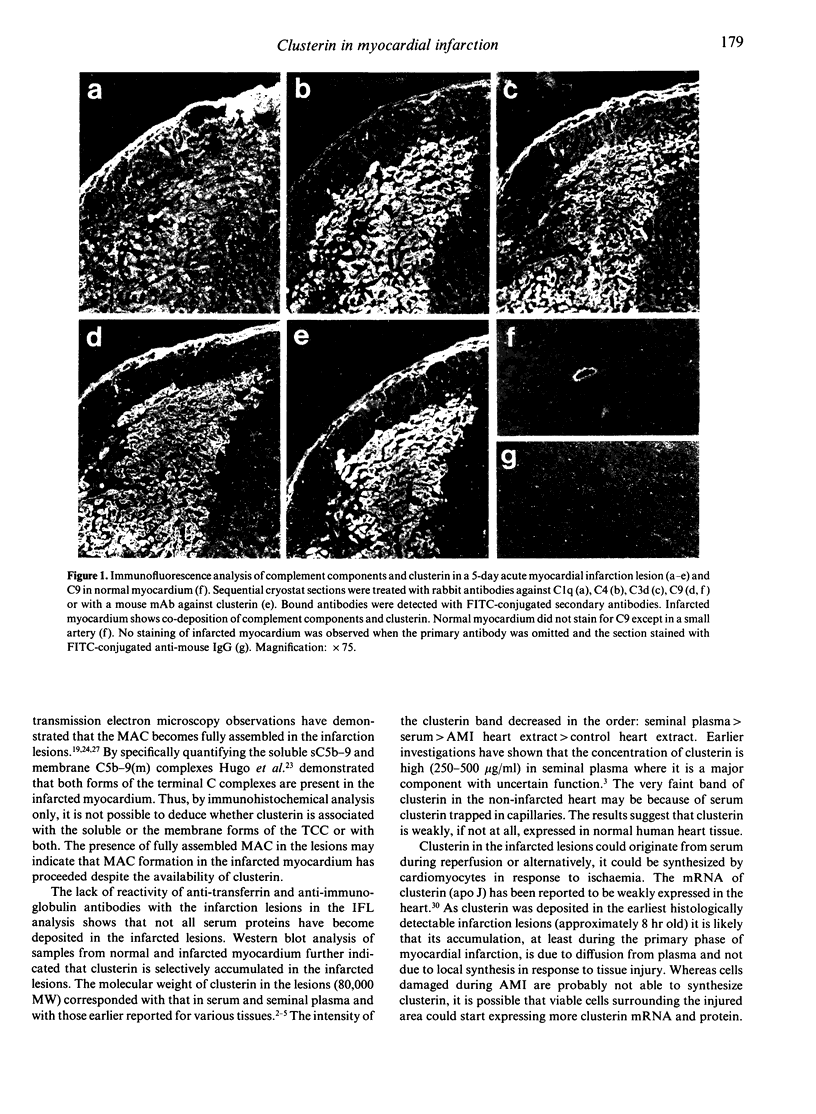
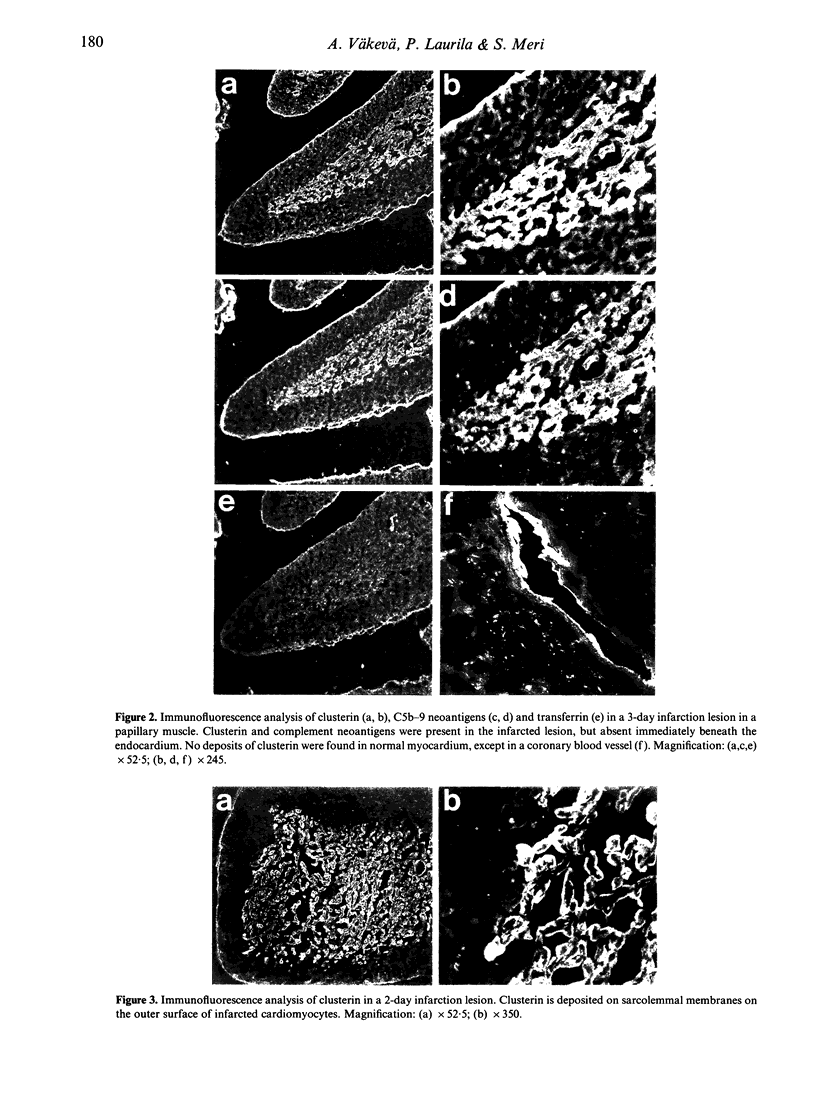
Abstract
Clusterin is a multi-functional plasma glycoprotein that has been shown to inhibit formation of the complement membrane attack complex (MAC) by preventing the association of terminal complement complexes with target cell membranes. Recent studies have suggested that complement activation is involved in the development of tissue injury of myocardial infarction. In this study we observed that clusterin is selectively deposited in the infarcted areas of human myocardium. Clusterin deposits were observed in the heart tissue of 10 patients whose infarcted lesions were 8 hr to 14 days old, but not in patients who died from other causes. Clusterin co-localized with the MAC on the surface of damaged cardiomyocytes. In normal myocardium only endothelial lining of blood vessels occasionally stained positive for clusterin. The 80,000 MW clusterin was also detected by Western blot analysis in extracts of myocardial infarction lesions, but only faintly in extracts of normal heart. As clusterin has apparently failed in protecting myocardium against complement-mediated cell injury its main role might be to participate in the clearance of damaged and necrotic tissue together with the MAC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Käflein R., Halstensen T. S., Hugo F., Preissner K. T., Mollnes T. E. Complement S-protein (vitronectin) is associated with cytolytic membrane-bound C5b-9 complexes. Clin Exp Immunol. 1988 Dec;74(3):459–464. [PMC free article] [PubMed] [Google Scholar]
- Blaschuk O., Burdzy K., Fritz I. B. Purification and characterization of a cell-aggregating factor (clusterin), the major glycoprotein in ram rete testis fluid. J Biol Chem. 1983 Jun 25;258(12):7714–7720. [PubMed] [Google Scholar]
- Buttyan R., Olsson C. A., Pintar J., Chang C., Bandyk M., Ng P. Y., Sawczuk I. S. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989 Aug;9(8):3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C. Y., Mathur P. P., Grima J. Structural analysis of clusterin and its subunits in ram rete testis fluid. Biochemistry. 1988 May 31;27(11):4079–4088. doi: 10.1021/bi00411a026. [DOI] [PubMed] [Google Scholar]
- Choi N. H., Nakano Y., Tobe T., Mazda T., Tomita M. Incorporation of SP-40,40 into the soluble membrane attack complex (SMAC, SC5b-9) of complement. Int Immunol. 1990;2(5):413–417. doi: 10.1093/intimm/2.5.413. [DOI] [PubMed] [Google Scholar]
- Correa-Rotter R., Hostetter T. H., Nath K. A., Manivel J. C., Rosenberg M. E. Interaction of complement and clusterin in renal injury. J Am Soc Nephrol. 1992 Nov;3(5):1172–1179. doi: 10.1681/ASN.V351172. [DOI] [PubMed] [Google Scholar]
