Abstract
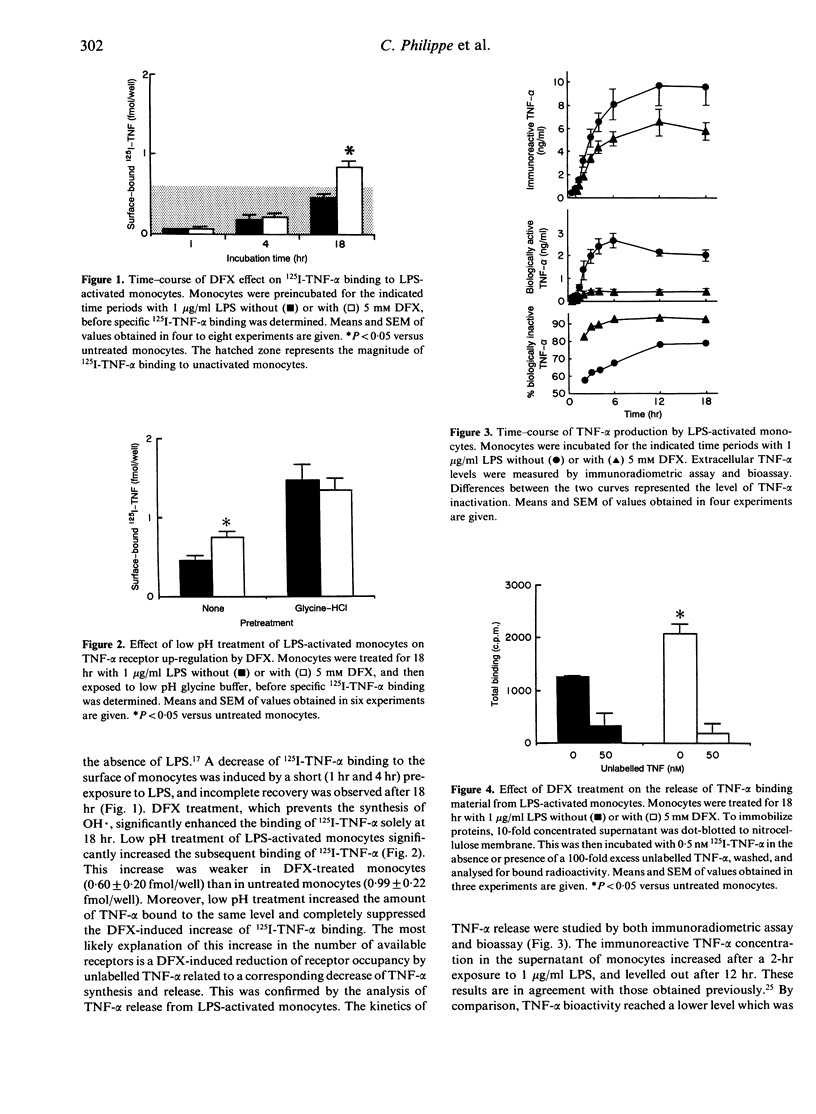
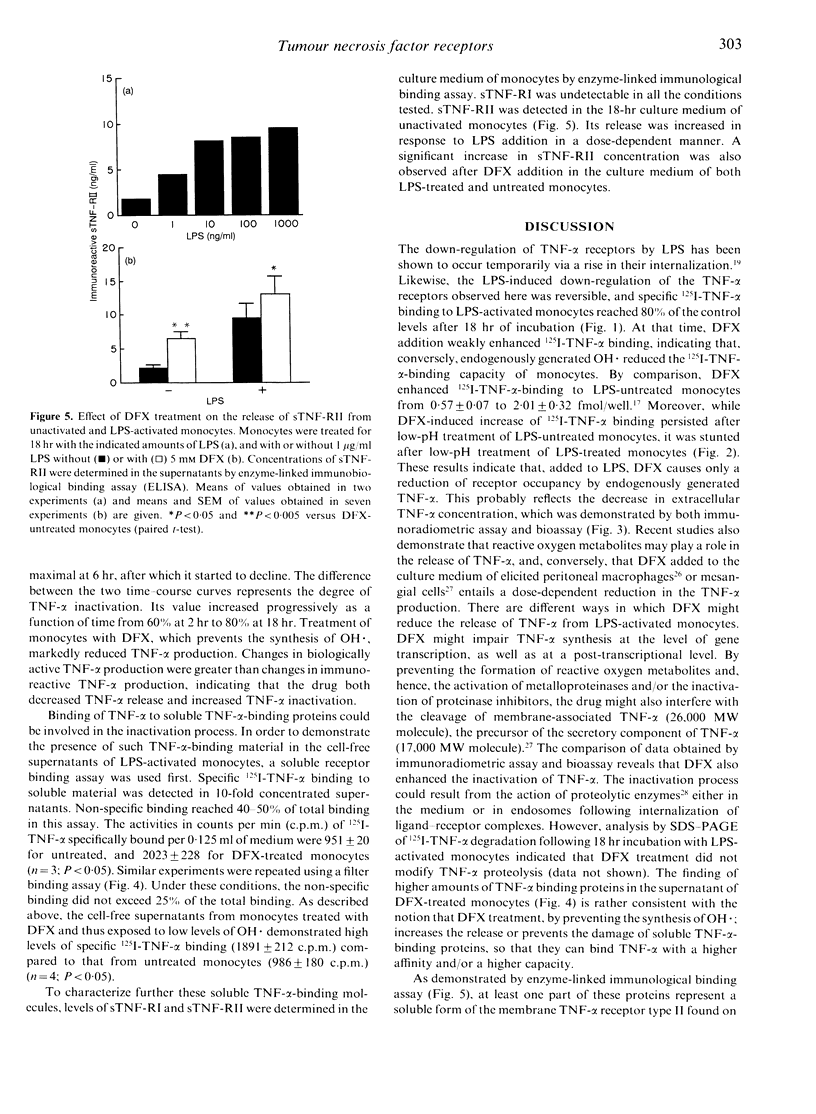
Previous studies have shown that desferrioxamine (DFX), an iron chelator preventing the synthesis of hydroxyl radical (OH.), up-regulates the cell-surface expression of tumour necrosis factor-alpha (TNF-alpha) receptors on unactivated human blood monocytes. In the present study, we investigated the regulatory action of DFX on 125I-TNF-alpha binding to monocytes upon exposure to bacterial lipopolysaccharide (LPS). Exposure to LPS (1 microgram/ml) resulted in almost complete loss of 125I-TNF-alpha binding to the surface of monocytes. This down-regulation was reversible and the recovery observed after 18 hr was enhanced by addition of DFX (5 mM). However, binding studies on monocytes pre-exposed to low pH suggested that the DFX-induced increase of 125I-TNF-alpha binding was not due to differences in the number of receptors available but was probably due to a reduction of receptor occupancy by endogenously generated TNF-alpha. Time-course studies of TNF-alpha release from monocytes confirmed the ability of DFX to reduce the extracellular concentration of bioactive TNF-alpha through a decrease of its synthesis and an increase of its inactivation. The latter process was associated with an increased expression of the soluble form of TNF-alpha receptor type II. These results indicate that, in the presence of LPS, DFX increases the release of soluble TNF-alpha receptors from monocytes. Thus, conversely, OH. generated in situ could reduce the shedding of soluble TNF-alpha receptors and, hence, increase the widespread release of bioactive TNF-alpha.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affres H., Perez J., Hagege J., Fouqueray B., Kornprobst M., Ardaillou R., Baud L. Desferrioxamine regulates tumor necrosis factor release in mesangial cells. Kidney Int. 1991 May;39(5):822–830. doi: 10.1038/ki.1991.103. [DOI] [PubMed] [Google Scholar]
- Baud L., Affres H., Perez J., Ardaillou R. Reduction in tumor necrosis factor binding and cytotoxicity by hydrogen peroxide. J Immunol. 1990 Jul 15;145(2):556–560. [PubMed] [Google Scholar]
- Burchett S. K., Weaver W. M., Westall J. A., Larsen A., Kronheim S., Wilson C. B. Regulation of tumor necrosis factor/cachectin and IL-1 secretion in human mononuclear phagocytes. J Immunol. 1988 May 15;140(10):3473–3481. [PubMed] [Google Scholar]
