Abstract
Neurogenin 3 (Ngn3) is key for endocrine cell specification in the embryonic pancreas and induction of a neuroendocrine cell differentiation program by misexpression in adult pancreatic duct cells. We identify the gene encoding IA1, a zinc-finger transcription factor, as a direct target of Ngn3 and show that it forms a novel branch in the Ngn3-dependent endocrinogenic transcription factor network. During embryonic development of the pancreas, IA1 and Ngn3 exhibit nearly identical spatio-temporal expression patterns. However, embryos lacking Ngn3 fail to express IA1 in the pancreas. Upon ectopic expression in adult pancreatic duct cells Ngn3 binds to chromatin in the IA1 promoter region and activates transcription. Consistent with this direct effect, IA1 expression is normal in embryos mutant for NeuroD1, Arx, Pax4 and Pax6, regulators operating downstream of Ngn3. IA1 is an effector of Ngn3 function as inhibition of IA1 expression in embryonic pancreas decreases the formation of insulin- and glucagon-positive cells by 40%, while its ectopic expression amplifies neuroendocrine cell differentiation by Ngn3 in adult duct cells. IA1 is therefore a novel Ngn3-regulated factor required for normal differentiation of pancreatic endocrine cells.
Keywords: beta cell, development, diabetes, duct cell, transdifferentiation
Introduction
The first morphological signs of the primitive pancreas emerge as dorsal and ventral protrusions of the primitive gut epithelium (Slack, 1995) at embryonic day (E) 9.5 in the mouse. Subsequently, all lineages defining the various pancreatic cell types, comprising endocrine islet and exocrine acinar and duct cells, are formed from a multipotent progenitor cell pool expressing the transcription factor Pdx1 (Gu et al, 2002). This process is regulated by a cascade of transcription factors that initiate and maintain the distinct genetic programs (Wilson et al, 2003; Jensen, 2004). The basic helix–loop–helix transcription factor Ngn3 is transiently expressed in a subset of the pancreas progenitor cells from E9.5 to E18.5 and initiates the differentiation program of all islet cells (Apelqvist et al, 1999; Gradwohl et al, 2000; Jensen et al, 2000; Schwitzgebel et al, 2000; Gu et al, 2002). Homozygous Ngn3-null mice thus fail to develop endocrine islet cells (Gradwohl et al, 2000) and premature or ectopic expression of Ngn3 in embryonic endoderm is sufficient to initiate endocrine cell differentiation (Apelqvist et al, 1999; Schwitzgebel et al, 2000; Grapin-Botton et al, 2001). The specification of different islet cell types and the completion of the differentiation process require the activation of transcription factors that are downstream of Ngn3. Of these regulatory factors NeuroD1, Pax4 and Nkx2.2 are direct targets of Ngn3 (Huang et al, 2000; Smith et al, 2003; Watada et al, 2003). Together with Nkx6.1 and Arx they act early in the differentiation/specification process (Sander et al, 2000; Collombat et al, 2003, 2005). Arx and Pax4 are required for the specification of the α- and β-cell lineage, respectively (Sosa-Pineda et al, 1997; Collombat et al, 2003, 2005). Further differentiation and maintenance of the endocrine phenotype depends on the activity of other transcription factors such as Isl1 and Pax6 (Ahlgren et al, 1997; Sander et al, 1997; St-Onge et al, 1997). Despite these findings, the precise genetic program controlling the differentiation of islet progenitors into beta cells remains unclear. Such knowledge is essential to generate functional beta cells in vitro for cell replacement therapy in type I diabetes. Ectopic expression of Ngn3 in adult human pancreatic duct cells supported this concept by activating the genes encoding Pdx1, NeuroD1, Pax4, Pax6, Nkx6.1 and Nkx2.2 and transdifferentiating duct cells into a β-cell-like phenotype, albeit with low levels of insulin (Heremans et al, 2002).
In order to identify novel target genes of Ngn3, the transcriptome of adult human duct cells ectopically expressing Ngn3 was analysed on gene chips (Bonné et al, unpublished data). Among the genes most prominently induced by Ngn3 was that encoding the zinc-finger type transcription factor ‘insulinoma associated 1' (IA1 or INSM1). IA1 was previously shown to be expressed in insulinoma and other endocrine tumours and cell lines, human embryonic pancreas and mouse nervous system (Goto et al, 1992; Lan et al, 1993; Zhu et al, 2002; Breslin et al, 2003; Pedersen et al, 2003). So far neither the expression pattern of IA1 nor its function during pancreas development have been addressed directly. The present study reveals that IA1 is transiently expressed in similar cells as Ngn3 during pancreatic development and shows that the IA1 gene is directly regulated by Ngn3 but not by other endocrine lineage transcription factors. It also provides evidence that IA1 ensures an essential stimulatory signal for proper formation of β- and α-cells.
Results
The zinc-finger transcription factor IA1 is a Ngn3 target
Novel targets of the endocrinogenic master switch transcription factor Ngn3 were identified by analysis of the transcriptome of adult human pancreatic duct cells transduced with recombinant adenovirus expressing either Ngn3-GFP or GFP (Bonné et al, in preparation). IA1 (INSM1), a zinc-finger transcription factor in neoplastic β cells (Goto et al, 1992), was among the genes activated most strongly by Ngn3. Induction of IA1 preceded the expression of Pax4, NeuroD1 and Nkx2.2, which are direct targets of Ngn3 (Figure 1A) (Huang et al, 2000; Heremans et al, 2002; Smith et al, 2003; Watada et al, 2003) and essential for endocrinogenesis in the embryonic pancreas (Naya et al, 1997; Sosa-Pineda et al, 1997; Sussel et al, 1998). IA1 gene expression in adult duct cells becomes apparent at approximately 15–17 h following transduction, when the ectopic Ngn3 protein is first detected (Figure 1A and B). The appearance of IA1 mRNA in AdHANgn3-infected duct cells could be a consequence of Ngn3-induced activation of NeuroD1, since NeuroD1 is induced by Ngn3 (Huang et al, 2000) and because the proximal E3 box of the IA1 promoter is a reported target of the basic helix–loop–helix heterodimer NeuroD1/E47 (Breslin et al, 2003). However, IA1 was turned on by Ngn3 much earlier than NeuroD1 (Figure 1A) and IA1 transcripts were present at only low abundance 4 days following infection with AdNeuroD1 (Figure 1D). When expressed in adult human pancreatic duct cells at similar levels as Ngn3 or NeuroD1, other developmental transcription factors such as Foxa1, Foxa2, Pdx1, Pax4, Pax6, Nkx6.1 and Nkx2.2 failed to induce IA1 expression (Figure 1C). These data indicate that Ngn3 is a positive regulator of IA1, and this effect is unlikely to be mediated exclusively by NeuroD1 as an intermediary effector.
