 50 Gy of radiation over 5 weeks and were operated after a waiting period of 3 to 4 weeks. One of the two patients receiving the long regimen of neoadjuvant therapy developed an anastomotic stricture and needed anal dilatation. The functional result after dilatation of the very short, tense stricture was excellent. The functional results of the other patients were different from the group that did not undergo neoadjuvant therapy.
What about patients with diverticular disease? We always place the transverse coloplasty pouch as proximal as possible on the colon to have the best possible blood supply for the anastomosis. The left-sided colon is mobilized to the middle colic vessels, and usually the entire sigmoid colon and part of the descending colon is resected. Therefore, diverticular disease is not a problem with our technique of reconstruction.
In response to Dr. Hawley’s question about the patient with a proximal rectal cancer that underwent TME and transverse coloplasty pouch anal anastomosis, I mentioned in my presentation that this patient had a broad-based polyp at the dentate line. This was the indication for a complete rectal resection.
We do know that there is an ongoing randomized controlled trial under the direction of Frances Seow-Cheung from Singapore comparing the transverse coloplasty pouch with the standard colon–J-pouch. Our group is also preparing a randomized controlled trial. The results of these trials will allow us to validate the transverse coloplasty pouch and define the place for its clinical use.
Dr. Bell made a very important point about the functional principle of the transverse coloplasty pouch: is it the volume, or is it not the volume? Measuring pouch volume is rather difficult, but we have experimentally done this in our pig model. Using a standardized technique, the increase in neorectal volume is 40% compared with a straight coloanal anastomosis. Therefore, I think that the technique of transverse coloplasty does in fact form a small colon pouch. Whether the volume is the principle of action to improve stool function cannot be answered at present. In order to improve functional results, it seems to be very important to have a motility break on the propulsive action of the colon. This simple technique of reconfiguring a segment of colon adds an extra volume and seems to introduce such a propulsive break.
Footnotes
Correspondence: Markus W. Büchler, MD, Department of General Surgery, Ruprecht-Karls University, Im Neuenheimer Feld 110, 69120 Heidelberg.
E-mail: markus_buechler@med.uni-heidelberg.de
Accepted for publication April 2001.
References
- 1.Lazorthes F, Fages P, Chiotasso P, et al. Resection of the rectum with construction of a colonic reservoir and colo-anal anastomosis for carcinoma of the rectum. Br J Surg 1986; 73: 136–138. [DOI] [PubMed] [Google Scholar]
- 2.Parc R, Tiret E, Frileux P, et al. Resection and colo-anal anastomosis with colonic reservoir for rectal carcinoma. Br J Surg 1986; 73: 139–141. [DOI] [PubMed] [Google Scholar]
- 3.Hallbook O, Pahlman L, Krog M, et al. Randomized comparison of straight and colonic J pouch anastomosis after low anterior resection. Ann Surg 1996; 224: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho YH, Tan M, Seow-Choen F. Prospective randomized controlled study of clinical function and anorectal physiology after low anterior resection: comparison of straight and colonic J pouch anastomoses. Br J Surg 1996; 83: 978–980. [DOI] [PubMed] [Google Scholar]
- 5.Seow-Choen F, Goh HS. Prospective randomized trial comparing J colonic pouch-anal anastomosis and straight coloanal reconstruction. Br J Surg 1995; 82: 608–610. [DOI] [PubMed] [Google Scholar]
- 6.Lazorthes F, Chiotasso P, Gamagami RA, et al. Late clinical outcome in a randomized prospective comparison of colonic J pouch and straight coloanal anastomosis. Br J Surg 1997; 84: 1449–1451. [PubMed] [Google Scholar]
