Abstract
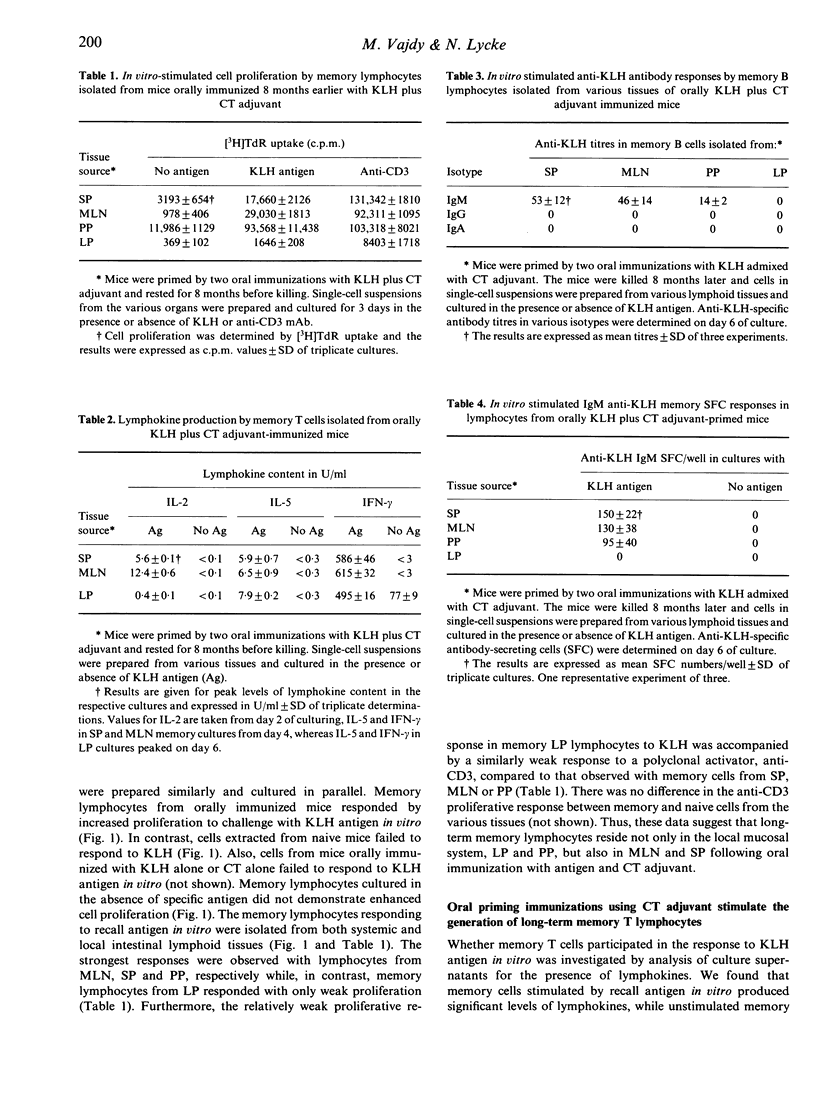
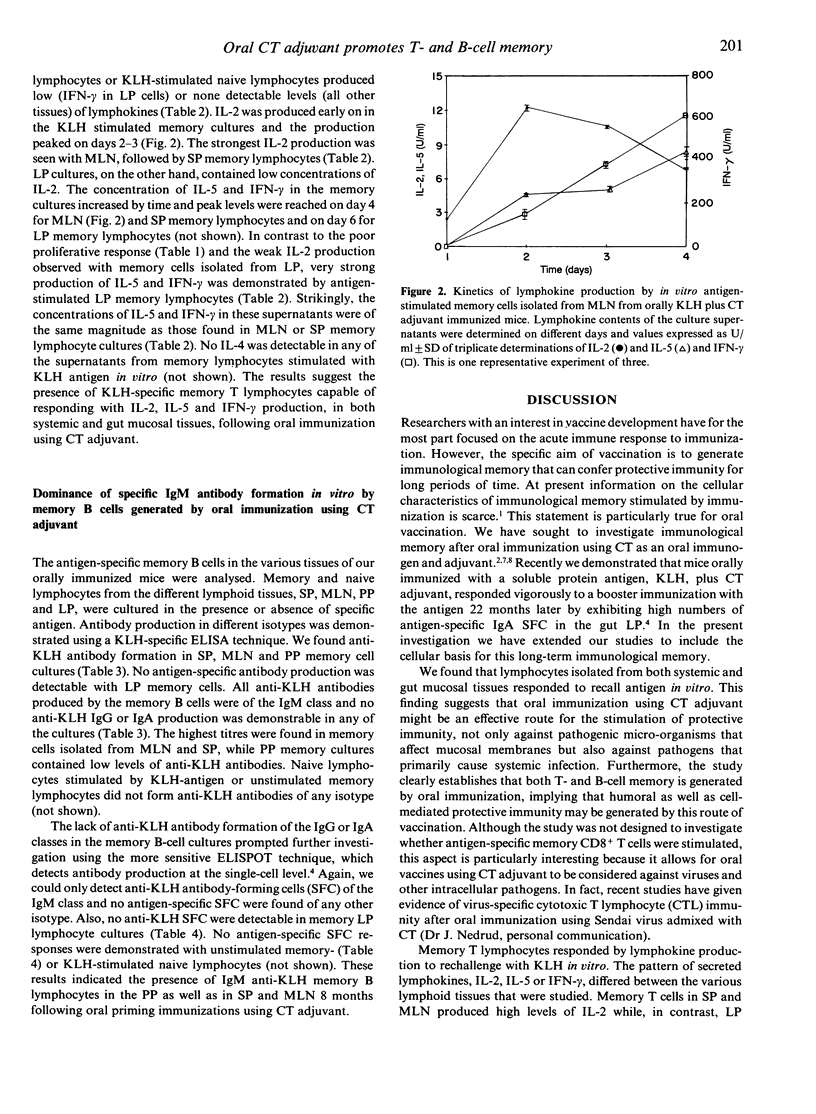
In the present study we investigated immunological memory at the cellular level following oral immunization using cholera toxin (CT) as the mucosal adjuvant. We found that memory cells, isolated from mice orally primed with keyhole limpet haemocyanin (KLH) admixed with CT adjuvant 8 months earlier, responded by increased proliferation to antigen-challenge in vitro. In contrast, unstimulated memory cells or KLH-stimulated cells from naive mice did not respond. Memory cells were isolated from different lymphoid tissues; spleen (SP), mesenteric lymph nodes (MLN), Peyer's patches (PP) as well as the intestinal lamina propria (LP). Thus, oral immunization using CT adjuvant promoted the generation of memory cells that were present in both systemic and local intestinal lymphoid tissues. The demonstration of lymphokine production in the KLH-responsive cultures indicated the presence of antigen-specific memory T cells. Lymphokine production early in culture was dominated by interleukin-2 (IL-2), which peaked on day 2-3, followed by IL-5 and, in particular, interferon-gamma (IFN-gamma) which increased over time. Lamina propria memory cells were found to proliferate poorly to recall antigen in vitro compared to lymphocytes from SP or MLN. In contrast, very significant production of IL-5 and, in particular, IFN-gamma was demonstrable in LP cell cultures. The use of CT adjuvant also stimulated the generation of antigen-specific memory B cells following oral immunization. This was evidenced by KLH-specific antibody production in antigen-challenged memory lymphocyte cultures. The memory B cells produced IgM anti-KLH, while no detectable antigen-specific IgG or IgA was found. Unstimulated memory cells or naive cells failed to produce anti-KLH antibodies. These in vitro findings provide evidence that oral immunization using CT adjuvant stimulates both antigen-specific memory T and B cells. Furthermore, our data suggest the existence of memory B cells following oral CT adjuvant immunization which have retained the ability to produce IgM and which therefore probably have not undergone terminal isotype switch differentiation to other isotypes and thus have not deleted the mu constant heavy-chain gene. Finally, our data also suggest that memory T and B cells, either sessile in the various lymphoid tissues or recirculating, can be activated by antigen in situ in, for example, lymph nodes and spleen and, more importantly, in the intestinal LP itself.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatelain R., Varkila K., Coffman R. L. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992 Feb 15;148(4):1182–1187. [PubMed] [Google Scholar]