- Cadranel J., Philippe C., Perez J., Milleron B., Akoun G., Ardaillou R., Baud L. In vitro production of tumour necrosis factor and prostaglandin E2 by peripheral blood mononuclear cells from tuberculosis patients. Clin Exp Immunol. 1990 Aug;81(2):319–324. doi: 10.1111/j.1365-2249.1990.tb03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri G., Clark I. A. Reactive oxygen species facilitate the in vitro and in vivo lipopolysaccharide-induced release of tumor necrosis factor. J Immunol. 1989 Aug 15;143(4):1290–1294. [PubMed] [Google Scholar]
- Ding A. H., Sanchez E., Srimal S., Nathan C. F. Macrophages rapidly internalize their tumor necrosis factor receptors in response to bacterial lipopolysaccharide. J Biol Chem. 1989 Mar 5;264(7):3924–3929. [PubMed] [Google Scholar]
- Giri J. G., Newton R. C., Horuk R. Identification of soluble interleukin-1 binding protein in cell-free supernatants. Evidence for soluble interleukin-1 receptor. J Biol Chem. 1990 Oct 15;265(29):17416–17419. [PubMed] [Google Scholar]
- Heller R. A., Song K., Villaret D., Margolskee R., Dunne J., Hayakawa H., Ringold G. M. Amplified expression of tumor necrosis factor receptor in cells transfected with Epstein-Barr virus shuttle vector cDNA libraries. J Biol Chem. 1990 Apr 5;265(10):5708–5717. [PubMed] [Google Scholar]
- Hohmann H. P., Brockhaus M., Baeuerle P. A., Remy R., Kolbeck R., van Loon A. P. Expression of the types A and B tumor necrosis factor (TNF) receptors is independently regulated, and both receptors mediate activation of the transcription factor NF-kappa B. TNF alpha is not needed for induction of a biological effect via TNF receptors. J Biol Chem. 1990 Dec 25;265(36):22409–22417. [PubMed] [Google Scholar]
- Hohmann H. P., Remy R., Brockhaus M., van Loon A. P. Two different cell types have different major receptors for human tumor necrosis factor (TNF alpha). J Biol Chem. 1989 Sep 5;264(25):14927–14934. [PubMed] [Google Scholar]
- Hohmann H. P., Remy R., Pöschl B., van Loon A. P. Tumor necrosis factors-alpha and -beta bind to the same two types of tumor necrosis factor receptors and maximally activate the transcription factor NF-kappa B at low receptor occupancy and within minutes after receptor binding. J Biol Chem. 1990 Sep 5;265(25):15183–15188. [PubMed] [Google Scholar]
- Kirchheimer J. C., Nong Y. H., Remold H. G. IFN-gamma, tumor necrosis factor-alpha, and urokinase regulate the expression of urokinase receptors on human monocytes. J Immunol. 1988 Dec 15;141(12):4229–4234. [PubMed] [Google Scholar]