- 7.Mortensen NJ, Ramirez JM, Takeuchi N, et al. Colonic J pouch-anal anastomosis after rectal excision for carcinoma: functional outcome. Br J Surg 1995; 82: 611–613. [DOI] [PubMed] [Google Scholar]
- 8.Seow-Choen F. Colonic pouches in the treatment of low rectal cancer. Br J Surg 1996; 83: 881–882. [DOI] [PubMed] [Google Scholar]
- 9.Nicholls RJ, Lubowski DZ, Donaldson DR. Comparison of colonic reservoir and straight colo-anal reconstruction after rectal excision. Br J Surg 1988; 75: 318–320. [DOI] [PubMed] [Google Scholar]
- 10.Dennett ER, Parry BR. Misconceptions about the colonic J-pouch: what the accumulating data show. Dis Colon Rectum 1999; 42: 804–811. [DOI] [PubMed] [Google Scholar]
- 11.Hida J, Yasutomi M, Fujimoto K, et al. Functional outcome after low anterior resection with low anastomosis for rectal cancer using the colonic J-pouch. Prospective randomized study for determination of optimum pouch size. Dis Colon Rectum 1996; 39: 986–991. [DOI] [PubMed] [Google Scholar]
- 12.Lazorthes F, Gamagami R, Chiotasso P, et al. Prospective, randomized study comparing clinical results between small and large colonic J-pouch following coloanal anastomosis. Dis Colon Rectum 1997; 40: 1409–1413. [DOI] [PubMed] [Google Scholar]
- 13.Z’graggen K, Maurer CA, Mettler D, et al. A novel colon pouch and its comparison with a straight coloanal and colon J-pouch–anal anastomosis: preliminary results in pigs. Surgery 1999; 125: 105–112. [PubMed] [Google Scholar]
- 14.Maurer CA, Z’graggen K, Zimmermann W, et al. Experimental study of neorectal physiology after formation of a transverse coloplasty pouch. Br J Surg 1999; 86: 1451–1458. [DOI] [PubMed] [Google Scholar]
- 15.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med 1997; 336: 980–987. [DOI] [PubMed] [Google Scholar]
- 16.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986; 1: 1479–1482. [DOI] [PubMed] [Google Scholar]
- 17.Z’graggen K, Maurer CA, Buchler MW. Transverse coloplasty pouch. A novel neorectal reservoir. Dig Surg 1999; 16: 363–366. [DOI] [PubMed] [Google Scholar]
- 18.Arndorfer RC, Steff JJ, Dodds WJ, et al. Improved infusion system for intraluminal esophageal manometry. Gastroenterology 1977; 73: 23–27. [PubMed] [Google Scholar]
- 19.Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 1998; 133: 894–899. [DOI] [PubMed] [Google Scholar]
- 20.von Flüe MO, Degen LP, Beglinger C, et al. Ileocecal reservoir reconstruction with physiologic function after total mesorectal cancer excision. Ann Surg 1996; 224: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallböök O, Johansson K, Sjödahl R. Laser-Doppler blood flow measurement in rectal resection for carcinoma: comparison between the straight and colonic J-pouch reconstruction. Br J Surg 1996; 83: 389–392. [DOI] [PubMed] [Google Scholar]
- 22.Moran B, Heald R. Anastomotic leakage after colorectal anastomosis. Semin Surg Oncol 2000; 18: 244–248. [DOI] [PubMed] [Google Scholar]
- 23.Merad F, Hay JM, Fingerhut A, et al. Is prophylactic pelvic drainage useful after elective rectal or anal anastomosis? A multicenter controlled randomized trial. French Association for Surgical Research. Surgery 1999; 125: 529–535. [PubMed] [Google Scholar]
- 24.Joo JS, Latulippe JF, Alabaz O, et al. Long-term functional evaluation of straight coloanal anastomosis and colonic J-pouch: is the functional superiority of colonic J-pouch sustained? Dis Colon Rectum 1998; 41: 740–746. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee AK, Parc R. Prediction of optimum dimensions of colonic pouch reservoir. Dis Colon Rectum 1996; 39: 1293–1295. [DOI] [PubMed] [Google Scholar]
- 26.Mantyh CR, Hull TL, Fazio VW. Coloplasty in low colorectal anastomosis. Manometric and functional comparison with straight and colonic J-pouch anastomosis. Dis Colon Rectum 2001; 44: 37–42. [DOI] [PubMed] [Google Scholar]