Abstract
Twinfilins are conserved actin-binding proteins composed of two actin depolymerizing factor homology (ADF-H) domains. Twinfilins are involved in diverse morphological and motile processes, but their mechanism of action has not been elucidated. Here, we show that mammalian twinfilin both sequesters ADP-G-actin and caps filament barbed ends with preferential affinity for ADP-bound ends. Twinfilin replaces capping protein and promotes motility of N-WASP functionalized beads in a biomimetic motility assay, indicating that the capping activity supports twinfilin's function in motility. Consistently, in vivo twinfilin localizes to actin tails of propelling endosomes. The ADP-actin-sequestering activity cooperates with the filament capping activity of twinfilin to finely regulate motility due to processive filament assembly catalyzed by formin-functionalized beads. The isolated ADF-H domains do not cap barbed ends nor promote motility, but sequester ADP-actin, the C-terminal domain showing the highest affinity. A structural model for binding of twinfilin to barbed ends is proposed based on the similar foldings of twinfilin ADF-H domains and gelsolin segments.
Keywords: actin, ADF homology domains, capping proteins, motility, twinfilin
Introduction
Twinfilin is an evolutionarily conserved actin-binding protein involved in motile and morphological processes from yeast to mammals. Twinfilin plays a nonessential regulatory role in turnover of cortical actin patches in Saccharomyces cerevisiae (Goode et al, 1998), is required for developmental processes in Drosophila (Wahlstrom et al, 2001), and is important in clathrin-mediated endocytosis and distribution of endocytic organelles in mammalian cells (Pelkmans et al, 2005). At least in yeast and mammals, twinfilin is a highly abundant protein and it localizes to regions of rapid actin dynamics in cells (Vartiainen et al, 2000, 2003; Palmgren et al, 2001).
Twinfilin is composed of two actin depolymerizing factor (ADF) homology (ADF-H) domains (Paavilainen et al, 2002). Like ADF, twinfilin shows preferential binding to ADP-actin, but at variance with ADF, it does not bind F-actin but instead makes a 1:1 complex with G-actin only (Ojala et al, 2002). Hence, twinfilin cannot act like ADF by accelerating filament treadmilling. Twinfilin inhibits actin polymerization, likely by sequestering G-actin (Ojala et al, 2002). Twinfilin also interacts with capping protein (CP) and at least in yeasts S. cerevisiae and Shizosaccharomyces pombe this interaction is essential for twinfilin's localization to the cortical actin cytoskeleton, although binding of twinfilin leaves the function of CP unchanged (Vartiainen et al, 2003; Falck et al, 2004; Kovar et al, 2005). However, G-actin sequestering and CP binding cannot satisfactorily explain how twinfilin contributes to actin dynamics and various motile processes in cells. Furthermore, the function of the N-terminal ADF-H domain of mammalian twinfilin has remained enigmatic, because both the CP binding and G-actin sequestering activities were shown to reside at the C-terminal half of the protein (Ojala et al, 2002; Falck et al, 2004), while G-actin binding by other twinfilins requires both ADF-H domains.
Here we analyze the effects of mammalian twinfilin on actin assembly dynamics in vitro and using biomimetic actin-based motility assays (Loisel et al, 1999; Romero et al, 2004). We demonstrate that it is by capping the barbed ends of filaments that mammalian twinfilin regulates motility, and show that both ADF-H domains are required for this activity. Our data also suggest that this property, which adds to the actin-sequestering activity of twinfilin, emerged at the vertebrate stage in evolution.
Results
Yeast, Drosophila and mouse twinfilins sequester ADP-G-actin in solutions of F-actin assembled at steady state in ATP
All twinfilins bind ADP-G-actin with higher affinity than ATP-G-actin; however, in the ATP-rich physiological medium, the form of actin that is sequestered by twinfilin is not known. The following experiment demonstrates that essentially ADP-G-actin is sequestered by yeast or mouse twinfilin (Figure 1, the same result was established for Drosophila twinfilin in an independent experiment). Filament growth was seeded by gelsolin-actin seeds in the presence or absence of twinfilin or thymosin β4 as a regular sequesterer of ATP-G-actin specifically (Carlier et al, 1993). Growth proceeded monotonously up to a steady-state plateau for actin alone and to a lower plateau in the presence of thymosin β4. In contrast, in the presence of twinfilin, the initial growth was followed by extensive depolymerization. This biphasic behavior is not compatible with sequestration of ATP-G-actin only. Since ATP is hydrolyzed as filament growth proceeds, the presence of dissociable ADP-actin during growth at filament pointed ends allows twinfilin to promote endwise depolymerization of filaments through tight binding and sequestration of ADP-G-actin. Actin is sequestered mainly in ADP-bound form because (1) twinfilin has ∼10-fold higher affinity for ADP- than for ATP-actin (Ojala et al, 2002); (2) nucleotide exchange is very slow in twinfilin-actin (Goode et al, 1998; Vartiainen et al, 2003); (3) twinfilin does not dissociate fast enough from actin to recycle ATP-actin (the dissociation rate constant for mouse twinfilin-1:ADP-G-actin complex under physiological ionic conditions at 20°C is 1.8 s−1 (Ojala et al, 2002); (4) twinfilin-ADP-actin, at variance with ADF-ADP-actin (Carlier et al, 1997), does not polymerize.
Figure 1.
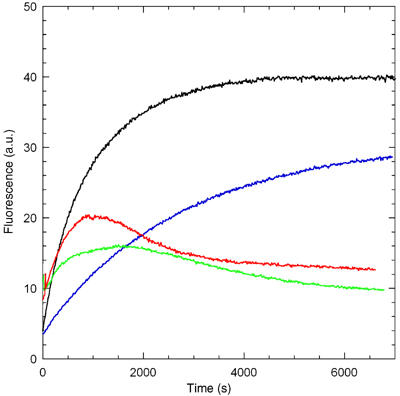
All twinfilins sequester predominantly ADP-G-actin at steady state in solutions of F-actin containing ATP. Polymerization of actin (2.5 μM) was seeded by gelsolin-actin (6 nM), with the following additions: black: none; blue: 5 μM thymosin β4; red: 5.4 μM mammalian twinfilin; green: 5.1 μM yeast twinfilin.
Twinfilin caps filament barbed ends
The effect of mouse twinfilin-1 on actin dynamics at the two ends of actin filaments was analyzed. Twinfilin inhibited growth totally at either end in a saturation manner (Figure 2A). Sequestering of ATP-G-actin by twinfilin well accounted for inhibition of pointed end growth. In addition, twinfilin showed a slight pointed end nucleation activity in the range of 0 to 1 μM. The data were consistent with the formation of a twinfilin-ATP-G-actin complex (KT of about 2–3 μM) that did not support pointed end growth. The value of KT is similar to the one derived from the two-fold increase in the fluorescence of pyrenyl-labeled ATP-G-actin upon binding twinfilin (Falck et al, 2004). In contrast, twinfilin inhibited barbed end growth in a range of concentrations substoichiometric to G-actin. These data are not consistent with the sequestration of actin by twinfilin observed at pointed ends and suggest that twinfilin could inhibit filament growth by blocking barbed ends.
Figure 2.