- Kohno T., Brewer M. T., Baker S. L., Schwartz P. E., King M. W., Hale K. K., Squires C. H., Thompson R. C., Vannice J. L. A second tumor necrosis factor receptor gene product can shed a naturally occurring tumor necrosis factor inhibitor. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8331–8335. doi: 10.1073/pnas.87.21.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz M., Gullberg U., Nilsson E., Olsson I. Characterization in vitro of a human tumor necrosis factor-binding protein. A soluble form of a tumor necrosis factor receptor. J Clin Invest. 1990 Nov;86(5):1396–1402. doi: 10.1172/JCI114853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. E., McCarthy S. P., Lorenzen J., McGee J. O. Differential effects of LPS, IFN-gamma and TNF alpha on the secretion of lysozyme by individual human mononuclear phagocytes: relationship to cell maturity. Immunology. 1990 Mar;69(3):402–408. [PMC free article] [PubMed] [Google Scholar]
- Lonnemann G., Endres S., Van der Meer J. W., Cannon J. G., Koch K. M., Dinarello C. A. Differences in the synthesis and kinetics of release of interleukin 1 alpha, interleukin 1 beta and tumor necrosis factor from human mononuclear cells. Eur J Immunol. 1989 Sep;19(9):1531–1536. doi: 10.1002/eji.1830190903. [DOI] [PubMed] [Google Scholar]
- Ming W. J., Bersani L., Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987 Mar 1;138(5):1469–1474. [PubMed] [Google Scholar]
- Philippe C., Fouqueray B., Perez J., Baud L. Up-regulation of tumour necrosis factor-alpha receptors on monocytes by desferrioxamine. Clin Exp Immunol. 1992 Mar;87(3):499–503. doi: 10.1111/j.1365-2249.1992.tb03026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellstab P., Schaffner A. Endotoxin suppresses the generation of O2- and H2O2 by "resting" and lymphokine-activated human blood-derived macrophages. J Immunol. 1989 Apr 15;142(8):2813–2820. [PubMed] [Google Scholar]
- Scheurich P., Köbrich G., Pfizenmaier K. Antagonistic control of tumor necrosis factor receptors by protein kinases A and C. Enhancement of TNF receptor synthesis by protein kinase A and transmodulation of receptors by protein kinase C. J Exp Med. 1989 Sep 1;170(3):947–958. doi: 10.1084/jem.170.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckinger P., Zhang J. H., Hauptmann B., Dayer J. M. Characterization of a tumor necrosis factor alpha (TNF-alpha) inhibitor: evidence of immunological cross-reactivity with the TNF receptor. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5188–5192. doi: 10.1073/pnas.87.13.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinas G. A., Bloesch D., Kaufmann M. T., Keller U., Dayer J. M. Induction of plasma inhibitors of interleukin 1 and TNF-alpha activity by endotoxin administration to normal humans. Am J Physiol. 1990 Nov;259(5 Pt 2):R993–R997. doi: 10.1152/ajpregu.1990.259.5.R993. [DOI] [PubMed] [Google Scholar]