Figure 1.
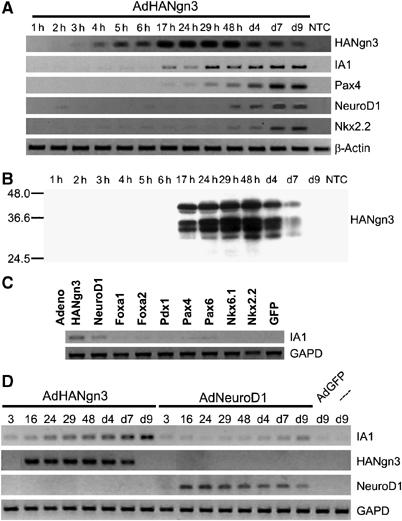
Rapid and specific induction of IA1 expression in AdHANgn3-transduced adult human duct cells. (A) RT–PCR analysis of gene expression in AdHANgn3-transduced duct cells. Exogenous Ngn3 is detected at 4 h following transduction. IA1 expression is noticed at 17 h following transduction, which is earlier than the proposed direct Ngn3 target genes Pax4, NeuroD1 and Nkx2.2. (B) Exogenous HANgn3 protein is detected at 17 h post-transduction. Expression levels rise until 48 h post-transduction, after which they gradually drop and become undetectable at day 9. (C) Activation of IA1 gene expression in adult human pancreatic duct cells is observed following transduction with either AdHANgn3 and, albeit much weaker, AdNeuroD1, but not with adenoviral Foxa1, Foxa2, Pdx1, Pax4, Pax6, Nkx6.1, Nkx2.2 and GFP. RNA was extracted at 7 days following transduction. (D) Time course of the induction of IA1 expression in adult human duct cells transduced with either AdHANgn3 or AdNeuroD1. Induction of endogenous IA1 starts at 16 h post-infection with AdHANgn3 until at least 9 days following transduction. In contrast, induction of endogenous IA1 by AdNeuroD1 is obvious only at 4 days following transduction. Background signal caused by DNA of the single exon IA1 gene. NTC, nontransduced control.
We next examined whether Ngn3 directly regulates the transcription of the IA1 gene. IA1 promoter-driven luciferase activity was increased in 293 cells when the reporter was cotransfected with a Ngn3 expression plasmid (Figure 2A). The extent of increase was similar to that observed in cells cotransfected with NeuroD1 and the reporter construct, in line with the observations by Breslin et al (2003). Supertransfection with cDNA encoding E47 did not influence the outcome of these experiments (data not shown). To establish whether Ngn3 binds directly to the IA1 gene promoter in vivo, chromatin immunoprecipitation was performed using adult human duct cells transduced with either AdHANgn3 or AdGFP. Genomic DNA was sheared to 200–1000 bp prior to immunoprecipitation (Figure 2B). Promoter regions of the IA1 and NeuroD1 genes but not control gene fragments from BRCA1 or CTLA4 were coimmunoprecipitated by HANgn3 (Figure 2C). Taken together, these results show that IA1 is a novel direct target of Ngn3, and hence becomes activated during the Ngn3-induced transdifferentiation program in adult human duct cells.
Figure 2.
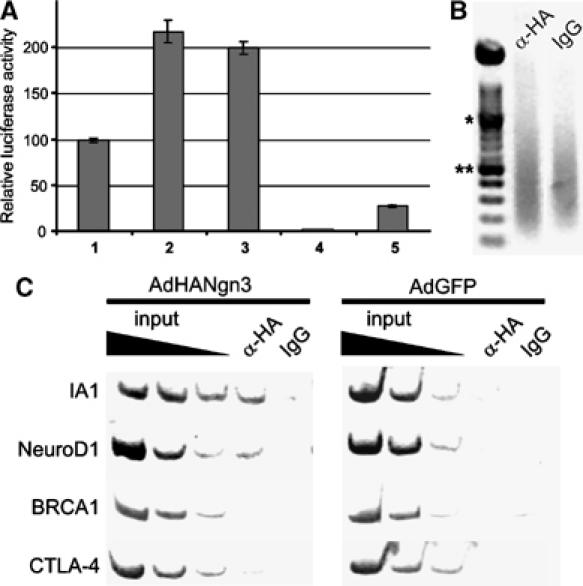
Ngn3 binds and activates the IA1 promoter. (A) The IA1 promoter is activated by Ngn3 and NeuroD1 in transient promoter–reporter assays in 293 cells. Cotransfection of an IA1 promoter–reporter construct with Ngn3 (2) or NeuroD1 (3) increases IA1 promoter activity by twofold as compared to the IA1 promoter only (1). The pGL3basic (4) and pGL3control (5) samples represent negative and positive controls for promoter activity, respectively. All experiments were performed in triplicate and repeated at least three times. (B, C) Ngn3 interacts with the 5′ flanking regions of the IA1 and NeuroD1 genes. (B) Ethidium bromide-stained agarose gel showing chromatin sonicated to an average length of 200–1000 bp. *1000 bp; **500 bp. (C) Chromatin immunoprecipitation with an anti-HA antibody or IgG was performed on chromatin derived from isolated human duct cells infected with either HANgn3 or GFP. DNA from input chromatin was serially diluted as a reference for semiquantitative PCR analysis. The figures are representative of four independent experiments. The IA1 gene promoter is coimmunoprecipitated in the HANgn3-transduced duct cells using an anti-HA antibody but not by addition of IgG. Similarly, NeuroD1, but not BRCA1 or CTLA-4, are specifically co-precipitated by the anti-HA antibody. IA1, NeuroD1, BRCA1 and CTLA-4 are not precipitated in the negative control cells transduced with AdGFP.