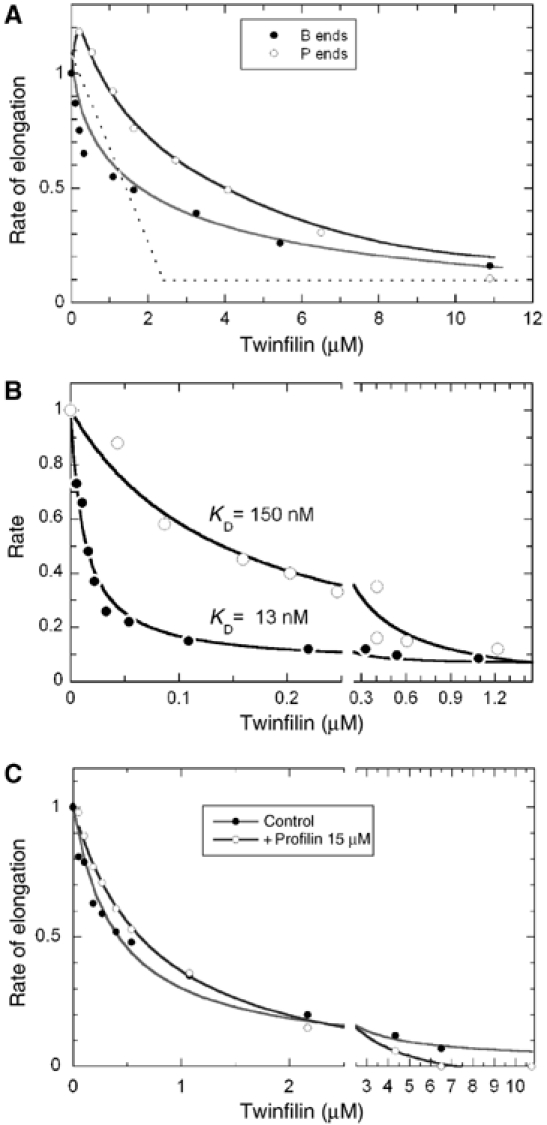
Twinfilin inhibits actin assembly with different mechanisms at barbed ends and pointed ends. (A) Barbed end growth (closed circles) and pointed end growth (open circles) were initiated with 2.5 μM G-actin (10% pyrenyl-labeled) and twinfilin as indicated. The initial rates were normalized to the value of 1 measured in the absence of twinfilin. Note that the observed inhibition is stronger than expected within this model in a range of low twinfilin concentration: all data points are below the dotted line that represents sequestration expected if twinfilin bound G-actin with infinite affinity. The red line is consistent with barbed end capping by twinfilin with a value of KF of 0.2 μM and ATP-G-actin sequestration with a value of KT of 2 μM (Equation (4)). In contrast, the effect of twinfilin at pointed ends is accounted for by nucleation of new pointed ends and sequestration of actin in a 1:1 complex with KT=2–3 μM. (B) Twinfilin inhibits dilution-induced depolymerization of F-ADP-actin (closed circles) or F-AMPPNP-actin (open circles) at barbed ends with high affinity. Filaments (2.5 μM F-actin, 75% pyrenyl-labeled) were diluted 80-fold in polymerization buffer containing twinfilin at the indicated concentrations. The line is the theoretical curve used to fit the data with a model in which twinfilin caps ADP-bound barbed ends (Equation (3)) with KF=13 nM, and AMPPNP-bound barbed ends with KF=150 nM. (C) Barbed end capping by twinfilin inhibits barbed end growth from profilin-actin. Barbed end growth was initiated by spectrin-actin seeds (conditions as in Figure 1a) in the presence of 3 μM G-actin with (open circles) or without (closed circles) 15 μM profilin and twinfilin as indicated. The curve fitting data in the absence of profilin is calculated according to Equation (4) assuming that twinfilin caps barbed ends (KF=0.2 μM) and sequesters ATP-G-actin (KT=4 μM). The curve fitting data in the presence of profilin is calculated according to Equation (6) assuming that twinfilin only caps barbed ends (KF=0.6 μM).
Like other barbed end CPs, twinfilin inhibited dilution-induced depolymerization of F-actin at the barbed ends (Figure 2B). The affinity of twinfilin for barbed ends was one order of magnitude higher in the depolymerization (KF=13 nM) than in the growth assay (KF=0.1–0.3 μM with different preparations of twinfilin), indicating that twinfilin caps barbed ends with a higher affinity when terminal F-actin subunits have bound ADP (in depolymerizing regime) than ATP or ADP-Pi (growth regime). Consistently, dilution-induced depolymerization of AMPPNP-F-actin in an F buffer containing AMPPNP (a non-hydrolyzable analog of ATP) was inhibited by twinfilin with a 10-fold lower affinity (KF=150 nM, Figure 2B).
The pointed end nucleating activity of twinfilin observed in growth assays (Figure 2A) is fully consistent with the barbed end capping activity of twinfilin.
To evaluate the barbed end capping of twinfilin independently of its G-actin sequestering activity, we measured the effect of twinfilin on barbed end growth from profilin-actin (Figure 2C). Profilin, which has a 20-fold higher affinity than twinfilin for ATP-G-actin, was used at a much larger concentration than twinfilin, so that all G-actin is profilin-actin (consistently, the data were identical in the presence of a twice larger amount of profilin). The rate of barbed end growth from profilin-actin is only 30% lower than from G-actin (Gutsche-Perelroizen et al, 1999). Once normalized, the dependence of the growth rate on twinfilin concentration was almost identical in the absence or presence of profilin, testifying that the capping activity of twinfilin accounts for about 80% of the inhibition of barbed end growth. The data were analyzed (see Materials and methods) assuming that twinfilin caps barbed ends, and the twinfilin–ATP–G-actin complex does not assemble at either barbed or pointed ends. The best fit was obtained for values of KT of 3 μM for binding of twinfilin to ATP-G-actin and KF of 0.2 μM for binding of twinfilin-actin to barbed ends. In the presence of an excess profilin, the data were well fitted assuming that twinfilin only caps barbed ends (KF=0.6 μM).
The effect of twinfilin on the steady state of actin assembly was measured when barbed ends were free or gelsolin-capped (Figure 3A). Twinfilin caused a linear decrease in F-actin and eventually totally depolymerized F-actin. When barbed ends were free, the data showed an initial steep decline until they reached and superimposed the straight line observed for depolymerization of gelsolin-capped filaments. A comprehensive interpretation of all data in Figures 1, 2 and 3 is that twinfilin caps barbed ends (the steep initial decline in F-actin is due to the increase in critical concentration) and sequesters G-actin mainly in the ADP-bound form, thus resulting in identical depolymerization in the presence or absence of gelsolin.
Figure 3.
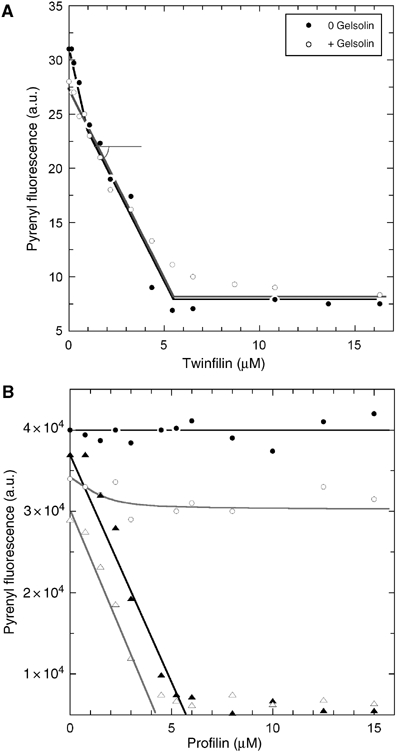
Combined effects of profilin and twinfilin on actin assembly at barbed and pointed ends. (A) Effect of twinfilin on actin assembly at steady state with free or capped barbed ends. Actin (3.1 μM, 5% pyrenyl-labeled) was polymerized in the absence (closed circles) or presence (open circles) of gelsolin at a 1:300 gelsolin:actin molar ratio, and twinfilin as indicated. (B) Twinfilin and profilin sequester actin in an additive manner when barbed ends are capped. In the absence of capping protein, the combined capping and sequestering activities of twinfilin allow profilin to maintain barbed end dynamics. Actin (3.45 μM) was assembled at steady state in the absence (circles) or in the presence (triangles) of gelsolin (10 nM). Closed symbols: no twinfilin; open symbols: 1 μM twinfilin.