- Chen K. S., Strober W. Cholera holotoxin and its B subunit enhance Peyer's patch B cell responses induced by orally administered influenza virus: disproportionate cholera toxin enhancement of the IgA B cell response. Eur J Immunol. 1990 Feb;20(2):433–436. doi: 10.1002/eji.1830200230. [DOI] [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- George A., Cebra J. J. Responses of single germinal-center B cells in T-cell-dependent microculture. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):11–15. doi: 10.1073/pnas.88.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Ishii R., Yamasaki K., Kishimoto T., Hardy R. R. Isolation of high-affinity memory B cells: phycoerythrin as a probe for antigen-binding cells. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1379–1383. doi: 10.1073/pnas.84.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Bacterial enteric infections and vaccine development. Gastroenterol Clin North Am. 1992 Jun;21(2):283–302. [PubMed] [Google Scholar]
- James S. P., Mullin G. E., Kanof M. E., Zeitz M. Role of lymphokines in immunoregulatory function of mucosal T cells in humans and nonhuman primates. Immunol Res. 1991;10(3-4):230–238. doi: 10.1007/BF02919698. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Miller R. A. Memory and anergy: challenges to traditional models of T lymphocyte differentiation. FASEB J. 1992 Apr;6(7):2428–2433. doi: 10.1096/fasebj.6.7.1563595. [DOI] [PubMed] [Google Scholar]
- Jäck H. M., McDowell M., Steinberg C. M., Wabl M. Looping out and deletion mechanism for the immunoglobulin heavy-chain class switch. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1581–1585. doi: 10.1073/pnas.85.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrenz D., Strober S., Vitetta E. The relationship between surface immunoglobulin isotype and the immune function of murine B lymphocytes. V. High affinity secondary antibody responses are transferred by both IgD-positive and IgD-negative memory B cells. J Immunol. 1981 Sep;127(3):867–872. [PubMed] [Google Scholar]
- Lafrenz D., Teale J. M., Klinman N. R., Strober S. Surface IgG-bearing cells retain the capacity to secrete IgM. J Immunol. 1986 Mar 15;136(6):2076–2079. [PubMed] [Google Scholar]
- Lebman D. A., Griffin P. M., Cebra J. J. Relationship between expression of IgA by Peyer's patch cells and functional IgA memory cells. J Exp Med. 1987 Nov 1;166(5):1405–1418. doi: 10.1084/jem.166.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Adoptive transfer of gut mucosal antitoxin memory by isolated B cells 1 year after oral immunization with cholera toxin. Infect Immun. 1989 Apr;57(4):1137–1141. doi: 10.1128/iai.57.4.1137-1141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Long-term cholera antitoxin memory in the gut can be triggered to antibody formation associated with protection within hours of an oral challenge immunization. Scand J Immunol. 1987 Apr;25(4):407–412. doi: 10.1111/j.1365-3083.1987.tb02207.