Transient expression of IA1 in islet progenitor cells of the mouse embryonic pancreas
Since Ngn3 is not expressed in normal adult human or mouse pancreas (Gradwohl et al, 2000; Jensen et al, 2000; Schwitzgebel et al, 2000), our findings on Ngn3-dependent induction of IA1 prompted characterization of IA1 gene expression in embryonic mouse pancreas. At embryonic day E10.5, IA1 is detected in scattered cells (Figure 3A). Its highest expression levels are reached at E15.5 and subsequently decrease below detection limits from E18.5 (Figure 3A–D), correlating well with the timing of Ngn3 expression (Gradwohl et al, 2000). At embryonic day E15.5, IA1 transcripts were also observed in the duodenum and stomach, as well as in thymus, thyroid and adrenal glands (Figure 3E). In addition, IA1 is expressed in regions of the developing forebrain, midbrain and hindbrain, and the spinal cord (Figure 3E). In situ hybridization on adjacent cryo-sections showed that the spatio-temporal expression pattern of IA1 is similar to that of Ngn3 and partially overlapping with NeuroD1 (Figure 3F–H). In agreement with this observation, some cells express Ngn3 but not yet IA1, and IA1 transcripts appeared in undifferentiated cells that contain Ngn3 protein (Figure 3I). However, while no Ngn3-positive cells coexpressed islet hormones (Gradwohl et al, 2000), rare IA1-positive cells contained insulin or glucagon (Figure 3C, arrow), suggesting that the appearance of IA1 lags behind that of Ngn3 in the islet precursor cells. Furthermore, in the embryonic pancreas rare IA1-expressing cells are actively cycling, as demonstrated by BrdU incorporation (Figure 3J).
Figure 3.
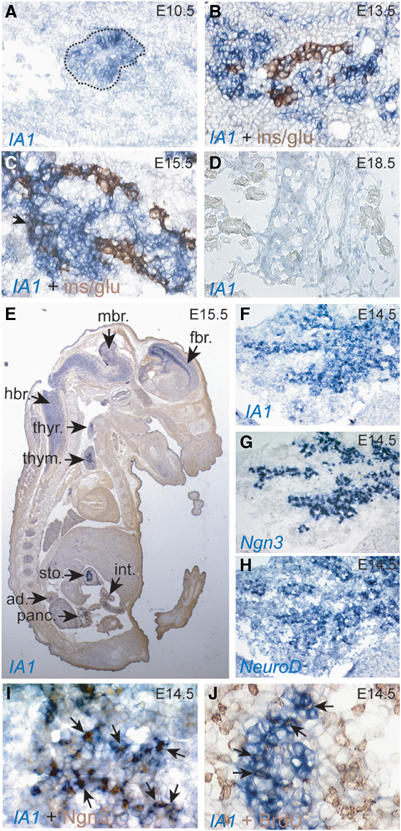
Overlapping expression patterns of IA1 and Ngn3 in islet progenitor cells of mouse embryo. (A) IA1 gene transcription in the pancreas starts at E10.5, as shown by in situ hybridization. Dashed line delimits the pancreatic epithelium. (B, C) Insulin and glucagon are present in few IA1-expressing cells (arrow in C). (D) The number of IA1-expressing cells increases until E15.5, then rapidly decreases and no IA1 expression can be detected at E18.5. (E) In E15.5, mouse IA1 transcripts (blue staining) are observed in the developing nervous system and endocrine glands as well as in the pancreas and gastrointestinal tract. (F–H) In situ hybridization on consecutive pancreas sections shows coexpression of IA1 and Ngn3 in islet precursor cells, while coexpression with NeuroD1 is limited. (I) IA1 (in situ hybridization, blue) and Ngn3 (immunohistochemistry, brown) are coexpressed in endocrine progenitor cells (arrows). (J) BrdU labels IA1-expressing cells (arrows). ad, adrenal gland; fbr, forebrain; hbr, hindbrain; int, intestine; mbr, midbrain; panc, pancreas; thym, thymus; thyr, thyroid; sto, stomach. Magnification A–D and F–H: × 40, E: × 5, I and J: × 63.
IA1 expression is selective in the endocrine lineage and depends on Ngn3
To determine conclusively whether IA1 is expressed selectively in islet progenitors or also in immature acinar or duct cells, IA1 expression was examined in Ngn3 knockout mice that lack all types of islet cells (Gradwohl et al, 2000). No IA1-expressing cells were detected in pancreas of the Ngn3 null mutants (Figure 4A and B), ascertaining that IA1 is exclusively expressed in the endocrine lineage of the embryonic pancreas. Furthermore, it supports that Ngn3 is necessary to induce IA1 not only in transdifferentiating adult duct cells in vitro but also in embryonic progenitor cells in vivo.
Figure 4.

IA1 is specifically expressed in the islet lineage, immediately downstream of the proendocrine gene Ngn3. (A–J) In situ hybridization with probes specific for IA1 and Ngn3 on consecutive cryosections of embryonic pancreas from mutant mice deficient for the Ngn3, NeuroD1, Pax4, Arx or Pax6 transcription factors, respectively. (A, B) No IA1 transcripts are detected in the pancreas of Ngn3-deficient mice. Expression of IA1 and Ngn3 is unaffected in the pancreas of NeuroD1 (C, D), Pax4 (E,F), Arx (G, H) and Pax6 (I, J) null mutant embryos. Signals for IA1 and Ngn3 overlap on consecutive sections of pancreas from the different mutant mice. Magnification A–J: × 40.