The effect of profilin on F-actin at steady state in the presence of twinfilin was measured with free or capped barbed ends (Figure 3B). When barbed ends were capped, profilin and twinfilin sequestered G-actin in an additive manner. When barbed ends were free, addition of increasing amounts of profilin failed to cause total depolymerization of F-actin, in the presence as well as in the absence of twinfilin. Instead, in the presence of twinfilin, F-actin was maintained by profilin-actin at a steady-state concentration. The steady level of F-actin at saturation by profilin decreased upon increasing the concentration of twinfilin (data not shown). These data indicate that twinfilin fails to cap 100% of the barbed ends. Hence, profilin does not sequester G-actin. The few barbed ends that remain free are maintained in a dynamic state by profilin-actin. In other words, twinfilin and profilin synergize in tuning the steady state of actin assembly. As will be explored in greater detail below, the maintenance of free barbed ends arises from the actin-sequestering activity of twinfilin.
Role of twinfilin in actin-based motility
The role of mammalian twinfilin in motility was addressed using a biomimetic motility assay. N-WASP-coated beads were placed in a motility mix (Loisel et al, 1999) containing actin, Arp2/3, a capping protein (e.g. gelsolin), ADF and profilin. These proteins are confirmed to be essential in lamellipodium motility (Rogers et al, 2003). Twinfilin was added to the standard medium, or challenged for its ability to replace a component of the medium.
Addidion of twinfilin to the optimized motility medium did not affect the rate of propulsion nor the morphology of actin tails (Figure 4A and D). Twinfilin was unable to replace ADF in the motility mix, thus confirming biochemical data (Ojala et al, 2002). In contrast, twinfilin replaced gelsolin or CP and induced actin-based propulsion of beads in a medium containing no capper, as efficiently as CP or gelsolin (Figure 4B and C and Supplementary movie M1). On addition of increasing amounts of twinfilin, as observed with gelsolin (Pantaloni et al, 2000), bead-associated actin structures changed from asters to actin tails with a fishbone pattern, followed by regular actin tails and rapid movement (Figure 4B). In the absence of both ADF and capper, only few beads (10%) moved very slowly (0.37±0.21 μm/min) and the actin tails elongated without depolymerizing; addition of either twinfilin or CP or gelsolin caused an identical slight increase in velocity (Figure 4C). In summary, twinfilin behaves as a bona fide barbed end capper in the above assays.
Figure 4.
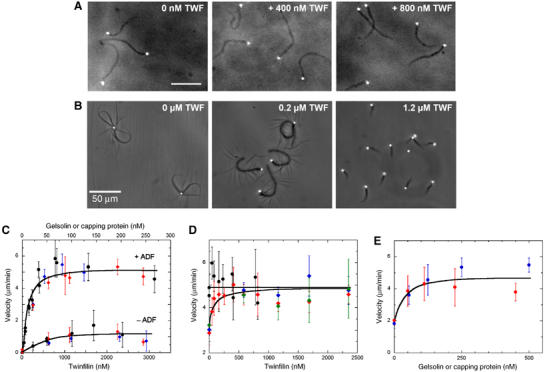
Mammalian twinfilin acts as a barbed end capping protein in motility. (A) Twinfilin does not change the morphology of actin tails when added to the optimized motility medium (90 nM gelsolin). Bar=50 μm. (B) Twinfilin supplements for a capping protein when it is added to the gelsolin-free motility medium. In the absence of gelsolin, N-WASP-coated beads do not move and initiate asters. Beads move with polarized actin tails upon addition of mammalian twinfilin. (C) Twinfilin (black) or capping protein (blue) or gelsolin (red) are equally efficient as barbed end cappers to promote bead movement in the absence (bottom curve) or presence (top curve) of 9 μM ADF. (D) Bead velocity is not affected by addition of twinfilin to the optimized motility medium containing 90 nM gelsolin (black circles), but equally supplements suboptimal amounts (20 nM) of either gelsolin (red), capping protein (blue), or CapG (green). The maximum velocity of ∼5 μm/min is reached upon addition of twinfilin. (E) Gelsolin (red) or capping protein (blue) equally supplement the suboptimal effect of twinfilin present at 150 nM in the motility medium.
Twinfilin is known to bind CP, but the functional significance of the interaction is unknown. To address this issue, twinfilin was added to a motility medium containing suboptimal amounts of either CP, gelsolin or CapG as standard cappers (Figure 4D). Twinfilin supplemented the low levels of any one of the three cappers and enhanced motility with equal efficiency, demonstrating that the effect of twinfilin in motility is independent of its interaction with CP. Consistently, addition of either gelsolin or CP to a suboptimal amount of twinfilin increases velocity identically (Figure 4E). At this point, the physiological significance of the interaction between twinfilin and CP remains enigmatic.
Barbed end capping and G-actin sequestering activities of mouse twinfilin finely tune formin-induced processive filament assembly
Actin-based motile processes are also driven by formins, which catalyze rapid processive barbed end assembly of filaments. Capping proteins play different roles in N-WASP-Arp2/3 and formin machineries. They are essential for maintenance of a densely branched actin network generated by WAVE- or N-WASP-Arp2/3 (Wiesner et al, 2003), but cause arrest of formin-induced movements by binding to barbed ends in competition with formins (Higashida et al, 2004; Romero et al, 2004). We addressed the role of twinfilin in formin-induced motility in comparison with CP, using the FH1–FH2 domain of mDia1 (Figure 5). FH1–FH2-coated beads initiated processive filament assembly in a solution of actin and profilin (Romero et al, 2004) and displayed a high density of attached filaments after 2 h (Figure 5A, left panel). When twinfilin was present, after 2 h about 100-fold less filaments were attached (6±3 filaments per bead, average of 20 beads), while many filaments are present in the solution (Figure 5A, right panel). The time course of filament dissociation from the bead after addition of twinfilin (Figure 5B and Movie M2) confirms that twinfilin caps filaments in competition with formin; however, processive assembly of a small number of filaments (0.9 μm/min) is still observed at late times.
Figure 5.
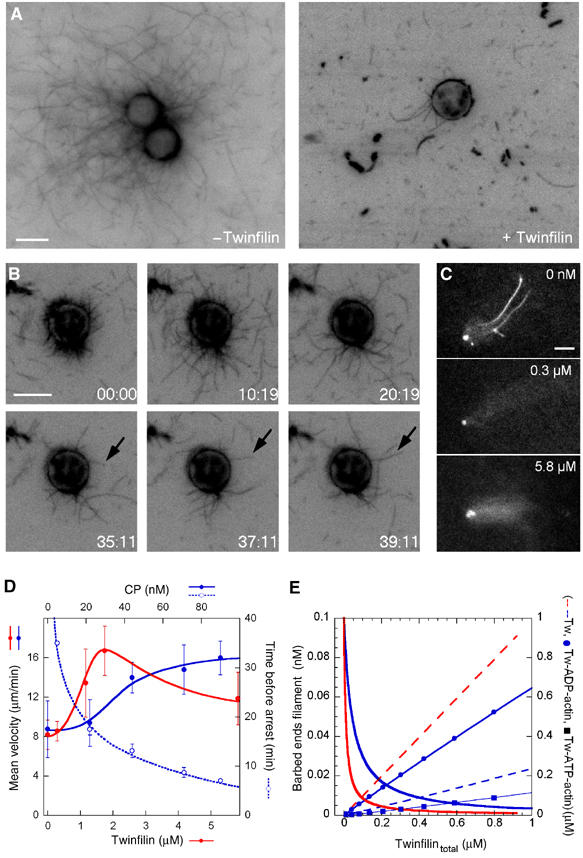
Twinfilin maintains formin-induced processive filament assembly and motility using its barbed end capping and ADP-actin sequestering activities. (A) Twinfilin inhibits processive filament assembly from profilin-actin catalyzed by bead-bound formin. FH1–FH2-coated beads (6 μm in diameter) were placed in 0.4 μM rhodamine-F-actin, 4 μM profilin, in the absence (left panel) or in the presence of 310 nM twinfilin (right panel), incubated for 2 h and observed in fluorescence microscopy. Bar=6 μm. (B) Time-lapse recording of the same beads, in the conditions described in panel A. Filaments gradually dissociate from the bead due to the capping activity of twinfilin; however, a small number of filaments remain bound and grow at late times. Arrows point to a growing individual filament. Time is in minutes, bar=6 μm. (C) Effect of twinfilin on FH1–FH2-coated bead motility. Beads (3 μm in diameter) were incubated in 7 μM F-actin, 14 μM ADF, 4 μM profilin without twinfilin or with 0.3 μM or 5.8 μM twinfilin. Movement is not arrested by twinfilin, but the density of actin tails first decreases (middle panel) then increases (bottom panel) upon increasing twinfilin. (D) Capping protein (blue) and Twinfilin (red) concentration dependence of the velocity (closed circles, continuous lines) and time of arrest (open circles, dotted line) of FH1–FH2 coated beads (no arrest of movement occurs with twinfilin). Other conditions are as under panel C. (E) Modeling of the combined effects of ADP-G-actin sequestration and barbed end capping activities of twinfilin (see Materials and methods). Free barbed ends with capping only (red continuous line) or capping and sequestration (blue continuous line); free twinfilin assuming capping only (red dashed line) or capping and sequestration of G-actin (blue dashed line), and concentrations of sequestered ADP-actin (DTw, blue circles) and ATP-actin (TTw, blue squares). Curves are calculated using KF=10 nM; KT=2 μM; KD=10 nM; k+B=10 μM−1 s−1; k−B=0.8 s−1; k−P=10 s−1, k+P=10 μM−1 s−1. The high values of rate constants at pointed ends account for the effect of ADF.