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986 Oct;59(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Dertzbaugh M. T., Eldridge J. H., Hirasawa M., Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10(2):75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Zubiaga A. M., Merrow M., Sauter N. P., Huber B. T. Cholera toxin discriminates between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990 Jul 1;172(1):95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter A. P., Gilbert W. Antibodies of the secondary response can be expressed without switch recombination in normal mouse B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7189–7193. doi: 10.1073/pnas.81.22.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirzer U. C., Schürmann G., Post S., Betzler M., Meuer S. C. Differential responsiveness to CD3-Ti vs. CD2-dependent activation of human intestinal T lymphocytes. Eur J Immunol. 1990 Oct;20(10):2339–2342. doi: 10.1002/eji.1830201025. [DOI] [PubMed] [Google Scholar]
- Quiding M., Nordström I., Kilander A., Andersson G., Hanson L. A., Holmgren J., Czerkinsky C. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-gamma production and evokes local immunological memory. J Clin Invest. 1991 Jul;88(1):143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Weinberg A. D., English M., Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990 Dec 1;145(11):3796–3806. [PubMed] [Google Scholar]
- Szakal A. K., Kosco M. H., Tew J. G. Microanatomy of lymphoid tissue during humoral immune responses: structure function relationships. Annu Rev Immunol. 1989;7:91–109. doi: 10.1146/annurev.iy.07.040189.000515. [DOI] [PubMed] [Google Scholar]
- Vajdy M., Lycke N. Y. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology. 1992 Mar;75(3):488–492. [PMC free article] [PubMed] [Google Scholar]
- Williams N. A., Wilson A. D., Bailey M., Bland P. W., Stokes C. R. Primary antigen-specific T-cell proliferative responses following presentation of soluble protein antigen by cells from the murine small intestine. Immunology. 1992 Apr;75(4):608–613. [PMC free article] [PubMed] [Google Scholar]
- Wilson A. D., Bailey M., Williams N. A., Stokes C. R. The in vitro production of cytokines by mucosal lymphocytes immunized by oral administration of keyhole limpet hemocyanin using cholera toxin as an adjuvant. Eur J Immunol. 1991 Oct;21(10):2333–2339. doi: 10.1002/eji.1830211007. [DOI] [PubMed] [Google Scholar]
- Yaoita Y., Kumagai Y., Okumura K., Honjo T. Expression of lymphocyte surface IgE does not require switch recombination. Nature. 1982 Jun 24;297(5868):697–699. doi: 10.1038/297697a0. [DOI] [PubMed] [Google Scholar]
- Yefenof E., Sanders V. M., Uhr J. W., Vitetta E. S. In vitro activation of murine antigen-specific memory B cells by a T-dependent antigen. J Immunol. 1986 Jul 1;137(1):85–90. [PubMed] [Google Scholar]
- Zeitz M., Schieferdecker H. L., Ullrich R., Jahn H. U., James S. P., Riecken E. O. Phenotype and function of lamina propria T lymphocytes. Immunol Res. 1991;10(3-4):199–206. doi: 10.1007/BF02919693. [DOI] [PubMed] [Google Scholar]