As mentioned earlier, data obtained by others (Breslin et al, 2003) and ourselves (Figure 1C and D) indicate that NeuroD1 may also activate IA1 transcription. We therefore analysed the expression pattern of IA1 and Ngn3 on adjacent cryosections of pancreas from mouse embryos that were null mutant for NeuroD1 (Figure 4C and D). We also assessed embryos deficient for other key developmental transcription factors acting downstream of Ngn3, namely Pax4, Arx and Pax6 (Figure 4E–J) (Sosa-Pineda et al, 1997; St-Onge et al, 1997; Huang et al, 2000; Collombat et al, 2003, 2005). IA1 and Ngn3 have a similar expression pattern both in NeuroD1 mutant and wild-type embryonic pancreas (Figure 4C and D versus Figure 3F and G). This observation demonstrates that NeuroD1 is not essential for the expression of both IA1 and Ngn3 (Figure 4C and D). In addition, the expression of mRNAs encoding IA1 or Ngn3 is also not affected in the islet precursors of Pax4−/− (Figure 4E and F), Arx−/− (Figure 4G and H) or Pax6−/− (Figure 4I and J) mutant mice. These results show that IA1 is exclusively expressed in the Ngn3-dependent cellular lineage and that its expression is dependent on Ngn3 but not on known downstream regulators. Together with data indicating that Ngn3 occupies the IA1 gene, these findings place IA1 at a discrete early step subsequent to Ngn3 in the regulatory cascade that drives pancreatic endocrine differentiation.
IA1 is an essential regulator of endocrine cell generation in the embryonic mouse pancreas
To further clarify the role of IA1 in pancreatic endocrine development, tissue explants from E12.5 pancreas were cultured under conditions that allow endocrine cell differentiation and islet cell formation (Miralles et al, 1998; Mellitzer et al, 2004). The pancreatic rudiments were isolated from mice expressing the enhanced green fluorescent protein (eGFP) as a reporter under control of the Ngn3 promoter and thus enable tracing of endocrine progenitor cells (data not shown). Explants were incubated with IA1-specific antisense oligonucleotides of the morpholino-type to inactivate IA1 translation. Although antisense oligonucleotide technology to knockdown specific gene expression in embryonic pancreas in vitro has been reported before (Prasadan et al, 2002; Li et al, 2004), we validated the uptake of biotinylated morpholinos by immunostaining (Figure 5B and B′) and observed a gradient towards the inside of the explant. Two antisense oligonucleotides of the morpholino-type were designed (sequence under Materials and methods) to target different segments of the IA1 mRNA sequence. Antisense or control oligonucleotides were incubated with the pancreatic explants during the entire period of culture. As compared to nontreated explant cultures, presence of the control morpholino oligonucleotides (Figure 5A–E) did not alter growth rate (unpublished data) or normal cell differentiation. However, while differentiation of exocrine cells was not affected by the antisense morpholinos, as shown by immunostaining of amylase-positive cells (Figure 5E′), both anti-IA1 oligos reduced the total number of cells containing immunoreactive glucagon or insulin to a similar extent, that is, 40% (Figure 5D′ and F).
Figure 5.

Inhibition of IA1 translation blocks islets cell differentiation. E12.5 dorsal pancreatic epithelia were isolated from Ngn3-promoter-driven eGFP transgenic embryos, the surrounding mesenchyme was dissected away and the remaining epithelial cells were cultured for 4 days in the presence of standard missense control morpholinos (A–E) or IA1-specific morpholinos (A′–E′). (A, A′) Bright field image showing normal growth of pancreatic rudiments. (B, B′) Peroxidase staining (brown) shows the uptake and distribution of biotinylated antisense oligonucleotides in the pancreatic rudiments. (C, C′) eGFP fluorescence of Ngn3+ endocrine progenitor cells in living explants. (D, D′) eGFP fluorescence combined with immunodetection of insulin and glucagon (red) on cryosections of pancreatic explants at day 4 of culture. Cell nuclei are counterstained with DAPI. (E, E′) Immunodetection of amylase (red) on cryosections of pancreatic explants at day 4 of culture. Cell nuclei are counterstained with DAPI. Magnification A–E: × 20. (F) Quantification of Ngn3-eGFP and insulin- and glucagon-expressing cells in standard missense control-treated or IA1 antisense-treated pancreatic explants shows a significant decrease (morpholino 1 P<0.02, morpholino 2 P<0.05) in islet cell numbers in IA1 antisense-treated explants. Each figure represents the mean±s.e.m. of four (morpholino 1) or three (morpholino 2) independent experiments with 3–4 explants per individual experiment. Statistical significance of the data was determined by unpaired, two-tailed Student's t-test.
IA1 enhances transdifferentiation of adult human pancreatic duct cells
As ectopic expression of Ngn3 recapitulates embryonic endocrinogenesis in adult pancreatic duct cells (Heremans et al, 2002), the capacity of the direct Ngn3 target IA1 to induce or improve duct cell transdifferentiation was investigated. Ectopic IA1 alone did not activate the genes encoding the developmental transcription factors NeuroD1, Pax4 and Nkx2.2, Delta-Notch signalling cascade components DLL1, DLL4, Hes6 and Hes1, or neuroendocrine marker genes such as synaptophysin (SYP), chromogranin A (CHGA), PC1/3 and INS (Figure 6A). As IA1 was originally characterized as a transcriptional repressor (Breslin et al, 2002), its interaction with Ngn3-induced gene expression mediating endocrine cell development or function was analysed by normal (Figure 6B) and real time (Figure 6C) RT–PCR. Coexpression of Ngn3 and IA1 in duct cells enhanced the induction of the Ngn3 target genes NeuroD1, Pax4 and Nkx2.2 by 2–6-fold depending on the target gene (n=3, P<0.05) (Figure 6C). These observations thus suggest that IA1 and Ngn3 act in concert to stimulate the endocrinogenic program in the pancreas.
Figure 6.