In a second experiment, FH1–FH2-coated beads moved rapidly in a solution of F-actin maintained at a steady state in the presence of profilin and ADF. CP, like gelsolin (Romero et al, 2004), transiently enhanced propulsion, by increasing the steady-state concentration of G-actin linked to barbed end capping in the medium, and eventually caused arrest of movement due to capping of the formin-bound barbed ends. Addition of twinfilin also increased the rate of propulsion, consistent with twinfilin capping the barbed ends of filaments in the medium; however, movement was never arrested, even though the number of filaments that formed the actin tail decreased (Figure 5C and D). The phenotype of maintained movement differentiates twinfilin from other barbed end capping proteins. On further increasing twinfilin up to concentrations that caused complete depolymerization of filaments in the medium, efficient processive actin assembly from FH1–FH2 beads resumed, large actin tails were again formed causing bead propulsion (Figure 5C, bottom panel).
A straightforward explanation for the above observations is derived from twinfilin's additive ADP-actin sequestering and barbed end capping activities. The initial increase in velocity and concomitant decrease in the number of bead-attached filaments is consistent with the capping activity of twinfilin. In this experimental context, twinfilin additionally causes depolymerization of filaments present in the motility medium and increasingly sequesters ADP–G-actin. The sequestering activity of twinfilin buffers the concentration of free twinfilin and establishes a ‘ground level' of free barbed ends, resulting in maintenance of active processive barbed end growth and motility of formin-coated beads. This effect is quantitatively modeled in Figure 5E (see Materials and methods for model). When enough twinfilin has been added to totally depolymerize the filaments, which were initially maintained at a steady state in the motility medium, ADP-actin is not longer produced. ATP-G-actin then forms, it binds less tightly than ADP-actin to twinfilin and interacts with profilin to feed rapid processive growth off the formin-coated beads.
Both ADF-H domains of twinfilin are required for barbed end capping
Twinfilin is composed of two actin-binding ADF-H domains separated by a short linker and followed by a 35-residue C-terminal extension that binds CP (Falck et al, 2004). The strong G-actin binding and sequestering activity reside in twinfilin's C-terminal ADF-H domain, whereas the N-terminal domain has a relatively modest affinity for G-actin (Ojala et al, 2002). Insight into the putative role of each of the two ADF-H domains in the barbed end capping activity of full-length mouse twinfilin was obtained by assaying the effect of each domain on actin dynamics at barbed and pointed ends. Constructs used included the N-terminal ADF-H domain with the linker, and the C-terminal ADF-H domain with or without the linker. All three ADF-H constructs inhibited barbed and pointed end growth with almost identical efficiencies, consistent with sole ATP-G-actin sequestering activity and absence of barbed end capping activity. The N-terminal ADF-H domain showed about 10-fold lower affinity than the C-terminal ADF-H domains for ATP-G-actin (Figure 6A). Steady-state measurements of F-actin confirmed that both the N-terminal and C-terminal ADF-H domains caused depolymerization of F-actin (Figure 6B and C). Consistent with the polymerization assays, neither the N-terminal nor the C-terminal ADF-H domains of twinfilin affected actin-based motility of N-WASP-coated beads in the standard motility assay, nor were they able to induce bead movement, at concentrations up to 2 μM, in a medium containing no CP (Supplementary Figure S1). Neither one was able to replace ADF in the motility assay.
Figure 6.
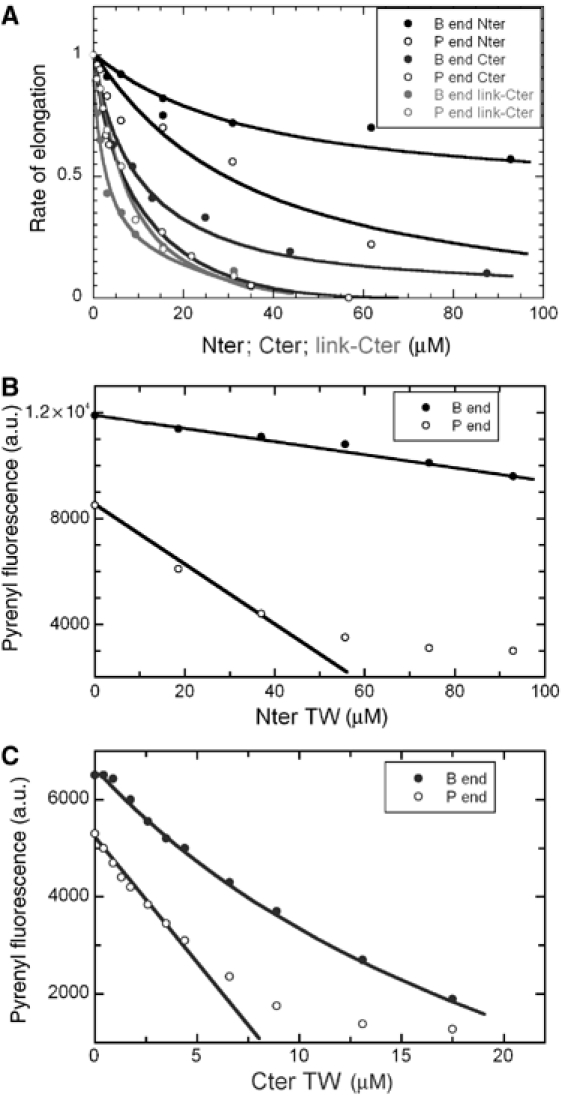
The N-terminal and C-terminal ADFH domains of twinfilin sequester actin but do not cap filaments. (A) The two ADF-H domains of twinfilin inhibit actin assembly at barbed and pointed ends by sequestering actin. Initial rates of barbed (closed circles) or pointed end (open circles) growth were measured as under Figure 1A in the presence of twinfilin N-terminal ADF-H domain (black) or C-terminal ADF-H domain (blue) or the C-terminal domain with the linker (red). Note the 10-fold higher affinity of the C-terminal domain (see Table I). (B) The N-terminal ADF-H domain of twinfilin possesses only a G-actin sequestering function. The steady-state amount of F-actin (1.5 μM) was measured in the absence (closed circles) or presence (open circles) of gelsolin and the N-terminal ADF-H domain as indicated. The two lines drawn through the data points are consistent with sequestration of ATP-actin by the N-terminal ADF-H domain, with KT=28 μM, because the slopes are consistent with the equation [SA]=[S0]. Ac/(Ac+KT), with KT=28 μM, Ac=0.1 μM at barbed ends and Ac=0.6 μM at pointed ends. (C) The C-terminal ADF-H domain of twinfilin sequesters actin when barbed ends are capped or noncapped. Conditions as under panel B, 2 μM actin. Depolymerization at pointed ends is consistent with sequestration of ATP-G-actin and KT=2.6 μM, in agreement with barbed end growth data (Frame A and Table I). Depolymerization at barbed ends is slightly more efficient, suggesting sequestration of G-actin in small part in ADP bound form also occurs.