- Spinas G. A., Keller U., Brockhaus M. Release of soluble receptors for tumor necrosis factor (TNF) in relation to circulating TNF during experimental endotoxinemia. J Clin Invest. 1992 Aug;90(2):533–536. doi: 10.1172/JCI115891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szefler S. J., Norton C. E., Ball B., Gross J. M., Aida Y., Pabst M. J. IFN-gamma and LPS overcome glucocorticoid inhibition of priming for superoxide release in human monocytes. Evidence that secretion of IL-1 and tumor necrosis factor-alpha is not essential for monocyte priming. J Immunol. 1989 Jun 1;142(11):3985–3992. [PubMed] [Google Scholar]
- Taylor L., Menconi M. J., Polgar P. The participation of hydroperoxides and oxygen radicals in the control of prostaglandin synthesis. J Biol Chem. 1983 Jun 10;258(11):6855–6857. [PubMed] [Google Scholar]
- Unglaub R., Maxeiner B., Thoma B., Pfizenmaier K., Scheurich P. Downregulation of tumor necrosis factor (TNF) sensitivity via modulation of TNF binding capacity by protein kinase C activators. J Exp Med. 1987 Dec 1;166(6):1788–1797. doi: 10.1084/jem.166.6.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valone F. H., Epstein L. B. Biphasic platelet-activating factor synthesis by human monocytes stimulated with IL-1-beta, tumor necrosis factor, or IFN-gamma. J Immunol. 1988 Dec 1;141(11):3945–3950. [PubMed] [Google Scholar]
- Van Zee K. J., Kohno T., Fischer E., Rock C. S., Moldawer L. L., Lowry S. F. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4845–4849. doi: 10.1073/pnas.89.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Moldawer L., Chan B. Macrophage/monocyte receptor for nonenzymatically glycosylated protein is upregulated by cachectin/tumor necrosis factor. J Clin Invest. 1989 Dec;84(6):1813–1820. doi: 10.1172/JCI114366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzen R., Wallach D., Engelmann H., Nophar Y., Brakebusch C., Kemper O., Resch K., Holtmann H. Selective decrease in cell surface expression and mRNA level of the 55-kDa tumor necrosis factor receptor during differentiation of HL-60 cells into macrophage-like but not granulocyte-like cells. J Immunol. 1992 Jun 1;148(11):3454–3460. [PubMed] [Google Scholar]
- van Kessel K. P., van Strijp J. A., Verhoef J. Inactivation of recombinant human tumor necrosis factor-alpha by proteolytic enzymes released from stimulated human neutrophils. J Immunol. 1991 Dec 1;147(11):3862–3868. [PubMed] [Google Scholar]