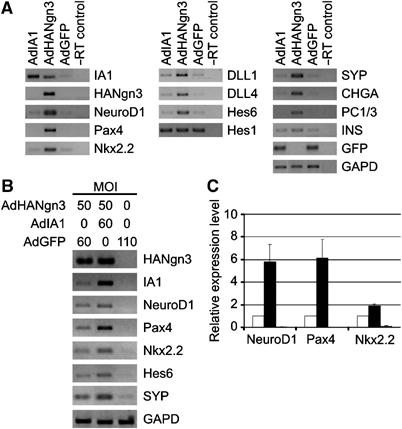
Profiling gene expression in adult human pancreatic duct cells following ectopic expression of Ngn3 and IA1. (A) Duct cells were transduced with either AdHANgn3, AdIA1 or AdGFP and processed for RT–PCR analysis at 7 days following transduction. Exogenous HANgn3, IA1 and Ngn3-induced endogenous IA1 are readily detected in AdIA1 and AdHANgn3 samples, respectively, but not in the AdGFP control. Viral Ngn3 contains a HA-tag and is detected in the AdHANgn3 samples only. Ectopic IA1 expression does not induce endogenous NeuroD1, Pax4 or Nkx2.2 transcription factor expression, in contrast to Ngn3. Induction of Delta-Notch signaling components DLL1, DLL4 and Hes6 by Ngn3 is not recapitulated by IA1, but remains at expression levels comparable to the GFP control sample. IA1 does not influence Hes1 mRNA abundance. The endocrine marker genes encoding SYP, CHGA, prohormone convertase 1/3 (PC1/3) and, albeit to a lesser extent, insulin (INS) are induced by ectopic Ngn3 expression but remain similar to control levels in IA1-expressing cells. Control RT–PCRs detect transcripts for GFP and GAPD. (B) Cotransduction of Ngn3 and IA1 enhances Ngn3-mediated transdifferentiation in adult human duct cells. Cells were transduced with AdHANgn3 and AdGFP, AdHANgn3 and AdIA1 or AdGFP only at a constant total MOI of 110, and processed for RT–PCR analysis at 3 days following transduction. Exogenous HANgn3, IA1 and Ngn3-induced endogenous IA1 are readily detected. Expression of the Ngn3 target genes NeuroD1, Pax4 and Nkx2.2 is enhanced in the AdHANgn3 and AdIA1 cotransduced samples as compared to the AdHANgn3 sample. RT–PCR results also indicate higher expression of the Delta-Notch component Hes6 and the endocrine marker SYP in Ngn3 and IA1 cotransduced cells. A control GAPD RT–PCR indicates comparable amounts of cDNA input. (C) The level of transcripts shown in (B) was quantified by real-time RT–PCR using specific Taqman probes as described in ‘Materials and methods' and revealed a 2–6-fold increase in target mRNA following cotransduction of adult duct cells with Ngn3 and IA1 as compared to transduction with Ngn3 alone (n=3). Statistical significance of the data was determined by unpaired, two-tailed Student's t-test.
Discussion
The development of the pancreas requires the sequential expression of a number of transcription factors. While Pdx1 defines the region of the primitive gut tube that will give rise to the pancreas, Ngn3 is subsequently expressed in a subset of pancreas progenitor cells and is essential for their differentiation into endocrine cells (Apelqvist et al, 1999; Gradwohl et al, 2000; Schwitzgebel et al, 2000; Grapin-Botton et al, 2001). Specification and further differentiation of the islet cell types is controlled by target genes of Ngn3, many of which are still unknown. The present paper describes the IA1 gene as a novel, direct Ngn3 target and the encoded zinc-finger transcription factor as a positive regulator of endocrine specification. In addition, we provide evidence for the essential role of IA1 for β- and α-cell differentiation in the embryonic pancreas and position IA1 downstream of Ngn3 and parallel with NeuroD1 in the network of developmental transcription factors that regulate endocrine differentiation.
IA1 is an immediate Ngn3 target transiently expressed in the endocrine progenitor cells of the pancreas
In an effort to understand the precise mechanisms whereby Ngn3 activates the formation of β cells, we screened for intermediary effectors of Ngn3 function. By micro-array analysis, IA1 was identified as an Ngn3-induced gene in duct cells expressing exogenous Ngn3. Indeed, IA1 was found to be expressed in a similar subset of cells as Ngn3 in E14.5 pancreas, however without a complete overlap: in Ngn3+IA1− cells the IA1 gene was activated (Ngn3+IA1+) and its transcript remained present in cells where Ngn3 mRNA has already disappeared (Ngn3−IA1+), suggesting the existence of a positive (auto)regulatory feedback loop. Moreover, a strong enrichment of the IA1 transcript was also observed in gene chip experiments on eYFP+ islet progenitor cells purified from E15.5 embryonic pancreas of Ngn3eYFP/+ mice (Mellitzer et al, 2004; G Mellitzer and G Gradwohl unpublished data). Remarkably, previous reports did not observe IA1 expression in Ngn3-enriched islet progenitor cells of mouse (Gu et al, 2004) or in Ngn3-transduced transdifferentiating mPAC L20 cells (Gasa et al, 2004). Of all gene chips used in these studies, only the MU74Av2 microarray that was used in some experiments by Gu et al (2004) contains one set of probes complementary to IA1.