In conclusion, the isolated ADF-H domains of twinfilin possess the actin-sequestering but not the capping activity of twinfilin and fail to act like twinfilin in motility. Thus, the barbed end capping activity of twinfilin requires the two ADF-H domains and is essential in motile processes.
Mouse twinfilin contributes to actin-dependent motility of endocytic vesicles
In vivo experiments addressed the physiological consequences of the barbed end capping activity of twinfilin in motility. Twinfilin localizes to the regions of rapid actin dynamics such as the leading edge of motile cells, but its possible contribution to motile processes has not been demonstrated. Instead, RNAi studies on Drosophila S2 cells provided evidence that twinfilin is not crucial to actin-dependent lamella formation (Rogers et al, 2003). Because twinfilin regulates the motility of N-WASP-coated beads (Figure 4) and because N-WASP is involved in actin-based vesicular transport (Innocenti et al, 2005), we examined twinfilin's contribution to transferrin uptake. In NIH 3T3 cells, endogenous twinfilin was found in dot-like structures that co-localized with actin tails of transferrin-positive particles. Virtually, all (>95%) transferrin particles that were positive for F-actin, labeled with twinfilin. In contrast, twinfilin was absent from transferrin particles that did not co-localize with F-actin, indicating that twinfilin is specifically associated with endosomes that are driven by actin-based motility (Figure 7A). Together with recent studies showing that subcellular distribution of endocytotic vesicles was severely defective in twinfilin knockdown cells (Pelkmans et al, 2005), these data suggest that twinfilin is involved in actin-driven internalization or motility of endocytic vesicles.
Figure 7.
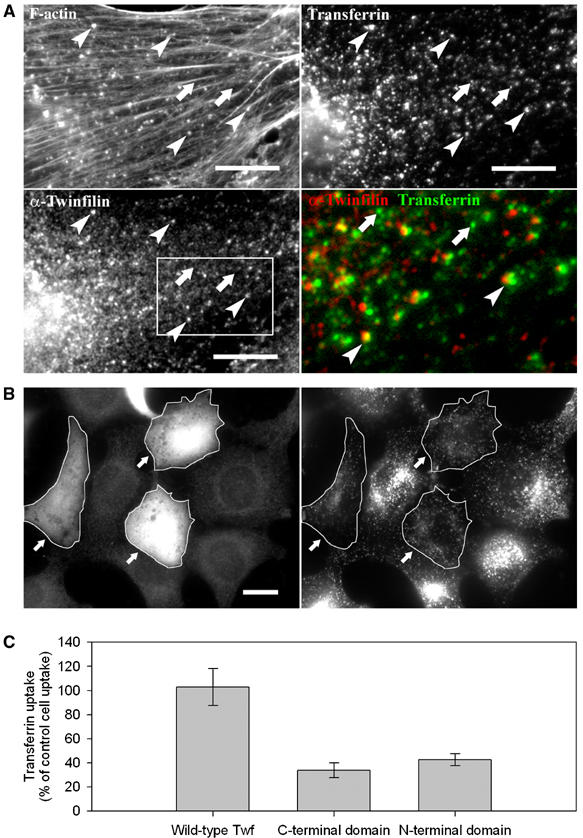
Twinfilin is involved in receptor-mediated endocytosis. (A) Endogenous twinfilin-1 localizes to F-actin-rich transferrin particles (examples indicated with arrowheads), but is absent from transferrin particles that are not associated with F-actin (arrows). NIH 3T3 cells were incubated for 20 min in 15 μg/ml rhodamine-transferrin (upper right panel), and stained with phalloidin (upper left panel), and anti-twinfilin-1 antibody (bottom left panel). A higher magnification overlay of twinfilin (red) and transferrin (green) from the boxed region is presented in the bottom right panel. Bar: 5 μm. (B) Overexpression of twinfilin's N-terminal ADF-H domain results in defects in receptor-mediated endocytosis. After 20 min incubation in 15 μg/ml transferrin, wild-type cells showed efficient uptake and accumulation of transferrin to the perinuclear region. In contrast, the cells overexpressing twinfilin's isolated ADF-H domain (arrows) showed punctate cytoplasmic staining and reduced transferrin uptake. Bar: 5 μm. (C) Intensity of rhodamine-transferrin fluorescence was quantified by TINA software from 10 cells overexpressing wild-type twinfilin, N-terminal ADF-H domain, or C-terminal half of the protein and compared with the intensity of rhodamine fluorescence of an adjacent wild-type cell from the same frame. The efficiency of transferrin uptake was reduced to approximately 30 and 40% in cells overexpressing twinfilin's C-terminal half and N-terminal ADF-H domain, respectively. Transferrin uptake in cells overexpressing full-length twinfilin was very similar as compared to wild-type cells. s.e.m.s are indicated in the graph.
To elucidate the specific role of twinfilin in endocytosis, we examined the effects of various twinfilin constructs on transferrin uptake. Myc-tagged twinfilin constructs were transfected to NIH 3T3 cells and expressed for 16 h under CMV promoter. Based on immunofluorescence experiments using an anti-myc antibody, all constructs were expressed at comparable levels in cells. Overexpression of wild-type twinfilin did not significantly affect transferrin uptake (Figure 7C). However, overexpression of either twinfilin's isolated N-terminal ADF-H domain or C-terminal half (including the C-terminal ADF-H domain and the tail-region) interfered with subcellular distribution of transferrin vesicles. Transferrin showed perinuclear accumulation in wild-type cells, but punctate cytoplasmic staining in cells overexpressing twinfilin's N-terminal or C-terminal ADF-H domain. The efficiency of transferrin uptake was also severely reduced in these cells as compared to cells overexpressing wild-type twinfilin (Figure 7B and C). These data suggest that twinfilin's isolated ADF-H domains, which do not cap barbed ends, inhibit endocytosis by depolymerizing F-actin to a detrimental extent, due to their actin monomer sequestering activity. In support to this view, the effect of the isolated domains on endocytosis is similar to the effect of Latrunculin A, which efficiently sequesters G-actin and inhibits endocytosis (Fujimoto et al, 2000). Twinfilin, in contrast, does not inhibit endocytosis when it is overexpressed, because it not only sequesters G-actin but also caps the filaments. While the failure to cap barbed ends like full-length twinfilin, and the known actin-sequestering activity of the isolated domains of twinfilin are sufficient to account for the results, we cannot eliminate other not known effects of these domains.
Yeast and Drosophila twinfilins sequester ADP-G-actin but do not cap filament barbed ends
We examined whether the twinfilins of lower eukaryotes like S. cerevisiae or Drosophila also possess a weak barbed end capping activity in addition to their known G-actin sequestering activity. S. cerevisiae and Drosophila twinfilins both failed to show the high-affinity inhibition of barbed end growth and depolymerization that characterized the barbed end capping activity of mammalian twinfilin. Only low-affinity sequestration of ATP-G-actin was measured in a range of 0–50 μM (Table I). Finally, the yeast and fly twinfilins did not induce actin-based motility of N-WASP-coated beads in the reconstituted motility assay in the absence of CP (Supplementary Figure S2). In steady-state measurements of F-actin assembled in the presence or absence of gelsolin, yeast twinfilin depolymerized actin by sequestration of ADP-actin, exactly as found for mouse twinfilin (displayed in Figure 3), and in agreement with data shown in Figure 1.
Table 1.