- Crawford M. H., Grover F. L., Kolb W. P., McMahan C. A., O'Rourke R. A., McManus L. M., Pinckard R. N. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circulation. 1988 Dec;78(6):1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- Eddy A. A., Fritz I. B. Localization of clusterin in the epimembranous deposits of passive Heymann nephritis. Kidney Int. 1991 Feb;39(2):247–252. doi: 10.1038/ki.1991.29. [DOI] [PubMed] [Google Scholar]
- Ehnholm C., Bozas S. E., Tenkanen H., Kirszbaum L., Metso J., Murphy B., Walker I. D. The apolipoprotein A-I binding protein of placenta and the SP-40,40 protein of human blood are different proteins which both bind to apolipoprotein A-I. Biochim Biophys Acta. 1991 Nov 27;1086(3):255–260. doi: 10.1016/0005-2760(91)90167-g. [DOI] [PubMed] [Google Scholar]
- French L. E., Polla L. L., Tschopp J., Schifferli J. A. Membrane attack complex (MAC) deposits in skin are not always accompanied by S-protein and clusterin. J Invest Dermatol. 1992 May;98(5):758–763. doi: 10.1111/1523-1747.ep12499946. [DOI] [PubMed] [Google Scholar]
- French L. E., Tschopp J., Schifferli J. A. Clusterin in renal tissue: preferential localization with the terminal complement complex and immunoglobulin deposits in glomeruli. Clin Exp Immunol. 1992 Jun;88(3):389–393. doi: 10.1111/j.1365-2249.1992.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer G. V., Taylor C. E. Improved mountant for immunofluorescence preparations. J Clin Pathol. 1974 Mar;27(3):254–256. doi: 10.1136/jcp.27.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo F., Hamdoch T., Mathey D., Schäfer H., Bhakdi S. Quantitative measurement of SC5b-9 and C5b-9(m) in infarcted areas of human myocardium. Clin Exp Immunol. 1990 Jul;81(1):132–136. doi: 10.1111/j.1365-2249.1990.tb05303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne D. E., Lowin B., Peitsch M. C., Böttcher A., Schmitz G., Tschopp J. Clusterin (complement lysis inhibitor) forms a high density lipoprotein complex with apolipoprotein A-I in human plasma. J Biol Chem. 1991 Jun 15;266(17):11030–11036. [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Clusterin: the intriguing guises of a widely expressed glycoprotein. Trends Biochem Sci. 1992 Apr;17(4):154–159. doi: 10.1016/0968-0004(92)90325-4. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7123–7127. doi: 10.1073/pnas.86.18.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagiyama A., Savage H. E., Michael L. H., Hanson G., Entman M. L., Rossen R. D. Molecular basis of complement activation in ischemic myocardium: identification of specific molecules of mitochondrial origin that bind human C1q and fix complement. Circ Res. 1989 Mar;64(3):607–615. doi: 10.1161/01.res.64.3.607. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Carpenter C. B., Chiariello M., Fishbein M. C., Radvany P., Knostman J. D., Hale S. L. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest. 1978 Mar;61(3):661–670. doi: 10.1172/JCI108978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P. C., Lampert-Etchells M., Johnson S. A., Poirier J., Masters J. N., Finch C. E. Dynamics of gene expression for a hippocampal glycoprotein elevated in Alzheimer's disease and in response to experimental lesions in rat. Neuron. 1990 Dec;5(6):831–839. doi: 10.1016/0896-6273(90)90342-d. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Kawamata T., Walker D. G. Distribution of clusterin in Alzheimer brain tissue. Brain Res. 1992 May 8;579(2):337–341. doi: 10.1016/0006-8993(92)90071-g. [DOI] [PubMed] [Google Scholar]
- Murphy B. F., Kirszbaum L., Walker I. D., d'Apice A. J. SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988 Jun;81(6):1858–1864. doi: 10.1172/JCI113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinckard R. N., O'Rourke R. A., Crawford M. H., Grover F. S., McManus L. M., Ghidoni J. J., Storrs S. B., Olson M. S. Complement localization and mediation of ischemic injury in baboon myocardium. J Clin Invest. 1980 Nov;66(5):1050–1056. doi: 10.1172/JCI109933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. E., Paller M. S. Differential gene expression in the recovery from ischemic renal injury. Kidney Int. 1991 Jun;39(6):1156–1161. doi: 10.1038/ki.1991.146. [DOI] [PubMed] [Google Scholar]
- Schäfer H., Mathey D., Hugo F., Bhakdi S. Deposition of the terminal C5b-9 complement complex in infarcted areas of human myocardium. J Immunol. 1986 Sep 15;137(6):1945–1949. [PubMed] [Google Scholar]
- Väkevä A., Laurila P., Meri S. Loss of expression of protectin (CD59) is associated with complement membrane attack complex deposition in myocardial infarction. Lab Invest. 1992 Nov;67(5):608–616. [PubMed] [Google Scholar]
- Väkevä A., Laurila P., Meri S. Regulation of complement membrane attack complex formation in myocardial infarction. Am J Pathol. 1993 Jul;143(1):65–75. [PMC free article] [PubMed] [Google Scholar]
- Weisman H. F., Bartow T., Leppo M. K., Marsh H. C., Jr, Carson G. R., Concino M. F., Boyle M. P., Roux K. H., Weisfeldt M. L., Fearon D. T. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990 Jul 13;249(4965):146–151. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- de Silva H. V., Harmony J. A., Stuart W. D., Gil C. M., Robbins J. Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990 Jun 5;29(22):5380–5389. doi: 10.1021/bi00474a025. [DOI] [PubMed] [Google Scholar]
- de Silva H. V., Stuart W. D., Duvic C. R., Wetterau J. R., Ray M. J., Ferguson D. G., Albers H. W., Smith W. R., Harmony J. A. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J Biol Chem. 1990 Aug 5;265(22):13240–13247. [PubMed] [Google Scholar]
- de Silva H. V., Stuart W. D., Park Y. B., Mao S. J., Gil C. M., Wetterau J. R., Busch S. J., Harmony J. A. Purification and characterization of apolipoprotein J. J Biol Chem. 1990 Aug 25;265(24):14292–14297. [PubMed] [Google Scholar]