Earlier studies based on in vitro binding assays and transient transfection analysis of a 1.7 kb IA1 promoter–reporter fragment indicated that IA1 expression can be regulated by NeuroD1 (Zhu et al, 2002; Breslin et al, 2003). Our results provide several arguments that compellingly demonstrate that Ngn3, rather than NeuroD1 or other intermediary factors, directly acts as a major regulator of IA1 expression in differentiating pancreatic cells. First, the pattern of expression of IA1 in the embryonic pancreas closely correlates with that of Ngn3, and only to a lesser extent with that of NeuroD1. Second, IA1 expression is lost in the Ngn3 knockout mouse but appears normal in the developing endocrine pancreas and central nervous system (data not shown) of NeuroD1 null mutant mice. Similarly, IA1 expression is not affected in embryonic pancreas of Arx, Pax4 and Pax6 knockout mice. Third, although ectopic expression of NeuroD1 can activate an IA1 promoter–reporter in transient transfection assays, there is only minor induction of endogenous IA1 gene expression after NeuroD1 transduction in adult pancreatic duct cells. This effect is in contrast to the robust response elicited by Ngn3. Fourth, activation of the IA1 gene by ectopic expression of Ngn3 precedes the induction of previously reported direct target genes of Ngn3 such as Pax4, NeuroD1 and Nkx2.2 (Huang et al, 2000; Smith et al, 2003; Watada et al, 2003). With the exception of weak activation of endogenous IA1 by NeuroD1, ectopic expression of pancreatic developmental transcription factors other than Ngn3 did not induce IA1 in adult human duct cells. Finally, NeuroD1-independent activation and binding of the IA1 promoter by Ngn3 was supported by transient promoter-reporter assays and chromatin immunoprecipitation analysis. These findings thus identify IA1 as an entirely novel limb of the Ngn3-dependent network. Nonetheless, although NeuroD1 is not essential for IA1 gene activity in pancreatic cells, our results and earlier data suggest that NeuroD1 can act as a positive regulator of IA1. This suggests a feedforward loop whereby Ngn3 activates both IA1 and NeuroD1, and the latter may further enhance Ngn3-induced IA1 gene activation.
IA1 expression in endocrine cell progenitors is necessary for optimal differentiation into insulin- and glucagon-expressing islet cells
In the rat amphicrine cell line AR42J, IA1 expression correlates with its conversion to insulin-positive cells and the appearance of NeuroD1 and other β-cell-specific transcription factors (Zhu et al, 2002). IA1 transcripts are also observed in human embryonic pancreas (Zhu et al, 2002) and in progenitors of endocrine mouse pancreas (present study). Although in earlier studies IA1 has been described as a repressor of NeuroD1 expression (Breslin et al, 2002), it is obvious from the present study that cotransduction of IA1 and Ngn3 in duct cells enhanced rather than inhibited the Ngn3-associated effects of transdifferentiation, resulting in higher expression levels of proendocrine genes and (neuro)endocrine markers including NeuroD1. However, as compared to ectopic expression of Ngn3 alone, Ngn3 and IA1 together did not increase the amount of insulin in the transduced adult duct cells. Moreover, Ngn3-inducible genes were not affected by IA1 transduction alone, excluding that IA1 simply acts as an immediate downstream relay of Ngn3 function. Direct evidence for a role of IA1 in endocrine differentiation during embryonic development was provided by a severely decreased number of insulin- and glucagon-positive cells in embryonic explant cultures when incubated with IA1-specific antisense oligonucleotides.
In conclusion, the activation of IA1 immediately downstream of Ngn3, but independently of NeuroD1 and other Ngn3-dependent regulators, uncovers a new regulatory branch in the program of endocrine differentiation in pancreas. This finding underscores the complexity of the Ngn3-dependent endocrinogenic network, as opposed to a simple lineal hierarchy. How exactly IA1 activates the differentiation program in the endocrine pancreas and whether it functions primarily as a transcriptional activator or repressor may become clear by identification of its genomic targets.
Materials and methods
Culture and genetic manipulation of adult duct cells and embryonic pancreas
Adult human duct cells were obtained and cultured as described previously (Heremans et al, 2002). Duct cells were transduced with recombinant adenovirus containing the full-length cDNA of human IA1 subcloned in pAdTrackCMV that also encodes eGFP (He et al, 1998). AdHANgn3 encodes murine Ngn3 fused at the 5′ end to a HA-tag and subcloned in pAdTrackCMV or in pShuttleCMV that does not express GFP. Adenoviral multiplicity of infection (MOI) was 50, unless stated otherwise.
Dorsal pancreases were dissected from E12.5 Ngn3-eGFP embryos and pancreatic epithelium was separated from its surrounding mesenchyme as described (Mellitzer et al, 2004). Epithelium was supplemented with biotinylated IA1 antisense 1 (5′-GCATGTTGGCGCGGTGAAAAGGGCG-3′), IA1 antisense 2 (5′- GGTCCCACCTCCGTGCTCGGCCCTG-3′) or standard missense (5′-CCTCTTACCTCAGTTACAATTTATA-3′) oligonucleotides (20 μM) of the morpholino-type (Gene Tools, LCC) and partial medium changes were performed daily for 4 days.
Construction of Ngn3-eGFP mice
To generate the Ngn3 promoter-eGFP construct, a 6.87 kb XbaI–XhoI fragment (6696 bp of 5′ genomic and untranslated region sequences and 176 bp of Ngn3 coding region) of mouse Ngn3 genomic DNA (Gradwohl et al, 2000) was cloned upstream of the IRES-EGFP-pA sequence. The 8.46 kb Ngn3-eGFP insert was released by SalI digestion and microinjected into murine oocyte pronuclei at the ICS (Mouse Clinical Institute), Illkirch (France). Three independent transgenic lines were generated and maintained by crossing into a CD1 outbred background. Transgenic progeny were identified by PCR as in Mellitzer et al (2004).
Protein analysis
Immunohistochemistry and immunofluorescence on cryosections of mice embryos and explants of embryonic pancreas as well as immunoblots were performed as described previously (Heremans et al, 2002; Mellitzer et al, 2004; Martin et al, 2005). The following primary antibodies (dilution) were used: rabbit anti-GFP (1:500, Molecular Probes), guinea-pig anti-insulin (1:1000, Sigma), guinea-pig anti-glucagon (1:2000, Sigma), rabbit anti-α-amylase (1:1000, Sigma) and rabbit anti-HA (1:1000, Clontech). Secondary antibodies were Alexa 488 anti-rabbit (1:1000, Molecular Probes), Cy3 anti-guinea-pig (1:1000, Jackson ImmunoResearch Labs) and horseradish peroxidase-coupled anti-guinea-pig (1:200, Vector Labs) and anti-rabbit (1:1000, GE Healthcare). For immunofluorescence, nuclei were stained with DAPI at 1:10 000 and mounted in Aqua-polymount (Polysciences). For immunohistochemistry, endogenous peroxidase activity was blocked by incubation in 0.5% H2O2 diluted in Methanol. The signal was detected using a Vectastain Elite ABC Kit (Vector Laboratories) in combination with a DAB chromogen (DakoCytomation). Slides were dehydrated and mounted in Eukitt (Euromedex). Imaging and quantitative analyses were as in Mellitzer et al (2004) or using Zeiss Axiophot equipped with an Olympus Colour View camera and Olympus DP-Soft5.0. Each value in Figure 5 represents the mean absolute number of insulin-, glucagon- and eGFP-positive cells (±standard error) of, respectively, 4 (morpholino 1) and 3 (morpholino 2) independent experiments, 3–4 explants per experiment. Final cell counts were normalized to the measured surface area of the explant.