Binding parameters of the different twinfilins for ATP-G-actin in physiological conditions
| Species | |||||
|---|---|---|---|---|---|
| S. cerevisiae | Drosophila | Mouse Twf1 | Mouse N-ter ADF-H | Mouse C-ter ADF-H (±linker) | |
| KT | 22 μM | 2 μM | 2–4 μM | 25 μM | 2.5–4 μM |
Discussion
Twinfilin has unique original properties as compared to known actin binding proteins. All twinfilins, from yeast to mammals, sequester ADP-G-actin with high affinity under physiological conditions, that is, when filaments are assembled in the presence of ATP. Mammalian twinfilin, in addition, caps filament barbed ends with a higher affinity for ADP-bound terminal subunits. The barbed end cappping activity develops in the range of physiological concentrations of twinfilin (1–5 μM, Vartiainen et al, 2003). Using yeast twinfilin and the isolated ADF-H domains of mouse twinfilin as references that only sequester G-actin, we show that the barbed end capping activity of mouse twinfilin is essential for its role in motile processes. In vitro motility assays based on N-WASP-Arp2/3 show that twinfilin's effect is mediated by its capping activity. Similarly, we show that twinfilin's role in internalization/transport of vesicles, which is also N-WASP-Arp2/3 dependent (Innocenti et al, 2005), is consistent with a capping function. If twinfilin simply sequestered actin, the complex would be freely diffusable and twinfilin would not localize in actin tails of endocytic vesicles. Thus, twinfilin is associated to F-actin either by capping barbed ends itself or by binding CP at barbed ends. In the future, twinfilin mutants with specific defects in barbed end capping will have to be identified. These site-directed mutants can then be used in RNAi and rescue experiments to confirm the role of twinfilin's activities in motile processes.
The barbed end capping activity of twinfilin emerged late in evolution. However, this conclusion relies on results obtained with skeletal muscle actin. The possibility that yeast and Drosophila twinfilins cap the barbed ends of filaments only when they are assembled from yeast or Drosophila actins, respectively, is not totally excluded. The evolution of twinfilin activity at the vertebrate stage correlates with the ability of isolated ADF-H domains of mouse twinfilin to sequester actin individually, while the two ADF-H domains of yeast twinfilin are required to sequester ADP-G-actin (Goode et al, 1998; Palmgren et al, 2001).
Many barbed end CPs are involved in motile processes. Twinfilin adds up to the growing family of proteins that control actin dynamics at barbed ends. Eps8 caps barbed ends in a signal-controlled, spatially defined manner, and formins switch from leaky capping to processive growth catalysis in a profilin-dependent manner (Disanza et al, 2004; Kovar and Pollard, 2004; Romero et al, 2004). Twinfilin therefore is not essential, but may help adjust the extent of barbed end capping to the optimum level for motility. Identically cumulative capping properties are at work when twinfilin is added to suboptimal levels of either CP or CapG or gelsolin.
Uniquely, twinfilin has both actin sequestering and barbed end capping activities, and it sequesters ADP-G-actin specifically. These distinctive properties have interesting functional consequences. First, the ADP-G-actin sequestering and barbed end capping activities develop concomitantly, which limits the extent of barbed end capping and buffers free barbed ends at a low level (Figure 6). Twinfilin thus allows the maintenance of a basal level of formin-based processive filament assembly in a biomimetic assay and of dynamic barbed ends in the presence of profilin (Figure 3B). This property is specific to twinfilin, is not observed with standard CPs and may have physiological relevance. Second, in sequestering ADP-G-actin specifically, twinfilin builds a pool of unassembled actin, hence decreases the average length of filaments. The size of this pool is not governed by the concentration of free ATP-G-actin at steady state, as in the case of β-thymosins, which sequester ATP-G-actin specifically (Carlier et al, 1993). Instead, the amount of ADP-G-actin sequestered by twinfilin at steady state depends on the number of filaments (Pantaloni et al, 1984). Hence, twinfilin promotes the maintenance of actin meshworks formed of many capped short filaments, thus regulating filament length.
The structural mechanism of barbed end capping by twinfilin is an open issue. Assuming a single molecule of twinfilin caps a barbed end by binding to the terminal actin subunit, the two ADF-H domains of twinfilin, which when isolated bind G-actin in competition with ADF, must interact with different regions of this subunit. Remarkably, the two ADF-H domains of twinfilin display structural similarity to gelsolin domains (r.m.s.d. value of 3.0 Å for twinfilin's N-terminal domain and gelsolin segment-1), although these proteins do not display detectable sequence homology to each other (Figure 8). The six structurally related domains of gelsolin are involved in actin filament severing and capping through sequential interactions with distinct interfaces of actin filament subunits (Choe et al, 2002; Burtnick et al, 2004). Twinfilin may cap a barbed end by a similar mechanism to gelsolin S1–S2 fragment (reviewed in Weeds and Maciver, 1993). Kinetic studies of binding of twinfilin and its isolated domains to G-actin (Ojala et al, 2002) as well as the present data show that the C-terminal ADF-H domain has a higher affinity for actin, and may bind actin like ADF. Our results thus suggest the following general model for barbed end capping by proteins that have repeated modules. The N-terminal domain of twinfilin binds first to the barbed face of actin subdomain 1 exposed at the end of the filament, thus allowing positioning of the C-terminal domain at the ADF/cofilin binding site at the outside of the filament (Figure 8C). Structural analysis of twinfilin and its individual ADF-H domains in complex with actin (manuscript in preparation) should reveal the individual roles of the two ADF-H domains in barbed end capping.
Figure 8.
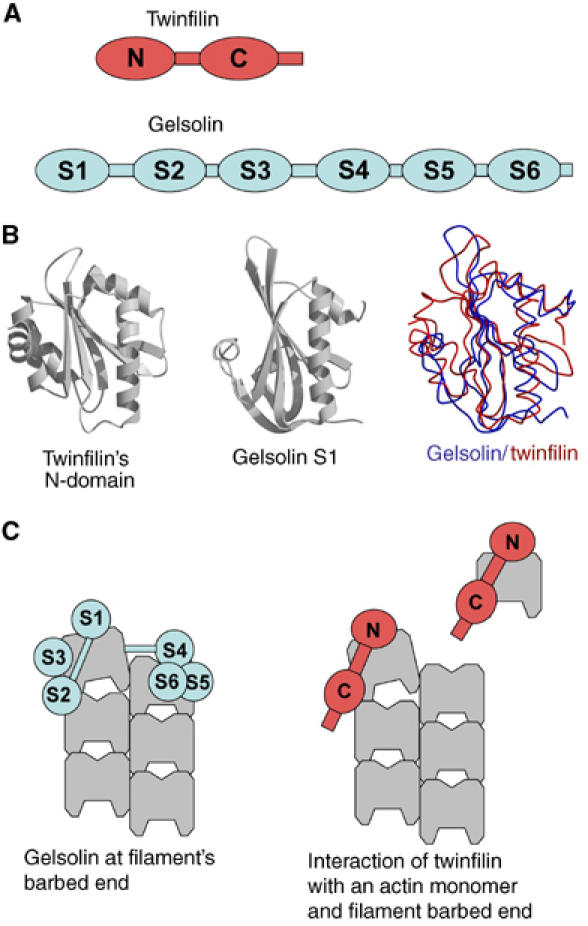
Twinfilin and gelsolin may cap filament barbed ends by structurally similar mechanisms. (A) Domain structures of twinfilin and gelsolin. Twinfilin is composed of two (N terminal and C terminal) ADF-H domains separated by a ∼30 residue linker-region and followed by a ∼35 residue C-terminal tail-region. Gelsolin is composed of six homologous domains (segments 1–6). (B) The ADF-H domains of twinfilin are structurally homologous to gelsolin domains. A comparison of the structures of twinfilin's N-terminal ADF-H domain and gelsolin segment 1 (McLaughlin et al, 1993; Paavilainen et al, 2002). (C) The six domains of gelsolin interact with different sites of the three terminal subunits of an actin filament (modified from Burtnick et al, 2004). The two structurally homologous twinfilin domains may cap the filament barbed end by a structurally similar mechanism to S1–S2, which is the minimal capping unit of gelsolin.