RNA analysis
RNA isolation, cDNA synthesis and RT–PCR were carried out as described previously (Heremans et al, 2002). For primer sequence and amplicon length on a cDNA or genomic DNA template, see Supplementary Table I. Real-time RT–PCR used Taqman technology on cDNA from three independent transduction experiments, an ABI Prism 7700 Sequence Detector (Applied Biosystems), and data were analysed using the Sequence Detection Systems Software, Version 1.9.1 (Applied Biosystems). Human-specific Assays on Demand (Applied Biosystems) were used for analysis of NeuroD1, Pax4, Nkx2.2 and β-actin.
In situ hybridization was carried out on 10 μm cryostat sections as described in Cau et al (1997), and in some cases followed by immunohistochemistry. cRNA probes specifically recognized Ngn3 (Gradwohl et al, 2000), NeuroD1 (Fode et al, 1998) and IA1 (transcribed from a 1.7 kb mouse cDNA; Image clone 570515, 12703_h20). BrdU incorporation (12 h pulse) and detection experiments were carried out as in Martin et al (2005).
Promoter–reporter assays
The dual luciferase reporter system was used for promoter activity measurements according to the manufacturer's instruction (Promega, Madison, WI). A 1687-bp human IA1 promoter fragment (−1656 to +30) was cloned in the pGL3 vector driving expression of the firefly luciferase reporter gene. pRLCMV encodes Renilla luciferase and was used as an internal control for transfection efficiency. Expression constructs contained mouse HA-tagged Ngn3 cDNA or nontagged rat NeuroD1. 293 cells were seeded at 70% confluency and transfected with appropriate plasmids using Lipofectamine 2000 (Invitrogen). The total amount of plasmid was kept constant between transfections. All transfections were carried out in triplicate and repeated at least three times. Cells were lysed 48 h following transfection and processed for analysis.
Chromatin immunoprecipitation assay
Approximately 2 × 106 transduced human duct cells were used per immunoprecipitation assay as described (Parrizas et al, 2001), with several modifications. After fixation in 1% formaldehyde, cells were washed twice with cold phosphate-buffered saline and swelled on ice for 10 min in 25 mM HEPES, pH 8, 1.5 mM MgCl2, 10 mM KCl, 0.1% NP-40, 1 mM DTT and 1 × protease inhibitor cocktail (Roche). Following dounce homogenization, the nuclei were collected and resuspended in 1 ml sonication buffer containing 50 mM HEPES, pH 8, 140 mM NaCl, 1 mM EDTA, 0.1% sodium deoxycholate, 0.1% SDS and 1 × protease inhibitor cocktail, and DNA was sonicated on ice to an average length of 200–1000 bp. After addition of 1% Triton X-100, samples were centrifuged at 13 000 r.p.m. and precleared with 90 μl mix of protein A+G-Sepharose (1:1). Precleared chromatin was immunoprecipitated with 5 μg mouse anti-HA (Roche) overnight at 4°C, after which 3 μg rabbit anti-mouse IgG (Sigma) was added for 4 h at 4°C. Control immunoprecipitations were performed with rabbit anti-mouse IgG. Immune complexes were collected by adsorption to 30 μl of protein A+G-Sepharose for 3 h at 4°C. Beads were washed and immunocomplexes were eluted essentially as described (Parrizas et al, 2001). Chromatin was precipitated with ethanol, treated with 20 μg proteinase K and purified with Qiaquick PCR purification columns (Qiagen). Immunoprecipitated DNA (2 μl) and serial dilutions of the 10% input DNA (1:15, 1:45 and 1:135) were analysed by PCR under nonsaturating conditions using primers encompassing functional E-boxes from the 5′ flanking regions of the human NeuroD1 and IA1 genes, or from the BRCA1 and CTLA4 genes. For primer sequences, see Supplementary Table II.
Supplementary Material
Supplementary Table 1
Supplementary Table 2
Acknowledgments
We are grateful to Michèle Kedinger for discussions and support, to Veerle Laurysens, Karen Sterck, Mélanie Messmer, Viviane Hauer, Christophe Orvain, Jan De Jonge and Erik Quartier for technical assistance. Financial support was from the Fund for Scientific Research (FWO)-Flanders, Belgium (#G.0064.02 to HH and post-doctoral fellowships to HH and SB), HSFP (post-doctoral fellowship to GM), the Juvenile Diabetes Research Foundation (JDRF Center Grant; Career Development Award to HH), Inserm (Avenir Grant to GG), the Institut Benjamin Delessert (Prix de Projets de Recherches to GG), Instituto de Salud Carlos III (to JF), the EU (Integrated project 6th FP ‘Betacelltherapy' to the JDRF Center) and the NIH Beta Cell Biology Consortium (DK072495-01 to HH and GG).