Whether other ADF-H containing proteins act at barbed ends like twinfilin is an issue of structural, functional and evolutional interest. Coactosin and Abp1p are the two other members of the ADF-H family. Coactosin consists of a single ADF-H domain that binds F-actin, shows a fold similar to gelsolin and villin (Liu et al, 2004) and prevents binding of CPs to barbed ends (Rohrig et al, 1995). The binding of actin to the ADF-H domain of Abp1p is required for the Arp2/3-dependent filament branching activity of Abp1p (Goode et al, 2001). The present data open a new perspective on the possible function of the ADF-H domain inserted in a modular protein.
Materials and methods
Proteins
Actin was purified from rabbit muscle and pyrenyl-labeled (Egile et al, 1999). Recombinant S. cerevisiae and Drosophila twinfilins were purified (Goode et al, 1998; Wahlstrom et al, 2001). His-tagged full-length mouse twinfilin-1 and its C-terminal (Twf169–350 and Twf140–350) ADF-H domains were expressed in Escherichia coli and purified (Vartiainen et al, 2003), and its N-terminal ADF-H domain (Twf1–174) was expressed as GST-fusion protein, purified, GST was removed by thrombin digestion (Ojala et al, 2002). Recombinant human ADF, gelsolin (Carlier et al, 1997), CP (Falck et al, 2004) and mDia1 FH1–FH2 domain (Romero et al, 2004) were purified as described. Profilin was purified from bovine spleen (Gutsche-Perelroizen et al, 1999), Arp2/3 complex from bovine brain (Egile et al, 1999). Human his-tagged N-WASP was expressed in Sf9 cells.
Actin polymerization assays
Actin assembly was monitored by the increase in pyrenyl-actin fluorescence (excitation and emission wavelengths 366 and 407 nm, respectively). Initial rates of barbed end growth were measured using spectrin-actin seeds (Casella et al, 1986). Gelsolin-actin complexes (0.4 μM gelsolin and 1 μM CaATP-G-actin mix) were used to seed pointed end growth. Growth assays were monitored using 2.5 μM G-actin (5% pyrenyl-labeled) and twinfilin as indicated. Only fresh solutions of twinfilin were used, since the protein loses rapidly actin-binding activity upon storage on ice. The initial rate of filament growth was normalized to the value of 1 measured in the absence of twinfilin.
Steady-state measurements of F-actin were carried out by measuring the fluorescence of pyrenyl-labeled F-actin incubated overnight at 4°C with twinfilin, profilin and gelsolin as indicated.
Data modeling
Barbed end growth assays. Data were analyzed using models in which twinfilin (Tw) binds ATP-G-actin (T) in a 1:1 nonpolymerizable TTw complex with an equilibrium dissociation constant KT and caps barbed ends forming a FTw complex with an equilibrium dissociation constant KF. The following equations were used.
The rate of filament growth from spectrin-actin seeds is
![]()
where [F] and [F0] are the concentrations of free barbed and pointed ends, [T0] is the total concentration of ATP-G-actin. TCB and TCP are the critical concentrations at the barbed and pointed ends respectively. To simplify the resolution of the equation, we will assume below that TCB and TCP are negligible as compared to the total G-actin concentration [T0], and that [FTw] is negligible as compared to free (Tw) and G-actin-bound (TTw) twinfilin. The total concentrations of filaments and twinfilin are [F0] and [Tw0].
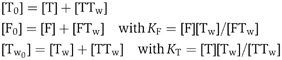
Equation (1) can be written as
![]()
In the absence of twinfilin, the growth rate is V0:
![]()
where k+P=α k+B
Equation (2) then becomes
![]()
where
![]()
In the presence of profilin in large amounts, twinfilin only caps barbed ends and no growth takes place at pointed ends. Then Equation (4) reduces to
![]()
Steady-state motility assays. The effect of twinfilin (barbed end capping+actin sequestration) on the movement of formin-coated beads was accounted for by the combination of the ADP-G-actin sequestering and barbed end capping activities of twinfilin, as follows. The model stipulates that filaments treadmill rapidly, ADP-G-actin (D) is produced by rapid pointed end depolymerization due to the effect of ADF (Carlier et al, 1997), ATP-G-actin (T) is produced from ADP-actin via nucleotide exchange with rate constant ke, twinfilin (Tw) sequesters ADP- and ATP-G-actin with equilibrium dissociation constants KD and KT, and caps barbed ends with equilibrium dissociation KF.
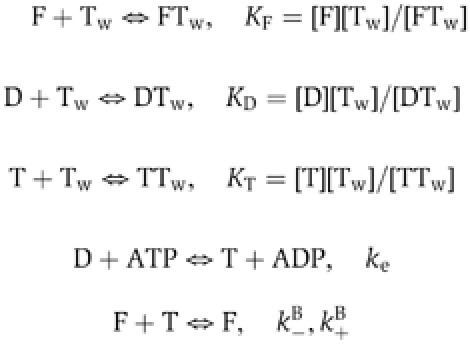
At steady state,
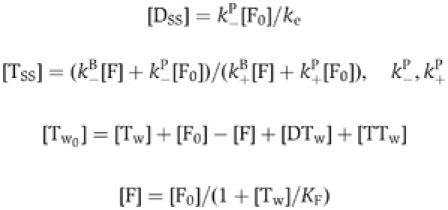
where k+B, k+P, k−B, k−P are the rate constants for association of ATP-actin to barbed ends of ADP-actin to pointed ends, dissociation of ATP-actin from barbed ends and dissociation of ADP-actin from pointed ends, respectively.
Motility assays
Motility assays were carried out as described (Wiesner et al, 2003; Romero et al, 2004). Carboxylated polystyrene microspheres (2 μm diameter, Polysciences Inc., 2% solids) were incubated with either 400 nM N-WASP or 10 μM FH1–FH2 in Xb buffer (10 mM HEPES, pH 7.8, 0.1 M KCl, 1 mM MgCl2, 1 mM ATP, 0.1 mM CaCl2, and 0.01% NaN3) for 1 h on ice. BSA (10 mg/ml) was added for 15 min. Beads were washed, stored on ice in Xb buffer with 1 mg/ml BSA and used for 4–5 days.
The standard motility medium for N-WASP-coated beads consisted of 7 μM F-actin in Xb buffer, 9 μM ADF, 2.4 μM profilin, 90 nM gelsolin, and 90 nM Arp2/3, except when indicated. The steady state of actin assembly was reached in 10 min. In the absence of ADF, steady state was reached in 6 h. Beads (0.02% solids final) were added to the steady motility medium. The medium for FH1FH2-coated beads consisted of 7 μM F-actin (5% rhodamine-labeled)), 14 μM ADF, 4 μM profilin and twinfilin as indicated. Samples of 5 μl were placed between a slide and a coverslip. Valap-sealed samples were observed in phase contrast or fluorescence microscopy (AX70, Olympus, equipped with a × 20 phase objective (NA 0.5) or a × 100 objective (NA 1.35), a motorized stage (Märzhäuser) and a camera (Cascade, Photometrics). Using Metamorph 6.0 (Universal Imaging Corp.) for microscope control and image acquisition, synchronous movies of up to five fields were recorded, following a dead time of about 10 min for selecting observation fields and entering their positions in the recognition-based tracking program. About 10 moving beads were selected and tracked. Average velocities were calculated for each set of beads.
Endocytosis assays
Myc-tagged wild-type mouse twinfilin-1, the N-terminal ADF-H domain (Twf1–142) and the C-terminal half (Twf169–350) constructs were expressed in NIH 3T3 cells and detected by immunofluorescence microscopy using a polyclonal anti-myc-antibody (Vartiainen et al, 2000). The efficiency of receptor-mediated endocytosis in these cells was measured (Bertling et al, 2004). In co-localization experiments, F-actin and endogenous twinfilin were visualized using Alexa488-phalloidin (Molecular Probes) and polyclonal anti-twinfilin-1 antibody (Vartiainen et al, 2003).
Supplementary Material
Supplementary movie M1
Supplementary movie M2
Supplementary Material
Acknowledgments
We thank Robert Robinson for sending the gelsolin-F-actin model. PL is supported by Academy of Finland and Emil Aaltonen Foundation. MFC is supported in part by the Ligue Nationale contre le Cancer (équipe labellisée) and by a HFSP research grant RGP0072/2003-C. SR is supported by a fellowship from the Ligue Nationale contre le Cancer.
References
- Bertling E, Hotulainen P, Mattila PK, Matilainen T, Salminen M, Lappalainen P (2004) Cyclase-associated protein 1 (CAP1) promotes cofilin-induced actin dynamics in mammalian nonmuscle cells. Mol Biol Cell 15: 2324–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick LD, Urosev D, Irobi E, Narayan K, Robinson RC (2004) Structure of the N-terminal half of gelsolin bound to actin: role in severing, apoptosis and FAF. EMBO J 23: 2713–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Jean C, Rieger KJ, Lenfant M, Pantaloni D (1993) Modulation of the interaction between G-actin and thymosin beta 4 by the ATP/ADP ratio: possible implication in the regulation of actin dynamics. Proc Natl Acad Sci USA 90: 5034–5038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M-F, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D (1997) Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol 136: 1307–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella JF, Maack DJ, Lin S (1986) Purification and initial characterization of a protein from skeletal muscle that caps the barbed ends of actin filaments. J Biol Chem 261: 10915–10921 [PubMed] [Google Scholar]
- Choe H, Burtnick LD, Mejillano M, Yin HL, Robinson RC, Choe S (2002) The calcium activation of gelsolin: insights from the 3A structure of the G4–G6/actin complex. J Mol Biol 324: 691–702 [DOI] [PubMed] [Google Scholar]
- Disanza A, Carlier M-F, Stradal TE, Didry D, Frittoli E, Confalonieri S, Croce A, Wehland J, Di Fiore PP, Scita G (2004) Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat Cell Biol 6: 1180–1188 [DOI] [PubMed] [Google Scholar]
- Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, Sansonetti PJ, Carlier M-F (1999) Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J Cell Biol 146: 1319–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck S, Paavilainen VO, Wear MA, Grossmann JG, Cooper JA, Lappalainen P (2004) Biological role and structural mechanism of twinfilin-capping protein interaction. EMBO J 23: 3010–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto LM, Roth R, Heuser JE, Schmid SL (2000) Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 1: 161–171 [DOI] [PubMed] [Google Scholar]
- Goode BL, Drubin DG, Lappalainen P (1998) Regulation of the cortical actin cytoskeleton in budding yeast by twinfilin, a ubiquitous actin monomer-sequestering protein. J Cell Biol 142: 723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode BL, Rodal AA, Barnes G, Drubin DG (2001) Activation of the Arp2/3 complex by the actin filament binding protein Abp1p. J Cell Biol 153: 627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutsche-Perelroizen I, Lepault J, Ott A, Carlier M-F (1999) Filament assembly from profilin-actin. J Biol Chem 274: 6234–6243 (2005) [DOI] [PubMed] [Google Scholar]
- Higashida C, Miyoshi T, Fujita A, Oceguera-Yanez F, Monypenny J, Andou Y, Narumiya S, Watanabe N (2004) Actin polymerization-driven molecular movement of mDia1 in living cells. Science 303: 2007–2010 [DOI] [PubMed] [Google Scholar]
- Innocenti M, Gerboth S, Rottner K, Lai FP, Hertzog M, Stradal T, Frittoli E, Didry D, Polo S, Disanza A, Benesch S, Di Fiore PP, Carlier M-F, Scita G (2005) Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat Cell Biol 7: 969–976 [DOI] [PubMed] [Google Scholar]
- Kovar DR, Pollard TD (2004) Insertional actin assembly of actin filament barbed ends in association with formins produces piconewton forces. Proc Nat Acad Sci USA 101: 14725–14730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar DR, Wu JQ, Pollard TD (2005) Profilin-mediated competition between capping protein and formin Cdc12p during cytokinesis in fission yeast. Mol Biol Cell 16: 2313–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin P, Gooch JT, Mannherz HG, Weeds AG (1993) Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature 364: 685–692 [DOI] [PubMed] [Google Scholar]
- Liu L, Wei Z, Wang Y, Wan M, Cheng Z, Gong W (2004) Crystal structure of human Coactosin-like protein. J Mol Biol 344: 317–323 [DOI] [PubMed] [Google Scholar]
- Loisel TP, Boujemaa R, Pantaloni D, Carlier M-F (1999) Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 401: 613–616 [DOI] [PubMed] [Google Scholar]
- Ojala PJ, Paavilainen VO, Vartiainen MK, Tuma R, Weeds AG, Lappalainen P (2002) The two ADF-H domains of twinfilin play functionally distinct roles in interactions with actin monomers. Mol Biol Cell 13: 3811–3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavilainen VO, Merckel MC, Falck S, Ojala PJ, Pohl E, Wilmanns M, Lappalainen P (2002) Structural conservation between the actin monomer-binding sites of twinfilin and actin-depolymerizing factor (ADF)/cofilin. J Biol Chem 277: 43089–43095 [DOI] [PubMed] [Google Scholar]
- Palmgren S, Ojala PJ, Wear MA, Cooper JA, Lappalainen P (2001) Interactions with PIP2, ADP-actin monomers and capping protein regulate the activity and localization of yeast twinfilin. J Cell Biol 155: 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaloni D, Carlier M-F, Coué M, Lal AA, Brenner SL, Korn ED (1984) The critical concentration of actin in the presence of ATP increases with the number of filaments and approaches the critical concentration of ADP-actin. J Biol Chem 259: 6274–6283 [PubMed] [Google Scholar]
- Pantaloni D, Boujemaa R, Didry D, Gounon P, Carlier MF (2000) The Arp2/3 complex branches filament barbed ends: functional antagonism with capping proteins. Nat Cell Biol 2: 385–391 [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M (2005) Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature 436: 78–86 [DOI] [PubMed] [Google Scholar]
- Rogers SL, Wiedemann U, Stuurman N, Vale RD (2003) Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J Cell Biol 162: 1079–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrig U, Gerisch G, Morozova L, Schleicher M, Wegner A (1995) Coactosin interferes with the capping of actin filaments. FEBS Lett 374: 284–286 [DOI] [PubMed] [Google Scholar]
- Romero S, Le Clainche C, Didry D, Egile C, Pantaloni D, Carlier MF (2004) Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 119: 419–429 [DOI] [PubMed] [Google Scholar]
- Vartiainen M, Ojala PJ, Auvinen P, Peränen J, Lappalainen P (2000) Mouse A6/twinfilin is an actin monomer-binding protein that localizes to the regions of rapid actin dynamics. Mol Cell Biol 20: 1772–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen MK, Sarkkinen EM, Matilainen T, Salminen M, Lappalainen P (2003) Mammals have two twinfilin isoforms whose subcellular localizations and tissue distributions are differentially regulated. J Biol Chem 278: 34347–34355 [DOI] [PubMed] [Google Scholar]
- Wahlstrom G, Vartiainen M, Yamamoto L, Mattila PK, Lappalainen P, Heino TI (2001) Twinfilin is required for actin-dependent developmental processes in Drosophila. J Cell Biol 155: 787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A, Maciver S (1993) F-actin capping proteins. Curr Opin Cell Biol 5: 63–69 [DOI] [PubMed] [Google Scholar]
- Wiesner S, Helfer E, Didry D, Ducouret G, Lafuma F, Carlier M-F, Pantaloni D (2003) A biomimetic motility assay provides insight into the mechanism of actin-based motility. J Cell Biol 160: 387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary movie M1
Supplementary movie M2
Supplementary Material