References
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H (1997) Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature 385: 257–260 [DOI] [PubMed] [Google Scholar]
- Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H (1999) Notch signalling controls pancreatic cell differentiation. Nature 400: 877–881 [DOI] [PubMed] [Google Scholar]
- Breslin MB, Zhu M, Lan MS (2003) Neurod1/E47 regulates the E-box element of a novel zinc finger transcription factor, IA-1, in developing nervous system. J Biol Chem 278: 38991–38997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin MB, Zhu M, Notkins AL, Lan MS (2002) Neuroendocrine differentiation factor, IA-1, is a transcriptional repressor and contains a specific DNA-binding domain: identification of consensus IA-1 binding sequence. Nucleic Acid Res 30: 1038–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Fode C, Guillemot F (1997) Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development 124: 1611–1621 [DOI] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sorensen J, Broccoli V, Krull J, Ponte I, Mundiger T, Smith J, Gruss P, Serup P, Mansouri A (2005) The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the (alpha)- and (beta)-cell lineages in the mouse endocrine pancreas. Development 132: 2969–2980 [DOI] [PubMed] [Google Scholar]
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P (2003) Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 17: 2591–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, Lemeur M, Goridis C, Guillemot F (1998) The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron 20: 483–494 [DOI] [PubMed] [Google Scholar]
- Gasa R, Mrejen C, Leachman N, Otten M, Barnes M, Wang JH, Chakrabarti S, Mirmira R, German M (2004) Proendocrine genes coordinate the pancreatic islet differentiation program in vitro. Proc Natl Acad Sci USA 101: 13245–13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Desilva MG, Toscani A, Prabhakar BS, Notkins AL, Lan MS (1992) A novel human insulinoma-associated cDNA, IA-1, encodes a protein with zinc-finger DNA-binding motifs. J Biol Chem 267: 15252–15257 [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F (2000) Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA 97: 1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapin-Botton A, Majithia AR, Melton DA (2001) Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev 15: 444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA (2002) Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129: 2447–2457 [DOI] [PubMed] [Google Scholar]
- Gu G, Wells JM, Dombkowski D, Preffer F, Aronow B, Melton DA (2004) Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development 131: 165–179 [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95: 2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans Y, Van De Casteele M, Veld PI, Gradwohl G, Serup P, Madsen O, Pipeleers D, Heimberg H (2002) Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol 159: 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HP, Liu M, El-Hodiri HM, Chu K, Jamrich M, Tsai MJ (2000) Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol Cell Biol 20: 3292–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J (2004) Gene regulatory factors in pancreatic development. Develop Dynam 229: 176–200 [DOI] [PubMed] [Google Scholar]
- Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P (2000) Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes 49: 163–176 [DOI] [PubMed] [Google Scholar]
- Lan MS, Russell EK, Lu J, Johnson BE, Notkins AL (1993) IA-1, a new marker for neuroendocrine differentiation in human lung-cancer cell-lines. Cancer Res 53: 4169–4171 [PubMed] [Google Scholar]
- Li Z, Manna P, Kobayashi H, Spilde T, Bhatia A, Preuett B, Prasadan K, Hembree M, Gittes GK (2004) Multifaceted pancreatic mesenchymal control of epithelial lineage selection. Dev Biol 269: 252–263 [DOI] [PubMed] [Google Scholar]
- Martin M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dolle P, Gradwohl G (2005) Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol 284: 399–411 [DOI] [PubMed] [Google Scholar]
- Mellitzer G, Martin M, Sidhoum-Jenny M, Orvain C, Barths J, Seymour PA, Sander M, Gradwohl G (2004) Pancreatic islet progenitor cells in neurogenin 3-yfp knock-add-on mice. Mol Endocrinol 18: 2765–2776 [DOI] [PubMed] [Google Scholar]
- Miralles F, Czernichow P, Scharfmann R (1998) Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development 125: 1017–1024 [DOI] [PubMed] [Google Scholar]
- Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ (1997) Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev 11: 2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrizas M, Maestro MA, Boj SF, Paniagua A, Casamitjana R, Gomis R, Rivera F, Ferrer J (2001) Hepatic nuclear factor 1-alpha directs nucleosomal hyperacetylation to its tissue-specific transcriptional targets. Mol Cell Biol 21: 3234–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N, Mortensen S, Sorensen SB, Pedersen MW, Rieneck K, Bovin LF, Poulsen HS (2003) Transcriptional gene expression profiling of small cell lung cancer cells. Cancer Res 63: 1943–1953 [PubMed] [Google Scholar]
- Prasadan K, Daume E, Preuett B, Spilde T, Bhatia A, Kobayashi H, Hembree M, Manna P, Gittes GK (2002) Glucagon is required for early insulin-positive differentiation in the developing mouse pancreas. Diabetes 51: 3229–3236 [DOI] [PubMed] [Google Scholar]
- Sander M, Neubuser A, Kalamaras J, Ee HC, Martin GR, German MS (1997) Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev 11: 1662–1673 [DOI] [PubMed] [Google Scholar]
- Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M (2000) Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development 127: 5533–5540 [DOI] [PubMed] [Google Scholar]
- Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS (2000) Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 127: 3533–3542 [DOI] [PubMed] [Google Scholar]
- Slack JM (1995) Developmental biology of the pancreas. Development 121: 1569–1580 [DOI] [PubMed] [Google Scholar]
- Smith SB, Gasa R, Watada H, Wang JH, Griffen SC, German MS (2003) Neurogenin3 and hepatic nuclear factor 1 cooperate in activating pancreatic expression of Pax4. J Biol Chem 278: 38254–38259 [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P (1997) The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature 386: 399–402 [DOI] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P (1997) Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature 387: 406–409 [DOI] [PubMed] [Google Scholar]
- Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS (1998) Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development 125: 2213–2221 [DOI] [PubMed] [Google Scholar]
- Watada H, Scheel DW, Leung J, German MS (2003) Distinct gene expression programs function in progenitor and mature islet cells. J Biol Chem 278: 17130–17140 [DOI] [PubMed] [Google Scholar]
- Wilson ME, Scheel D, German MS (2003) Gene expression cascades in pancreatic development. Mech Develop 120: 65–80 [DOI] [PubMed] [Google Scholar]
- Zhu M, Breslin MB, Lan MS (2002) Expression of a novel zinc-finger cDNA, IA-1, is associated with rat AR42J cells differentiation into insulin-positive cells. Pancreas 24: 139–145 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1
Supplementary Table 2


